Abstract
Multiple myeloma (MM) is a progressive disease that is thought to result from multiple genetic insults to the precursor plasma cell that ultimately affords the tumor cell with proliferative potential despite its differentiated phenotype and resistance to undergoing apoptosis. Altered expression of antiapoptotic factors as well as growth factors have been described in a significant number of patients. However, the key regulatory elements that control myeloma development and progression remain largely undefined. Because of the knowledge that B-lymphocyte stimulator (BLyS), a tumor necrosis factor (TNF) family member shown to be critical for maintenance of normal B-cell development and homeostasis, promotes the survival of malignant B cells, we began a coordinated study of BLyS and its receptors in MM. All MM cells studied expressed one or more of 3 known receptors (B-cell maturation antigen [BCMA], transmembrane activator and CAML interactor [TACI], and B-cell activating factor receptor [BAFF-R]) for BLyS; however, the pattern of expression was variable. Additionally, we provide evidence that BLyS can modulate the proliferative capacity and survival of MM cells. Finally, we provide evidence that BLyS is expressed by MM cells and is present in the bone marrow of patients with MM. Expression of BCMA, TACI, and BAFF-R by MM taken together with the ability of BLyS to support MM cell growth and survival has exciting implications because they may be potential therapeutic targets.
Introduction
Multiple myeloma (MM) is a universally fatal disease characterized by the significant accumulation of malignant plasma cells in the bone marrow. Currently, there is no curative treatment for this disease, underscoring the need to achieve a better understanding of the mechanisms underlying myeloma cell growth and survival control. An important clinically relevant aspect of myeloma cell biology is the mechanism that underlies malignant plasma cell resistance to apoptosis leading to prolonged survival and tumor cell accumulation. Our current lack of knowledge concerning this process in myeloma is paralleled and amplified by our current lack of insight into signals that regulate survival of normal bone marrow resident, long-lived plasma cells.
B-lymphocyte stimulator (BLyS), also called B cell–activating factor (BAFF),1 tumor necrosis factor (TNF) homologue that activates apoptosis, nuclear factor κB (NF-κB), and c-Jun NH2-terminal kinase (THANK),2 TNF and apoptosis ligand-related leukocyte-expressed ligand 1 (TALL-1),3 or zTNF4,4 is a TNF family member critical for maintenance of normal B-cell development and homeostasis. BLyS shares significant homology with another TNF family member, a proliferation-inducing ligand (APRIL), which has been shown to stimulate tumor cell growth and is expressed by a variety of human cancers.5 Previous studies of BLyS suggest that it costimulates B-cell proliferation and immunoglobulin secretion.1,6 A role for BLyS in attenuating apoptosis was further substantiated because B cells isolated from BLyS transgenic mice have elevated Bcl-2 levels and prolonged survival.4,7 Transgenic overexpression of BLyS in mice results in elevated numbers of mature B cells, including plasma cells,7,8 and development of autoimmune-like manifestations reminiscent of systemic lupus erythematosus and Sjögren syndrome.7,9
BLyS is expressed by monocytes, macrophages, dendritic cells, and neutrophils10-12 and may be the mechanism by which macrophages and dendritic cells directly regulate human B-cell activation.12 Although most studies indicate that B cells do not express BLyS,1,13 we recently reported the novel observation that an autocrine BLyS pathway is present in the leukemic cells from a subset of patients with B-cell chronic lymphocytic leukemia.13 The mechanisms underlying regulation of BLyS expression remain to be clearly defined. However, the ability of interferon γ (IFN-γ), granulocyte-macrophage colony-stimulating factor (GM-CSF), and interleukin 10 (IL-10)10,11 to up-regulate BLyS expression suggests that inflammatory signals may in part regulate how and when BLyS is expressed.
Three receptors for BLyS have been identified: B-cell maturation antigen (BCMA),14 transmembrane activator and CAML interactor (TACI),4 and B cell–activating factor receptor (BAFF-R).15,16 BCMA and BAFF-R are predominantly expressed on B lymphocytes, whereas TACI can be found on B cells and activated T cells.4,14-16 TACI and BCMA can also bind to APRIL, whereas BAFF-R is specific for BLyS. A key role for BAFF-R in BLyS binding has been suggested by studies demonstrating that A/WySnJ mice, which carry a mutation in the BAFF-R, have a loss of follicular and marginal zone B cells in secondary lymphoid organs, a phenotype similar to BLyS-deficient mice.4,7,14 Thus, BAFF-R appears to be the primary BLyS-binding receptor responsible for B-cell development and survival. Emerging reports on the role of TACI in B-cell survival suggest an important negative regulatory role for this receptor in B-cell homeostasis and autoimmunity because TACI-deficient mice have an accumulation of splenic B cells and increased levels of serum immunoglobulin levels.17,18 The role of BCMA in B-cell function remains the most confusing. Although BLyS and APRIL can clearly bind to BCMA,14 studies of BCMA-/- mice have shown that this receptor appears to be dispensable for humoral immune responses.19 Additionally, there have been no genuine examples of BCMA cell surface expression on human lymphocytes because studies indicate that BCMA is predominantly expressed in the Golgi apparatus.20 Taken together, these studies implicate BLyS and its receptors as key players in B-cell biology; however, the precise role that each receptor plays in malignant B-cell biology, particularly terminally differentiated plasma cells, remains undefined.
The significance of BLyS in B-cell survival and homeostasis combined with the finding that malignant B cells express BLyS13 raises the possibility that BLyS and its receptors may be involved in the growth and survival of MM cells. Accordingly, we began a thorough analysis of BLyS receptor expression on MM cells and we investigated the effect of BLyS on MM cell growth and survival. In this study, we provide evidence that CD138+ human MM cells, as well as MM cell lines, variably express all 3 receptors for BLyS; TACI, BAFF-R, and BCMA on their cell surface. Additionally, we demonstrate that BLyS enhances cytokinestimulated MM cell proliferation and survival. Lastly, we find that BLyS is present in the bone marrow of patients with MM and we show that MM cells, similar to B-CLL cells, express BLyS. The novel finding that BCMA is expressed on MM cells, taken together with the presence of BLyS in MM bone marrow, suggests a potential role for this receptor/ligand system in the growth and survival of malignant plasma cells.
Materials and methods
Cells and reagents
The MM cell lines ANBL-6, DP-6, KAS-6/1, and KP-6 have been previously described.21 Peripheral blood mononuclear cells (PBMCs) and bone marrow or pleural aspirate mononuclear cells were isolated as previously described13 from healthy donors or patients with MM providing written informed consent. MM cells were purified using anti-CD138 microbeads (Miltenyi Biotec, Auburn, CA). Normal B lymphocytes were purified using anti-CD19 microbeads (Miltenyi Biotec). Zymogenetics provided anti-TACI, anti-BCMA, and anti–BAFF-R biotinylated antibodies and FLAG-tagged or biotinylated BLyS. RED670- and phycoerythrin (PE)–conjugated streptavidin were purchased from Gibco BRL (Carlsbad, CA) and Caltag (Burlingame, CA), respectively. Mouse IgG control was purchased from PharMingen (San Diego, CA). PE-conjugated anti-CD27, anti-CD5, and mouse IgG controls were purchased from Becton Dickinson (San Jose, CA).
Flow cytometry
Cells (1 × 106) were washed in phosphate-buffered saline (PBS) containing 0.5% bovine serum albumin (BSA) and incubated with 0.1 μg biotinylated recombinant BLyS, 0.5 μg biotinylated anti-BCMA, anti–BAFF-R, anti-TACI, or mouse Ig control alone or in combination with PE-conjugated anti-CD27 or anti-CD5 or for 30 minutes at 4°C. Cells were washed and incubated with streptavidin-PE or strepavidin-RED670 for 30 minutes at 4°C, washed, and analyzed using FACScalibur and CellQuest (Becton Dickinson).
Cell viability studies
To determine the effect of BLyS on cell viability, MM cells were cultured in RPMI plus 0.5% BSA alone or with the addition of 0.1 μg/mL BLyS. After 7 days, 1 × 106 cells were incubated with 100 μL Dulbecco PBS, 5 μL 500 μg/mL propidium iodide (PI), and 100 μL fluorescein diacetate at a final concentration of 2 μg/mL for 3 minutes at 37°C. Viable cells were counted using a fluorescent microscope (Olympus America, Melville, NY). Annexin/PI studies were performed by incubating 1 × 106 MM cells with 1 μg annexin V-fluorescein isothiocyanate (FITC; Caltag) for 20 minutes at 4°C. Cells were washed and 0.5 μg PI was added to each sample and immediately analyzed by fluorescence-activated cell sorting (FACS) as described (see “Flow cytometry”).
Proliferation assay
Serum-starved ANBL-6, KAS-6/1, and KP-6 myeloma cells were cultured in 96-well flat-bottom microtiter plates (Costar, Cambridge, MA) at a density of 2.5 × 104 cells/well in the presence of 1 ng/mL IL-6, 10 ng/mL insulin-like growth factor I (IGF-I), 0.1 μg/mL BLyS, alone or in combination, for 7 days at 37°C in the presence of 5% CO2. Cultures were pulsed with 1 μCi (0.037 MBq) tritiated thymidine (3H-TdR; 5.0 Ci/mmol [185 GBq/mmol], Amersham, Piscataway, NJ) for 18 hours, harvested, and 3H-TdR incorporation levels determined using a Beckman scintillation counter.
PCR analysis
For reverse transcription–polymerase chain reaction (RT-PCR), the TRIzol reagent (Invitrogen, Carlsbad, CA) was used to isolate total RNA from freshly isolated CD138+ MM cells and MM cell lines. RNA was converted into cDNA using the First-Strand cDNA Synthesis Kit (Amersham Pharmacia, Little Chalfont Buckinghamshire, United Kingdom) according to the manufacturer's instructions. BLyS and β-actin cDNAs were detected by PCR amplification with HotStarTaq (Qiagen, Valencia, CA) (steps of 1 minute each at 94°C, 60°C, and 72°C for 35 cycles) using primers previously described as being specific for BLyS1 (5′ GGA GAA GGC AAC TCC AGT CAG AAC and 3′ CAA TTC ATC CCC AAA GAC ATG GAC). The β-actin primers were designed using the published cDNA nucleotide sequences (5′ GGA TCC GAC TTC GAG CAA GAG ATG GCC AC and 3′ CAA TGC CAG GGT ACA TGG TG).
Immunohistochemistry
Cytospin preparations of ANBL-6, KAS-6/1, Jurkat, or BLyS-transfected Jurkat cells were fixed in acetone and air-dried. Slides were incubated 10 minutes in 0.1% azide/3% H2O2 to abolish any endogenous peroxidase activity and rinsed well with PBS. Prior to staining, the slides were preincubated with 10% powdered milk/Tris (tris(hydroxymethyl)aminomethane)–buffered saline (TBS) for 60 minutes to block nonspecific protein binding and washed twice with PBS. The preparations were incubated with 10 μg/mL mouse antihuman BLyS (clone 148725, R&D Systems, Minneapolis, MN), rat anti-BLyS (Buffy-2; Alexis Biotechnology, San Diego, CA) or isotype-matched mouse Ig control (Dako Cytomation, Carpinteria, CA) for 30 minutes at room temperature and rinsed twice with PBS. The preparations were then incubated for 30 minutes with biotinylated antimouse IgG (1:300; Dako Cytomation) and followed by an additional 30 minutes of incubation with horseradish peroxidase-conjugated streptavidin (1:300; Dako Cytomation). BLyS was visualized using 3,3′diaminobenzidine (Dako Cytomation) and counterstaining with hematoxylin. The slides were cover slipped and mounted with Cytoseal 280 (Stephens Scientific, Kalamazoo, MI).
Formalin-fixed, paraffin-embedded sections of normal bone marrow and marrow of MM (from archives of Mayo Clinic Tissue Registry) were deparaffinized with xylene and rehydrated to water through graded alcohol series. Endogenous peroxidase activity was blocked by incubation with 50:50 mixture of 3% H2O2/methanol for 10 minutes and rinsed well with water. The sections were pretreated by heating with 10 mM citrate buffer, pH 6.0, for 30 minutes with a 5-minute cooldown before rinsing well in running tap water. Immunohistochemical staining was performed as described with cytospin preparations. All slides were observed with light microscopy (Olympus America) with images being captured with a SPOT RT camera (Diagnostic Instruments, Burlingame, CA).
Results
Expression of BLyS receptors on human CD19+ B cells
We first wished to gain a better understanding of the BLyS receptor profile on normal human B cells. Therefore, we used flow cytometric analyses to determine cell surface expression of TACI, BCMA, and BAFF-R on various subsets of human peripheral blood CD19+ B cells (Figure 1). CD19+ B cells were costained with anti-CD27, to differentiate memory and naïve B cells,22 and antibodies to BAFF-R, BCMA, and TACI (Figure 1 top panel). Soluble biotinylated BLyS was used to determine total BLyS binding (Figure 1). Both memory and naïve B cells bind BLyS at high levels, express BAFF-R, and are deficient for BCMA expression. Both subsets also express TACI; however, CD27+ memory B cells express slightly higher levels. Expression of BLyS receptors was also detected on peripheral CD5+ B1 B cells (Figure 1 middle panel). Similar to memory and naïve B cells, CD5+ B cells bind BLyS, express BAFF-R and TACI, and express low to no BCMA. Jurkat T cells, which do not bind BLyS or express significant levels of BLyS receptors, serve as a negative control (Figure 1 lower panel). The lack of BCMA expression on the various subsets of peripheral blood B cells is notable and suggests that regulation of BCMA, which is thought to be expressed on mature B cells, may be dependent on the activation status and differentiation stage of the B cell.
Expression of BCMA, TACI, and BAFF-R on peripheral blood B cells. Freshly isolated CD19+ normal B cells were stained with biotin-conjugated anti-TACI, anti–BAFF-R, anti-BCMA, or BLyS for 30 minutes on ice, washed, and incubated with RED670-streptavidin and CD27-PE (top row) or CD5-PE (middle row). Jurkat T cells stained with biotin-conjugated anti-TACI, anti–BAFF-R, and anti-BCMA followed by PE-streptavidin served as negative controls (bottom row). Isotype and fluorochrome controls were done for each sample.
Expression of BCMA, TACI, and BAFF-R on peripheral blood B cells. Freshly isolated CD19+ normal B cells were stained with biotin-conjugated anti-TACI, anti–BAFF-R, anti-BCMA, or BLyS for 30 minutes on ice, washed, and incubated with RED670-streptavidin and CD27-PE (top row) or CD5-PE (middle row). Jurkat T cells stained with biotin-conjugated anti-TACI, anti–BAFF-R, and anti-BCMA followed by PE-streptavidin served as negative controls (bottom row). Isotype and fluorochrome controls were done for each sample.
MM cells express TACI, BCMA, and BAFF-R
We next examined the BLyS-binding and receptor profile on a panel of cytokine-responsive MM cell lines (Figure 2A). Four of the 5 myeloma lines consistently bound soluble BLyS and expressed BCMA and TACI. The cell surface expression pattern of BCMA by MM cells is a novel finding because it has been typically found as an intracellular protein.20 Surprisingly, all the lines examined lack significant expression of BAFF-R. The KP-6 cell line was unique in that it lacked expression of all 3 BLyS receptors and was unable to bind soluble BLyS. We next examined the BLyS receptor profile on freshly isolated CD138+ human MM cells (Figure 2B). All 3 samples bound soluble BLyS and expressed BCMA and TACI. However, unlike the MM lines, 2 of 3 fresh patient MM cells express BAFF-R. The lack of BAFF-R on the cell lines may be artifact due to their in vitro culture conditions. However, expression of BAFF-R was absent on one freshly isolated MM (Figure 2B bottom panel) and may indicate that BAFF-R expression may be lost in vivo as well. As previously shown, the BLyS-binding and BLyS receptor profile of normal peripheral blood memory B cells (CD19+/CD27+) is distinct from MM cells because they lack significant BCMA expression (Figure 2C). In summary, MM cell lines (4 of 5) and freshly isolated MM cells (3 of 3) consistently bind soluble BLyS, express BCMA and TACI, and have variable expression of BAFF-R.
Expression of BCMA, TACI, and BAFF-R on MM cells. (A) MM cell lines ANBL-6, DP-6, KAS-6/1, KP6, and JMW were stained with biotin-conjugated anti-TACI, anti–BAFF-R, anti-BCMA, or BLyS (gray histograms) for 30 minutes on ice, washed, and incubated with PE-streptavidin. Isotype and fluorochrome controls were done for each sample (open histograms). (B-C) CD138+ human MM cells (B) and CD19+/CD27+ memory B cells (C) were stained and analyzed as described for panel A..
Expression of BCMA, TACI, and BAFF-R on MM cells. (A) MM cell lines ANBL-6, DP-6, KAS-6/1, KP6, and JMW were stained with biotin-conjugated anti-TACI, anti–BAFF-R, anti-BCMA, or BLyS (gray histograms) for 30 minutes on ice, washed, and incubated with PE-streptavidin. Isotype and fluorochrome controls were done for each sample (open histograms). (B-C) CD138+ human MM cells (B) and CD19+/CD27+ memory B cells (C) were stained and analyzed as described for panel A..
Regulation of BCMA, TACI, and BAFF-R expression is poorly understood and a thorough analysis of human B-cell expression of these receptors along the differentiation continuum and following activation has yet to be accomplished. In accordance with our data, developmental regulation of BCMA is supported by recent gene profiling studies of B-cell subsets where BCMA was found to be up-regulated during the late stages of normal B-cell differentiation and was highly expressed on MM cells.23-25 In one study, the mean level of BCMA mRNA expression in bone marrow plasma cells from 31 patients was almost 10-fold higher than BCMA levels in tonsillar B cells, further emphasizing the dramatic up-regulation of this receptor in highly differentiated B cells.24 However, the mechanisms by which this occurs remains completely unknown. The definition of the precise role of BCMA expression on normal plasmactyes or plasma cells also remains to be determined.
The expression of TACI and BAFF-R by MM cells was expected because normal mature B cells express these receptors. The significance of BAFF-R expression, or lack of, in MM cells remains to be defined, but the finding of variable BAFF-R expression is of interest because it highly influences the lifespan of B cells.15,16 The ability of MM cell lines to bind BLyS regardless of BAFF-R expression does, however, suggest that in MM cells, TACI or BCMA can compensate for the loss of BAFF-R. It will be of utmost importance to thoroughly examine the expression levels of all 3 receptors on a larger cohort of MM samples to more definitively understand their contribution to MM biology. It is possible that altered expression of BLyS receptors may contribute to the progressive accumulation of malignant B cells characteristic of MM.
BLyS promotes survival and proliferation of MM cells
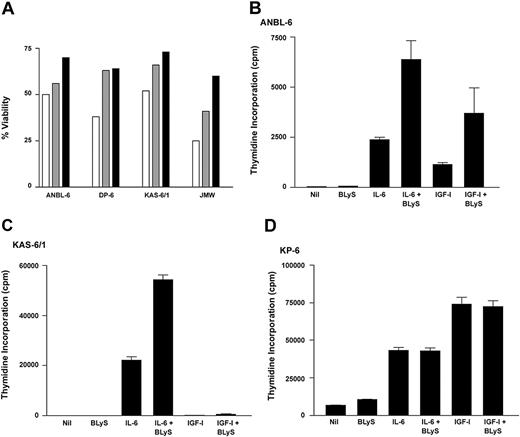
Because of the large number of reports that place BLyS as a central component of B-cell homeostasis and survival,8 we next examined the effect of BLyS on MM cell survival (Figure 3A) Similar to normal B cells, all MM cell lines cultured in the presence of BLyS had greater viability compared to cells cultured in media alone. IL-6, a known survival factor for MM cells, promoted MM cell survival at levels similar to BLyS. The increase in cell viability seen with the MM cell lines was relatively low compared to normal B cells (data not shown), and this is likely due to the presence of redundant survival mechanisms found in cell lines. However, the ability of BLyS to enhance cell survival was consistently seen using various methods of apoptosis detection (Figure 3 and data not shown).
BLyS promotes survival and proliferation of MM cells. (A) MM cells were cultured in RPMI plus 0.5% BSA alone (□) or with the addition of 0.1 μg/mL BLyS (▤) or 1 ng/mL IL-6 (▪), and cell viability was determined after 7 days. A minimum of 500 cells was counted for each condition. (B-D) MM cells were cultured in 96-well flatbottom microtiter plates in the presence of 1 ng/mL IL-6, 10 ng/mL IGF-I, 0.1 μg/mL BLyS, alone or in combination for 7 days at 37°C in the presence of 5% CO2. Values represent the mean of triplicate values of a representative experiment (n = 5).
BLyS promotes survival and proliferation of MM cells. (A) MM cells were cultured in RPMI plus 0.5% BSA alone (□) or with the addition of 0.1 μg/mL BLyS (▤) or 1 ng/mL IL-6 (▪), and cell viability was determined after 7 days. A minimum of 500 cells was counted for each condition. (B-D) MM cells were cultured in 96-well flatbottom microtiter plates in the presence of 1 ng/mL IL-6, 10 ng/mL IGF-I, 0.1 μg/mL BLyS, alone or in combination for 7 days at 37°C in the presence of 5% CO2. Values represent the mean of triplicate values of a representative experiment (n = 5).
The ability of BLyS to costimulate BCR and CD40L-mediated B-cell proliferation1,6 suggests that BLyS has the ability to influence the proliferative capacity of B cells. The effect of BLyS on plasma cell growth remains undefined; therefore, we examined the ability of BLyS to modulate cell proliferation mediated by IL-6 and IGF-I, 2 known growth factors for MM cells (Figure 3B-D). Using 3 cytokine-responsive MM lines as a model system, we saw that BLyS alone had no effect on MM cell proliferation. However, BLyS consistently enhanced IL-6–mediated cell proliferation in the ANBL-6 and KAS-6/1 lines. BLyS was also able to enhance IGF-I–mediated cell proliferation of ANBL-6 and to a lesser extent in KAS-6/1. BLyS was unable to enhance IL-6 and IGF-I proliferation in the KP-6 line. The difference in BLyS responsiveness between the cell lines reflects differential patterns in soluble BLyS binding and expression of BLyS receptors. As previously shown (Figure 2A), ANBL-6 and KAS-6/1 cells bind soluble BLyS and express BCMA and TACI, whereas KP-6 cells have low to no BLyS binding and lack expression of any of the 3 BLyS receptors. Taken together, these results suggest that BLyS has the potential to influence growth and survival of MM cells. The ability of BLyS to influence MM cell responsiveness to IL-6 or IGF-I is significant because these cytokines have been implicated as key regulators of MM cell growth and survival.26 Because BLyS alone had no effect on the proliferative capacity of MM cells, it is likely that attenuation of cell death is the mechanism by which BLyS affects the proliferative capacity of MM. The ability of BLyS to promote MM cell survival suggests a possible role for this protein in the maintenance and survival of malignant MM cells.
Expression of BLyS in MM cells
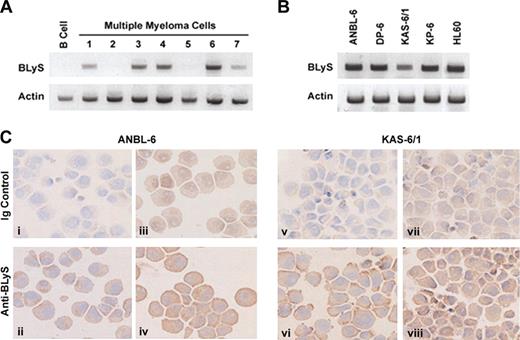
Current findings suggest that BLyS is predominantly expressed by activated monocytes and neutrophils.10,11 However, evidence indicates that BLyS can be expressed by tumor cells, including malignant B cells.13 Therefore, we next wished to determine whether primary MM cells or MM cell lines could express BLyS. To examine this possibility, we used RT-PCR to detect BLyS mRNA in purified CD138+ bone marrow mononuclear cells from patients with MM (Figure 4A) or our panel of MM cell lines (Figure 4B). Of interest, BLyS mRNA was detected in 5 of 7 patients (Figure 4A). By contrast, normal CD19+ peripheral blood B cells failed to express BLyS. Additionally, we found that all MM cell lines examined also expressed mRNAfor BLyS (Figure 4B). The PCR product for BLyS was excised and sequenced to confirm its identity (data not shown). The HL60 myelomonocytic cell line was used as a positive control (Figure 4B). The expression pattern of BLyS during normal B-cell differentiation is unclear and most studies suggest that BLyS is not expressed by normal resting or activated B cells.6,11,13 The evidence of BLyS mRNA expression by our panel of MM cell lines is clear and suggests the possibility of an autocrine feedback loop between BLyS and its receptors in MM. In support of these findings, recent gene profiling studies also indicate that MM cells express BLyS.25
Expression of BLyS in myeloma cells. Expression of BLyS mRNA was analyzed by RT-PCR in 7 CD138+ primary MM cell samples or CD19+ resting B cells (A) or in a panel of MM cell lines (B). The HL60 monocytic cell line was used as a positive control. (C) Immunohistochemical analysis of BLyS expression in ANBL-6 and KAS-6/1 MM cell lines was performed as described in “Materials and methods.” The antibody used in panels ii and vi was specific for membrane-bound BLyS (Alexis Biotechnology), whereas the antibody used in panels iv and viii was specific for soluble and membranebound BLyS (R&D Systems). Corresponding isotype controls are shown in panels i, iii, v, and vii. Original magnification, ×200.
Expression of BLyS in myeloma cells. Expression of BLyS mRNA was analyzed by RT-PCR in 7 CD138+ primary MM cell samples or CD19+ resting B cells (A) or in a panel of MM cell lines (B). The HL60 monocytic cell line was used as a positive control. (C) Immunohistochemical analysis of BLyS expression in ANBL-6 and KAS-6/1 MM cell lines was performed as described in “Materials and methods.” The antibody used in panels ii and vi was specific for membrane-bound BLyS (Alexis Biotechnology), whereas the antibody used in panels iv and viii was specific for soluble and membranebound BLyS (R&D Systems). Corresponding isotype controls are shown in panels i, iii, v, and vii. Original magnification, ×200.
To confirm our RT-PCR results we next examined MM cell lines for expression of BLyS protein by immunohistochemistry. ANBL-6 and KAS-6/1 cells were stained with 2 different antibodies to BLyS and their corresponding Ig controls (Figure 4C). BLyS was detected in both the ANBL-6 and KAS-6/1 cells. The pattern of staining varied between the reagents as the antibody used in Figure 4Cii,vi (Buffy-2; Alexis Biotechnology) is specific for membranebound BLyS, whereas the antibody used in Figure 4Civ,viii (clone 148725; R&D Systems) is specific for soluble and membranebound BLyS. The corresponding Ig controls are shown in the top panel (Figure 4Ci,iii,v,vii) for each cell type. In addition to the ANBL-6 and KAS-6/1 cell lines, we examined the expression of BLyS in KP-6 cells, which lack significant expression of BLyS receptors. Similar to the MM lines shown, BLyS expression was detected by KP-6 at low levels using both antibodies (data not shown). The mechanisms underlying regulation of BLyS expression, secretion, and binding by MM cells remains undefined and is currently under investigation. These findings confirm that MM cells express BLyS and provide evidence for an autocrine feedback loop that may afford MM cells a survival advantage.
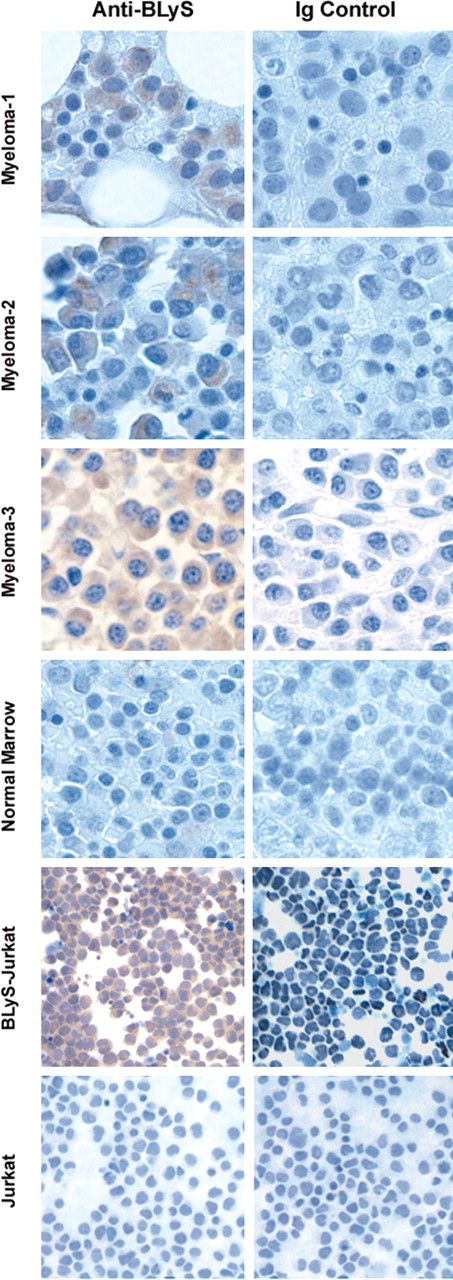
To our knowledge, BLyS expression levels in the bone marrow microenvironment of healthy individuals or patients with MM have not yet been reported. Therefore, we next examined bone marrow sections from MM patients for expression of BLyS using immunohistochemistry. MM or normal bone marrow tissue sections were stained with a BLyS-specific antibody or an isotype-matched control to determine BLyS expression. Jurkat T cells or Jurkat T cells stably expressing BLyS served as negative and positive controls, respectively. Bone marrow sections from MM patients stained brightly for BLyS, whereas there was low diffuse staining in normal marrows (Figure 5). Moreover, within the marrow microenvironment BLyS staining was localized to MM cells. The level of BLyS staining varied among patients, with some staining very highly (MM-3), whereas others had diffuse staining (MM-1). The results shown are representative of samples from 10 patients and 3 healthy individuals. These findings correlate with our PCR results in that there is variable expression of BLyS in marrows from MM patients. Although these findings do not specifically address which cell in the bone marrow produces BLyS, they do provide evidence that BLyS is present in the bone marrow of MM patients at higher levels compared to normal marrows. The ability of BLyS to attenuate apoptosis combined with the finding that BLyS binds to MM cells suggests a scenario in which MM cells use BLyS as a survival factor.
Expression of BLyS in primary myeloma cells. Immunohistochemical analysis of BLyS expression in normal bone marrow, MM bone marrow (MM 1-3), Jurkat, or BLyS-transfected Jurkat cells was performed with the anti-BLyS mAb from R&D Systems as described in “Materials and methods.” These results are representative of 3 normal and 10 MM samples. Original magnification, ×200.
Expression of BLyS in primary myeloma cells. Immunohistochemical analysis of BLyS expression in normal bone marrow, MM bone marrow (MM 1-3), Jurkat, or BLyS-transfected Jurkat cells was performed with the anti-BLyS mAb from R&D Systems as described in “Materials and methods.” These results are representative of 3 normal and 10 MM samples. Original magnification, ×200.
Discussion
The mechanism of action of BLyS remains poorly understood, in part because of the complexity introduced by multiple receptors. Study of signal transduction through these receptors is in its relative infancy; however, there is growing literature demonstrating a role for each receptor in activation of NF-κB (for a review, see Mackay et al27 ). The ability of BLyS to modulate NF-κB is interesting in the context of MM because increased NF-κB activity is associated with enhanced tumor cell survival.28,29
The high degree of similarity between the murine and human BLyS receptors, yet relative lack of homology between the 3 receptors within a species, suggests the possibility that these receptors may trigger both unique and common events following ligand binding. These differences may become particularly apparent if receptor composition changes as a function of B-cell differentiation, for example, increased BCMA expression in plasma cells.
In summary, we have shown for the first time that MM cell lines and fresh tumor samples from patients bind soluble BLyS and express BCMA, TACI, and BAFF-R. Additionally, we provide evidence that BLyS can modulate the proliferative capacity of cytokine-stimulated MM cells, likely through its ability to promote survival. Furthermore, we provide evidence that BLyS is expressed by MM cells and is present in the bone marrow of patients with MM. Expression of BCMA, TACI, and BAFF-R by MM taken together with the ability of BLyS to support MM cell growth and survival has exciting implications. Design of agents that block the interaction between BLyS and its receptors or target this receptor/ligand system30 may have therapeutic potential in MM and other B-cell malignancies.
Prepublished online as Blood First Edition Paper, September 25, 2003; DOI 10.1182/blood-2003-06-2043.
Supported by National Institutes of Health grant CA62228 and CA62442 (D.F.J.). A.J.N. was supported by a postdoctoral trainee award from National Institutes of Health (grant CA09441).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Steven Ziesmer and Linda Wellik for their help with immunohistochemistry and Dr Rick Bram for the use of reagents.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal