Abstract
Tumor necrosis factor (TNF) superfamily members BAFF, or B-cell activation factor of the TNF family, and APRIL, a proliferation-inducing ligand, are involved in normal B-cell survival and differentiation. They interact with 3 receptors: BAFF-R, specific to BAFF; and TACI and BCMA, which are shared by BAFF and APRIL. We tested the potential role of these proteins in B-cell chronic lymphocytic leukemia (B-CLL) resistance to apoptosis. TACI and BAFF-R mRNAs were found in leukemic B cells. BAFF and APRIL mRNAs and proteins were detected in B-CLL leukemic cells and normal blood or tonsil-derived B lymphocytes. Yet, in contrast to normal B lymphocytes, BAFF and APRIL were expressed at the membranes of leukemic cells. Adding soluble BAFF or APRIL protected B-CLL cells against spontaneous and drug-induced apoptosis and stimulated NF-κB activation. Conversely, adding soluble BCMA-Fc or anti-BAFF and anti-APRIL antibodies enhanced B-CLL apoptosis. Moreover, a soluble form of BAFF was detected using surface-enhanced laser desorption/ionization–time-of-flight mass spectrometry (SELDI-TOF MS) in the sera of B-CLL patients but not of healthy donors. Taken together, our results indicate that B-CLL cells can be rescued from apoptosis through an autocrine process involving BAFF, APRIL, and their receptors. Inhibiting BAFF and APRIL pathways may be of therapeutic value for B-CLL treatment.
Introduction
B-cell chronic lymphocytic leukemia (B-CLL), the most prevalent leukemia in Western countries, is characterized by the gradual accumulation in patients of small, mature B cells with the typical B-cell markers CD5, CD19, CD23, and CD20.1,2 Inasmuch as most tumoral cells are quiescent, this accumulation results from deficient apoptosis rather than from acute proliferation. However, a proliferating pool of cells has been described in lymph nodes and in bone marrow that might feed the accumulating pool in the blood.3 Numerous parameters contribute to the resistance of B-CLL to apoptosis, such as intrinsic defects in their apoptotic machinery or dysregulated production of survival signals from their microenvironment.4,5
To further understand this resistance, we concentrated our research on 2 genes implicated in B-cell survival, BAFF—B-cell activation factor of the tumor necrosis factor (TNF) family, known also as BlyS, B-lymphocyte stimulator; THANK, a TNF homologue that activates apoptosis, nuclear factor-κB, and c-jun NH2-terminal kinase; TALL-1, TNF- and ApoL-related leukocyte-expressed ligand-1, zTNF4; and TNFSF13B, TNF-superfamily member 13B6-10 —and APRIL—a proliferation-inducing ligand, also named TRDL-1α for TNF-related death ligand-1α, TALL-2, zTNF2, or TNFSF13.11,12 These molecules belong to a cluster of the TNF superfamily and share several biologic characteristics and functions, although their effects are not redundant.13 Both are homotrimeric type 2 transmembrane proteins, but they exist also as soluble molecules6,7,11 derived from the cleavage of transmembrane forms by a furinlike convertase.14,15 BAFF is expressed at the surfaces of myeloid cells and antigen-presenting cells (APCs)6,7,16 and induces B-lymphocyte proliferation and immunoglobulin secretion.7 Macrophage- and dendritic–cell-derived BAFF is a key molecule by which these APCs regulate human B-cell proliferative responses to T-cell–independent stimuli.16 APRIL, practically undetectable in normal tissues, is strongly up-regulated in many tumor cells in vivo and in vitro and stimulates tumor cell growth.11
Both BAFF and APRIL bind with high affinity 2 members of the TNF-receptor (TNF-R) superfamily, B-cell maturation antigen (BCMA) and transmembrane activator and calcium modulator and cyclophilin ligand-interactor (TACI).10,17-19 BCMA was discovered fused to the interleukin-2 (IL-2) gene by translocation in a malignant T-cell lymphoma.20 BCMA signaling involves the TNF-R–associated factors (TRAFs) 1, 2, 3, 5, and 6 and results in the activation of NF-κB, Elk-1, JNK (c-Jun NH2-terminal kinase), and p38 mitogen-activated protein kinase.8,21 The TACI intracellular domain also interacts with TRAF2, TRAF5, and TRAF6 and activates nuclear factor κB (NF-κB) and JNK.19 BAFF binding to human B cells results in NF-κB and Ets family transcription factor (ELF-1) activation and in Polo-like kinase mRNA induction.22 BAFF, but not APRIL, binds a third receptor named BAFF receptor (BAFF-R or BR3).23-25 BCMA, TACI, and BAFF-R are expressed on normal B lymphocytes23,26,27 and on a subset of activated T cells for TACI.27
This system of receptors/ligands is a key factor for B-cell survival and differentiation. In the presence of innate immune signals such as interferons, dendritic cells express more BAFF and APRIL, which, together with cytokines (IL-10, transforming growth factor-β [TGF-β], IL-4), induce immunoglobulin classswitch DNA recombination and plasmacytoid differentiation in a CD40-independent manner. BAFF and APRIL thus link innate and adaptive immune responses.28 Through the up-regulation of BAFF and APRIL on dendritic cells, innate immune signals can convert subliminal B-cell activation to a productive response at the risk of favoring autoantibody production.29
The BAFF system provides potential insight into the development of autoreactive B cells, but it also provides a paradigm of the interplay among survival, growth, and death that affects all cells.30 BAFF binds to B cells and promotes their survival and proliferation.6,7,31 Transgenic mice overexpressing BAFF in lymphoid tissues10,32,33 display hyperplasia of the mature B-cell compartment and, with age, develop symptoms of systemic lupus erythematosus (SLE) and Sjögren disease.34 Mice deficient in BAFF, like those presenting a mutation in the BAFF-R/BR3 gene, display a deficit in peripheral B lymphocytes.23,24,35,36 TACI-Fc or BAFF-R-Fc soluble forms can block B-cell proliferation induced by BAFF.19,23,24 APRIL stimulates in vitro proliferation of mouse primary B and T cells and increases spleen weight resulting from the accumulation of B cells in vivo and the percentage of activated T cells, suggesting a role for APRIL in lymphoid homeostasis.37 Soluble BCMA and TACI preventAPRIL-stimulated proliferation of primary B cells.37 Analysis of T cells revealed an activation-dependent increase in APRIL mRNA expression, and T cells from APRIL transgenic mice showed enhanced survival in vitro and reactive CD4+ T cells in vivo.38
In the present study, we have explored the contributions of BAFF, APRIL, and their receptors to the control of B-CLL apoptosis. Our results demonstrate that, though BAFF and APRIL mRNAs and proteins are detected to the same extent in purified CD19+ normal B lymphocytes and B-CLL leukemic cells, only the latter express the corresponding molecules at their membranes. Neutralizing the effects of these molecules with specific antibodies or with a soluble form of their common receptor, BCMA-Fc, leads to apoptosis induction in B-CLL cells. Conversely, adding exogenous soluble BAFF and APRIL to B-CLL cultures results in increased resistance to spontaneous or drug-induced apoptosis. Finally, a soluble form of BAFF was detected by surface-enhanced laser desorption/ionization–time-of-flight mass spectrometry (SELDI-TOF MS) in sera from patients with B-CLL but not from healthy donors. These results emphasize the protective role of BAFF and APRIL toward apoptosis and suggest the existence of autocrine or paracrine mechanisms for B-CLL survival.
Patients, materials, and methods
Patients and cells
After informed consent, peripheral blood samples from patients with untreated B-CLL (according to standard clinical and laboratory criteria) were obtained from the Hematology Departments of Hôtel Dieu and Institut Curie (both in Paris, France; courtesy of Dr C. Mathiot) (Table 1). The samples were collected after written informed consent was given, in accordance with the rules and tenets of the recently revised Helsinki protocol. Peripheral blood mononuclear cells (PBMCs) were collected after 30-minute centrifugation at 400g on Ficoll-Hypaque gradient (Pharmacia, Uppsala, Sweden). Residual monocytes were eventually depleted by adherence to plastic, as described.39
Clinical characteristics of B-CLL patients
Patient . | Sex . | Binet stage . | Age, y . | Lymphocyte count, mm3 . | Time from diagnosis . | VH mutational status . | Cytogenetics . |
|---|---|---|---|---|---|---|---|
| 1 | M | A | 72 | 80 000 | — | — | — |
| 2 | M | A | 73 | 110 000 | — | — | — |
| 3 | F | A | 46 | 257 000 | 5 y | — | Trisomy 12 |
| 4 | M | B | 72 | 122 000 | 6 mo | Unmutated | Trisomy 12 |
| 5 | F | A | 53 | 117 000 | 10 y | Mutated | Deletion 13 |
| 6A | M | B | 50 | 159 000 | 12 y | Mutated | Normal |
| 6B | 52 | 170 000 | 14 y | ||||
| 7 | F | C | 80 | 110 000 | 1 y | — | — |
| 8A | M | C | 65 | 64 000 | 4 y | — | — |
| 8B | 66 | 66 000 | 5 y | ||||
| 9 | M | C | 56 | 640 000 | 3 y | — | — |
| 10A | F | A | 70 | 92 000 | 3 y | Unmutated | — |
| 10B | 71 | 116 000 | 4 y | ||||
| 11 | F | A | 70 | 102 000 | > 20 y | — | — |
| 12 | F | A | 54 | 59 000 | First time | — | — |
| 13A | M | A | 66 | 37 000 | 1 y | — | Normal |
| 13B | 67 | 40 000 | 2 y | ||||
| 13C | 67 | 45 000 | 2 y | ||||
| 14 | M | A | 78 | 142 000 | 10 y | — | Deletions 13 + 11 |
| 15A | M | B | 60 | 25 000 | 3 y | Unmutated | Deletion 11 |
| 15B | 60 | 43 000 | 3 y | ||||
| 15C | 61 | 97 000 | 4 y | ||||
| 16 | F | A | 67 | 17 000 | 4 y | Mutated | — |
| 17 | F | B | 60 | 67 000 | 3 y | Mutated | — |
| 18A | F | A | 63 | 162 000 | 2 y | Mutated | Normal |
| 18B | 64 | 165 000 | 2 y | ||||
| 18C | 64 | 140 000 | 2 y | ||||
| 19 | F | A | 75 | 105 000 | — | — | — |
| 20 | M | B | 58 | 80 000 | 3 y | Mutated | Deletion 13 |
| 21 | M | A | 70 | 72 000 | 2 y | — | — |
| 22 | M | A | 51 | 19 000 | First time | — | — |
| 23 | M | A | 75 | 20 000 | 1 y | — | — |
Patient . | Sex . | Binet stage . | Age, y . | Lymphocyte count, mm3 . | Time from diagnosis . | VH mutational status . | Cytogenetics . |
|---|---|---|---|---|---|---|---|
| 1 | M | A | 72 | 80 000 | — | — | — |
| 2 | M | A | 73 | 110 000 | — | — | — |
| 3 | F | A | 46 | 257 000 | 5 y | — | Trisomy 12 |
| 4 | M | B | 72 | 122 000 | 6 mo | Unmutated | Trisomy 12 |
| 5 | F | A | 53 | 117 000 | 10 y | Mutated | Deletion 13 |
| 6A | M | B | 50 | 159 000 | 12 y | Mutated | Normal |
| 6B | 52 | 170 000 | 14 y | ||||
| 7 | F | C | 80 | 110 000 | 1 y | — | — |
| 8A | M | C | 65 | 64 000 | 4 y | — | — |
| 8B | 66 | 66 000 | 5 y | ||||
| 9 | M | C | 56 | 640 000 | 3 y | — | — |
| 10A | F | A | 70 | 92 000 | 3 y | Unmutated | — |
| 10B | 71 | 116 000 | 4 y | ||||
| 11 | F | A | 70 | 102 000 | > 20 y | — | — |
| 12 | F | A | 54 | 59 000 | First time | — | — |
| 13A | M | A | 66 | 37 000 | 1 y | — | Normal |
| 13B | 67 | 40 000 | 2 y | ||||
| 13C | 67 | 45 000 | 2 y | ||||
| 14 | M | A | 78 | 142 000 | 10 y | — | Deletions 13 + 11 |
| 15A | M | B | 60 | 25 000 | 3 y | Unmutated | Deletion 11 |
| 15B | 60 | 43 000 | 3 y | ||||
| 15C | 61 | 97 000 | 4 y | ||||
| 16 | F | A | 67 | 17 000 | 4 y | Mutated | — |
| 17 | F | B | 60 | 67 000 | 3 y | Mutated | — |
| 18A | F | A | 63 | 162 000 | 2 y | Mutated | Normal |
| 18B | 64 | 165 000 | 2 y | ||||
| 18C | 64 | 140 000 | 2 y | ||||
| 19 | F | A | 75 | 105 000 | — | — | — |
| 20 | M | B | 58 | 80 000 | 3 y | Mutated | Deletion 13 |
| 21 | M | A | 70 | 72 000 | 2 y | — | — |
| 22 | M | A | 51 | 19 000 | First time | — | — |
| 23 | M | A | 75 | 20 000 | 1 y | — | — |
VH status was defined as mutated if DNA sequences from leukemic cells differed 2% or more from germline. For some patients, blood samples were collected at different times as indicated by the letters. — indicates not tested.
Normal blood samples were obtained from the Institut Français du Sang as residues from platelet preparations. Small, resting, normal B lymphocytes were purified from PBMCs by positive selection on anti-CD19–coated magnetic beads (Miltenyi Biotec, Bergisch Gladbach, Germany; Dynal, Oslo, Norway). Their purity usually ranged from 92% to 95%, as estimated by labeling with an anti-CD20–phycoerythrin (PE) antibody, and monocyte contamination never exceeded 2%.40 Purified tonsillar B cells were isolated as described previously41 and consisted of memory and virgin B-cell populations.42
The EHEB cell line (DSMZ, Braunschweig, Germany) was established from a B-CLL patient.43 U937 is a human monocytic cell line, and RAJI is derived from Burkitt lymphoma.
Cells were cultured at 37°C in a humidified atmosphere of 5% CO2 in air in RPMI 1640 medium supplemented with 2 mM glutamine, 1 mM sodium pyruvate, 100 IU/mL penicillin, 100 μg/mL streptomycin, and 10% fetal calf serum (FCS) (Myoclone Super Plus; Invitrogen Life Technologies, Carlsbad, CA).
Reagents
Recombinant human soluble BAFF (amino acids 134-285, 17 kDa) and APRIL (extracellular domain [residues 110-250] fused to CD33 signal peptide) were from BioVision Research Products (Mountain View, CA) and R&D Systems (Minneapolis, MN), respectively. BCMA-Fc was a kind gift from Dr A. Tsapis (INSERM U131, Clamart, France). Goat anti-BAFF (C-16) and anti-APRIL (R-15) polyclonal antibodies (sc-5744 and sc-5739) and their corresponding blocking peptides were from Santa Cruz Biotechnology (Santa Cruz, CA). Normal goat immunoglobulin G (IgG) was from Caltag Laboratories (Burlingame, CA). Rabbit anti-BAFF polyclonal antibody was from BD Biosciences (Franklin Lakes, NJ). Normal rabbit IgG was from Calbiochem (Darmstadt, Germany). Flavopiridol was a kind gift fromAventis Pharmaceuticals (Bridgewater, NJ). All other reagents, including diethyl-dithiocarbamate (DETC), and chemicals were from Sigma (St Louis, MO).
Reverse transcription–polymerase chain reaction
Total RNA from B-CLL patients and healthy donor cells was isolated using an extraction kit (Qiagen, Courtaboeuf, France) and was quantified by spectrophotometry (Ultrospec 3000; Amersham Pharmacia, Piscataway, NJ). Purity was checked by migration on a 1% agarose gel.
BAFF, APRIL, TACI, BAFF-R, and β2-microglobulin mRNAs were detected by reverse transcription–polymerase chain reaction (RT-PCR) amplification. After RT of 1 μg total RNA into cDNA as previously described,39 PCR was performed using Taq DNA polymerase (Invitrogen Life Technologies) on cDNA diluted at 1:10 and was mixed with deoxynucleotide triphosphate (dNTP) (Sigma) and specific primers (0.5 μM) in a GeneAmp PCR thermocycler (System 9600; Perkin Elmer, Norwalk, CT) programmed with a 30-second denaturation step at 94°C, followed by 35 cycles of amplification and 10 minutes of final elongation at 72°C. Primers (synthesized by Sigma-Genosys, Cambridgeshire, United Kingdom) and cycles were as follows: BAFF—5′-CCTCACGGTGGTGTCTTTCT-3′, 5′-AAAGCTGAGAAGCCATGGAA-3′, 94°C at 45 seconds, 64°C at 45 seconds, 72°C at 60 seconds; APRIL—5′-GCTCATGCCAGCCTCATCTC-3′, 5′-CCAGGTGCAGGACAGAGTGCT-3′,44 94°C at 45 seconds, 67°C at 45 seconds, 72°C at 60 seconds; TACI—5′-GCAGTACTGGGATCCTCTGCTG-3′, 5′-GCTTCTGAGCCTCTGTGCTCCA-3′, 94°C at 45 seconds, 67°C at 45 seconds, 72°C at 60 seconds; BAFF-R—5′-CTGGTCCTGGTGGGTCTG-3′, 5′-TCTTGGTGGTCACCAGTTCA-3′, 94°C at 50 seconds, 57°C at 50 seconds, 72°C at 50 seconds; β2-microglobulin—5′-CATCCAGCGTACTCCAAAGA-3′, 5′-GACAAGTCTGAATGCTCCAC-3′,39 94°C at 45 seconds, 60°C at 45 seconds, 72°C at 60 seconds.
PCR fragments were separated on 2% agarose gels and visualized by ethidium bromide on an imager (Appligene Oncor, Illkirch, France).
Western blot analysis of BAFF and APRIL
Cells were lysed in 1% Triton buffer. After quantification by Bradford assay (Bio-Rad, Hercules, CA), proteins were separated using 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and were transferred to nitrocellulose membrane. Immunoblots were probed with rabbit anti-BAFF polyclonal antibody preincubated or not with recombinant human-soluble BAFF, goat anti-APRIL polyclonal antibody preincubated or not with blocking peptide, or mouse antiactin monoclonal antibody (ICN Biomedicals, Aurora, OH). They were revealed by enhanced chemiluminescence (Perkin Elmer).
Flow cytometry analysis
To analyze membrane expression of BAFF and APRIL, purified B cells were incubated with goat anti-BAFF or anti-APRIL polyclonal antibody or normal goat IgG. Binding was revealed by fluorescein-conjugated, affinitypurified donkey or rabbit F(ab')2 fragment antigoat IgG (H&L; Rockland, Gilbertsville, PA). For some patients, cells were preincubated with either human plasma IgG or FcγR blocking reagent (Miltenyi Biotec) to prevent nonspecific binding of the antibodies.
Cells were analyzed by flow cytometry (FACSort; Becton Dickinson, San Jose, CA) using the CellQuest program. Results were estimated by Kolmogorov-Smirnov testing. Statistical significance was estimated using the one-sided unpaired Student t test.
Nucleosome detection in cytoplasm
Apoptosis-induced DNA fragmentation was quantified using enzymelinked immunosorbent assay (ELISA) (Cell Death Detection ELISAPLUS; Roche Diagnostics, Basel, Switzerland) measuring the formation of mononucleosomes and oligonucleosomes in the cytoplasm. Results are expressed as cytoplasm nucleosome enrichment, in comparison with untreated cells taken as 1. Statistical significance was estimated using the one-sided paired Student t test.
NF-κB activation
NF-κB activation was tested using ELISA (TransAm NF-κB p50 and p65 transcription factors assay kits; Active Motif, Calsbad, CA) according to the manufacturer's instructions. After treatment, cells were lysed with the supplied buffer, and protein content was quantified using the Bradford assay. The NF-κB active form was detected by its fixation to the plate-immobilized consensus site oligonucleotide 5′-GGGACTTTCC-3′ and by an anti-p50 or an anti-p65 antibody that recognizes an epitope only accessible on activated NF-κB. Horseradish peroxide (HRP)–conjugated secondary antibody provides a colorimetric reading at 405 nm on a spectrophotometer WallacVictor2 (Perkin Elmer).
Detection of BAFF-soluble form by SELDI-TOF MS technique
SELDI-TOF MS is derived from matrix-assisted laser desorption/ionization (MALDI) and allows protein analysis on “affinity” surfaces that enhance their capture, desorption, or both. For each protein, the mass-charge ratio is estimated by its time of flight in a vacuum tube separating the chip surface and the detector. To detect BAFF in sera from healthy (n = 5) donors and B-CLL (n = 5) patients, PS20 preactivated ProteinChips (epoxideactivated amine surfaces) were coated with an anti-BAFF antibody. Two models were used and validated with recombinant BAFF protein, detected on PS20 ProteinChip or on golden chip (nonreactive surface used to evaluate purified proteins by conventional MALDI-TOF).
PS20 surface was coated with covalently bound G-protein, allowing an attachment of the rabbit anti-BAFF polyclonal antibody through its Fc fragment. Pooled sera from healthy donors or B-CLL patients (100 μg for each) were spotted on the chip and incubated overnight to achieve the formation of the antibody-antigen complex. After washings, the chip was overlaid with a saturated sinapinic acid solution (energy-absorbing molecule). Targeted proteins and eventual associated proteins were desorbed by laser illumination. Times of flight were recorded with a Protein Biology System II SELDI-Mass Spectrometer (Ciphergen Biosystems, Fremont, CA), with external calibration. Data were interpreted using the ProteinChip software 3.0.2.
Competition analyses between protein binding onto the goat anti-BAFF antibody and its blocking peptide were also performed. Goat anti-BAFF antibody was covalently linked to the PS20 array. Then pooled sera (from healthy donors or B-CLL patients) were spotted on the chip and incubated overnight. After washings, the blocking peptide was incubated for 4 hours (time during which protein samples and blocking peptide competed for binding to anti-BAFF antibody). Similar experiments were performed by first spotting the blocking peptide and then through competition with sera. Biochemical reagents and ProteinChips were from Ciphergen Biosystems.
Results
Expression of TACI and BAFF-R mRNAs in normal B lymphocytes and leukemic B cells
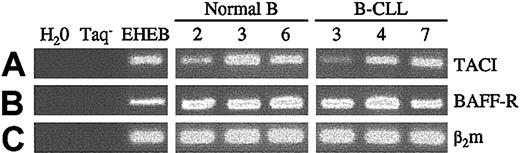
The presence of the 2 receptors shared by BAFF and APRIL, BCMA and TACI, and of a receptor specific for BAFF, BAFF-R, was previously reported on normal B lymphocytes.23,26,27 Using RT-PCR, the presence of TACI and BAFF-R mRNAs was found in nearly all leukemic B cells (7 of 9 patients for TACI, 9 of 9 patients for BAFF-R) and in blood-derived normal B cells (5 of 5 donors) (Figure 1), in agreement with the findings of Novak et al.45 The latter showed, in addition, that BCMA mRNA was detected in a subset of B-CLL patients. Moreover, by flow cytometry, they found significant levels of TACI protein at the surfaces of these cells; the lack of suitable antibodies made it difficult to check the membrane expression of BCMA and BAFF-R.45 These results indicated that leukemic B cells expressed at their surfaces the 2 receptors for BAFF and APRIL.
Expression of TACI and BAFF-R mRNAs in normal and leukemic B cells. Total RNA from normal blood-derived B lymphocytes, B-CLL leukemic B cells, and EHEB cells (control) was extracted as previously described, and cDNAs were subjected to PCR amplification with specific primers for TACI (lane A, 327 bp), BAFF-R (lane B, 256 bp), and β2-microglobulin as control (lane C, 169 bp). Negative controls were performed in the absence of cDNA and Taq.
Expression of TACI and BAFF-R mRNAs in normal and leukemic B cells. Total RNA from normal blood-derived B lymphocytes, B-CLL leukemic B cells, and EHEB cells (control) was extracted as previously described, and cDNAs were subjected to PCR amplification with specific primers for TACI (lane A, 327 bp), BAFF-R (lane B, 256 bp), and β2-microglobulin as control (lane C, 169 bp). Negative controls were performed in the absence of cDNA and Taq.
Expression of BAFF and APRIL mRNAs in normal B lymphocytes and leukemic B cells
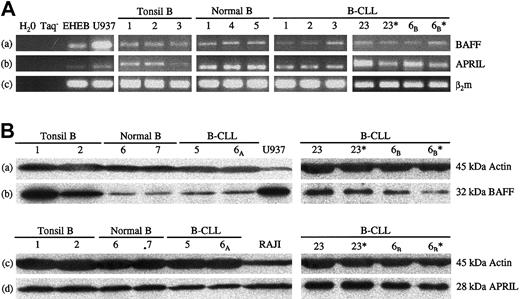
We tested the presence of BAFF and APRIL mRNAs in B-CLL cells, in comparison with B lymphocytes from healthy donors. B-CLL leukemic cells were found to express BAFF and APRIL mRNAs (13 of 13 patients), as detected by RT-PCR. Further purification of the leukemic B cells by CD19+ selection yielded similar results (Figure 2A). BAFF and APRIL mRNAs were also observed, at comparable levels, in normal CD19+ blood-derived (6 of 6 donors) and tonsil B lymphocytes (10 of 10 donors). The unexpected presence of BAFF and APRIL mRNAs in normal and leukemic B lymphocytes prompted us to study the distribution of the corresponding proteins in both cell types.
Expression of BAFF and APRIL mRNAs and proteins in normal blood and tonsil B lymphocytes and in B-CLL cells. (A) Total RNA was extracted from purified B lymphocytes from the blood of healthy volunteers or of patients with B-CLL or from tonsils. After RT, cDNAs were subjected to PCR amplification with specific primers for BAFF (lane a, 337 bp), APRIL (lane b, 365 bp), and β2-microglobulin as control (lane c, 169 bp). *Similar results were obtained after further purification of the leukemic B cells by CD19+ selection. U937 and EHEB cell lines were used as controls of expression. Negative controls were used in the absence of cDNA and of Taq. (B) Total lysates from purified B lymphocytes from the blood of healthy volunteers or B-CLL patients or from tonsils were analyzed by Western blotting and were revealed either with a rabbit anti-BAFF polyclonal antibody (lane b), with a goat anti-APRIL polyclonal antibody (lane d), or with a mouse antiactin monoclonal antibody as a control of roughly equal deposit of proteins (lanes a and c). *Similar results were obtained after further purification of the leukemic B cells by CD19+ selection. Lysates of U937 and RAJI cell lines were used as positive controls for BAFF and APRIL expression, respectively.
Expression of BAFF and APRIL mRNAs and proteins in normal blood and tonsil B lymphocytes and in B-CLL cells. (A) Total RNA was extracted from purified B lymphocytes from the blood of healthy volunteers or of patients with B-CLL or from tonsils. After RT, cDNAs were subjected to PCR amplification with specific primers for BAFF (lane a, 337 bp), APRIL (lane b, 365 bp), and β2-microglobulin as control (lane c, 169 bp). *Similar results were obtained after further purification of the leukemic B cells by CD19+ selection. U937 and EHEB cell lines were used as controls of expression. Negative controls were used in the absence of cDNA and of Taq. (B) Total lysates from purified B lymphocytes from the blood of healthy volunteers or B-CLL patients or from tonsils were analyzed by Western blotting and were revealed either with a rabbit anti-BAFF polyclonal antibody (lane b), with a goat anti-APRIL polyclonal antibody (lane d), or with a mouse antiactin monoclonal antibody as a control of roughly equal deposit of proteins (lanes a and c). *Similar results were obtained after further purification of the leukemic B cells by CD19+ selection. Lysates of U937 and RAJI cell lines were used as positive controls for BAFF and APRIL expression, respectively.
Expression of BAFF and APRIL proteins in normal and B-CLL B lymphocytes
Lysates of B cells from healthy donors and B-CLL patients were separated by SDS-PAGE, followed by Western blotting with specific rabbit anti-BAFF and goat anti-APRIL polyclonal antibodies. As shown in Figure 2B, comparable levels of BAFF and APRIL proteins were detected in lysates from normal B cells (4 of 4 donors) and leukemic (CD19+ enriched or not) B-CLL cells (12 of 12 patients for BAFF and 8 of 8 patients for APRIL). Similar experiments conducted with tonsillar B cells confirmed the presence of these proteins in B cells from other lymphoid tissues. BAFF levels were greater in tonsillar than in normal blood B cells, whereas APRIL levels were comparable in both cell types. Specificity was assessed by preincubating the anti-BAFF antibody with recombinant human soluble BAFF and the anti-APRIL antibody with its specific blocking peptide, before their addition to the membrane. In both cases, the chemiluminescence signal was totally abolished (not shown).
B-CLL leukemic B cells display BAFF and APRIL at their plasma membranes
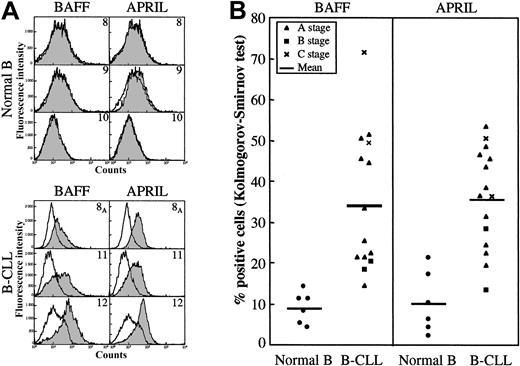
Leukemic B cells from B-CLL patients and purified CD19+ B lymphocytes from healthy donors were immunophenotyped for the surface expression of BAFF and APRIL. As evidenced in Figure 3A-B, significant percentages of B-CLL B cells were positive for BAFF (mean, 34.1%) and APRIL (mean, 35.5%). Nonspecific binding of the antibodies was excluded by FcγR blocking reagent competition, as shown in Table 2. In marked contrast, only a low percentage of normal B lymphocytes was found to express BAFF (mean, 8.8%) and APRIL (mean, 10%).
Membranous expression of BAFF and APRIL on B-CLL leukemic B cells. B cells purified from healthy blood donors (n = 6) or B-CLL patients (n = 15) were tested using immunofluorescence and flow cytometry for surface expression of BAFF and APRIL by labeling with specific antibodies or isotype control, and the percentages of positive cells were estimated using the Kolmogorov-Smirnov test, as described in “Patients, materials, and methods.” (A) Representative histograms of 3 healthy donors and 3 B-CLL patients. Open histograms indicate isotype control; gray histograms, BAFF (left) or APRIL (right) labeling. (B) Global distribution of BAFF- and APRIL-positive cells in healthy donors and B-CLL patients. Percentages for normal B lymphocytes: BAFF, mean 8.8% (range, 4%-14%); APRIL, mean 10% (range, 2%-21%). Percentages for B-CLL B lymphocytes: BAFF, mean 34.1% (range, 14%-71%) (P = .0001); APRIL, mean 35.5% (range, 13%-53%) (P = .0001).
Membranous expression of BAFF and APRIL on B-CLL leukemic B cells. B cells purified from healthy blood donors (n = 6) or B-CLL patients (n = 15) were tested using immunofluorescence and flow cytometry for surface expression of BAFF and APRIL by labeling with specific antibodies or isotype control, and the percentages of positive cells were estimated using the Kolmogorov-Smirnov test, as described in “Patients, materials, and methods.” (A) Representative histograms of 3 healthy donors and 3 B-CLL patients. Open histograms indicate isotype control; gray histograms, BAFF (left) or APRIL (right) labeling. (B) Global distribution of BAFF- and APRIL-positive cells in healthy donors and B-CLL patients. Percentages for normal B lymphocytes: BAFF, mean 8.8% (range, 4%-14%); APRIL, mean 10% (range, 2%-21%). Percentages for B-CLL B lymphocytes: BAFF, mean 34.1% (range, 14%-71%) (P = .0001); APRIL, mean 35.5% (range, 13%-53%) (P = .0001).
Determination of BAFF- and APRIL-positive cells with and without and after incubation with FcγR blocking reagent for 3 B-CLL samples
Patient . | BAFF-positive cells, % . | APRIL-positive cells, % . |
|---|---|---|
| 23 | ||
| - | 21 | 19 |
| + | 27 | 25 |
| 18c | ||
| - | 14 | 22 |
| + | 28 | 20 |
| 13c | ||
| - | 25 | 24 |
| + | 11 | 20 |
Patient . | BAFF-positive cells, % . | APRIL-positive cells, % . |
|---|---|---|
| 23 | ||
| - | 21 | 19 |
| + | 27 | 25 |
| 18c | ||
| - | 14 | 22 |
| + | 28 | 20 |
| 13c | ||
| - | 25 | 24 |
| + | 11 | 20 |
+ indicates with FcγR blocking reagent; and -, without FcγR blocking reagent.
Compared with Western blot experiments that showed similar levels of BAFF and APRIL proteins, these data may indicate a difference between normal and leukemic B cells in the control of their transport or stability at the surface. Detecting BAFF and APRIL, together with their receptors, on B-CLL cells thus suggested the existence of an autocrine loop of stimulation for their survival.
Exogenous BAFF and APRIL protect B-CLL cells from spontaneous and drug-induced apoptosis
We then studied whether these receptors could transduce signal(s) counteracting apoptosis in the leukemic cells. Recombinant soluble BAFF and APRIL were tested on apoptosis induction in B-CLL cells, either spontaneously or stimulated by the chemotherapeutic agent flavopiridol. Enrichment in cytoplasmic nucleosomes was measured in B-CLL cells after 72-hour stimulation. As shown in Table 3, adding soluble BAFF and APRIL elicited a reduction in nucleosome enrichment (spontaneous apoptosis) in 9 of 9 patients tested for BAFF and in 8 of 9 patients tested for APRIL, with no change for the last case. In some instances, a suppressive effect was observed when both agents were added together.
BAFF and APRIL protect B-CLL cells from spontaneous apoptosis
. | Patients . | . | . | . | . | . | . | . | . | . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | 6B . | 8B . | 13C . | 15C . | 18B . | 19 . | 21 . | 22 . | 23 . | Mean . | ||||||||
| Control | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||||||||
| BAFF | 0.45 | 0.69 | 0.43 | 0.5 | 0.85 | 0.2 | 0.79 | 0.6 | 0.43 | 0.55* | ||||||||
| APRIL | 0.43 | 0.85 | 0.59 | 0.69 | 0.55 | 0.09 | 0.98 | 0.78 | 0.16 | 0.57* | ||||||||
| BAFF + APRIL | ND | 0.51 | ND | ND | 0.74 | ND | 0.6 | ND | ND | 0.62† | ||||||||
. | Patients . | . | . | . | . | . | . | . | . | . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | 6B . | 8B . | 13C . | 15C . | 18B . | 19 . | 21 . | 22 . | 23 . | Mean . | ||||||||
| Control | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||||||||
| BAFF | 0.45 | 0.69 | 0.43 | 0.5 | 0.85 | 0.2 | 0.79 | 0.6 | 0.43 | 0.55* | ||||||||
| APRIL | 0.43 | 0.85 | 0.59 | 0.69 | 0.55 | 0.09 | 0.98 | 0.78 | 0.16 | 0.57* | ||||||||
| BAFF + APRIL | ND | 0.51 | ND | ND | 0.74 | ND | 0.6 | ND | ND | 0.62† | ||||||||
Cytoplasm nucleosome enrichment was measured after the addition of soluble BAFF and APRIL (0.25 μg/mL) to cultures of leukemic B cells for 72-hour incubation in the presence of medium alone for 9 patients with leukemia. Results are expressed as cytoplasm nucleosome enrichment compared with untreated cells taken as 1 (enrichment of nucleosomes in untreated cells increased approximately 5 times between days 0 and 3 of culture as a consequence of spontaneous apoptosis). Probability is expressed in comparison with the untreated cells (†). ND indicates not done.
.0001 ≤ P < .001.
.01 ≤ P < .05.
Flavopiridol at a suboptimal concentration (0.2 μM) induced marked cytoplasm nucleosome enrichment in 7 of 7 B-CLL patients tested. In all but 1 patient, adding soluble BAFF (0.25 μg/mL) elicited a marked reduction of this enrichment (Table 4). In 5 of 7 patients, this enrichment was also diminished in the presence of soluble APRIL, whereas it was slightly increased for the last 2 patients. For 3 of 6 patients, the protective effect of BAFF plus APRIL was better than with either agent alone.
BAFF and APRIL protect B-CLL cells from drug-induced apoptosis
. | Patients . | . | . | . | . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | 8B . | 10B . | 15C . | 18B . | 19 . | 20 . | 21 . | Mean . | ||||||
| Control | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| F | 2.2 | 2.26 | 4.22 | 1.61 | 6.33 | 5.03 | 1.24 | 3.27* | ||||||
| F + BAFF | 1.04 | 1.85 | 0.68 | 1.57 | 1.29 | 3.8 | 1.08 | 1.62† | ||||||
| F + APRIL | 1.57 | 2.71 | 0.66 | 2.3 | 5.26 | 4.46 | 0.82 | 2.54* | ||||||
| F + BAFF + APRIL | 0.95 | 2.1 | ND | 2.01 | 0.58 | 3.27 | 0.82 | 1.62 | ||||||
. | Patients . | . | . | . | . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | 8B . | 10B . | 15C . | 18B . | 19 . | 20 . | 21 . | Mean . | ||||||
| Control | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| F | 2.2 | 2.26 | 4.22 | 1.61 | 6.33 | 5.03 | 1.24 | 3.27* | ||||||
| F + BAFF | 1.04 | 1.85 | 0.68 | 1.57 | 1.29 | 3.8 | 1.08 | 1.62† | ||||||
| F + APRIL | 1.57 | 2.71 | 0.66 | 2.3 | 5.26 | 4.46 | 0.82 | 2.54* | ||||||
| F + BAFF + APRIL | 0.95 | 2.1 | ND | 2.01 | 0.58 | 3.27 | 0.82 | 1.62 | ||||||
Cytoplasm nucleosome enrichment was measured after the addition of soluble BAFF and APRIL (0.25 μg/mL) to cultures of leukemic B cells for 72 hours of incubation in the presence of medium containing 0.2 μM flavopiridol for 7 patients with leukemia. Results are expressed as cytoplasm nucleosome enrichment compared with untreated cells taken as 1 (enrichment of nucleosomes in untreated cells increased approximately 5 times between days 0 and 3 of culture as a consequence of spontaneous apoptosis). F indicates flavopiridol. Probability (.01 ≤ P < .05) is expressed in comparison with untreated (*) or flavopiridol-treated (†) cells.
These results were confirmed by direct counting of viable cells. The decrease in the percentage of viable cells treated with flavopiridol was partially or totally reverted in the presence of BAFF, APRIL, or both, yet we did not observe an increased proliferation of leukemic cells (not shown). Moreover, we confirmed for 1 patient that nucleosome data correlated with the percentage of annexin V–labeled cells (not shown).
These results indicated that, for most patients tested, adding either factor resulted in marked protection toward spontaneous and drug-elicited apoptosis. BAFF appeared more potent than APRIL, at least for some patients.
BAFF and APRIL stimulate NF-κB in B-CLL cells
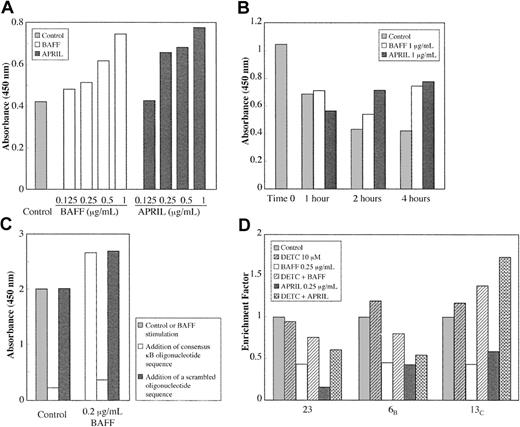
The 2 TNF homologues trigger normal B-lymphocyte survival in part through their capacity to stimulate the NF-κB “rescue” pathway from apoptosis. Leukemic B cells were thus incubated with soluble BAFF or APRIL, and the activation of NF-κB was estimated using ELISA. The latter transcription factor displays antiapoptotic properties and is already highly activated in B-CLL.46 Interestingly, adding BAFF and APRIL was still found to increase NF-κB activation slightly in a dose- and time-dependent manner, as shown in Figure 4A-B. This modest increase (25%-50%) in the presence of either BAFF or APRIL was regularly observed for the 6 patients tested. Binding specificity was assessed by total inhibition in the presence of the corresponding oligonucleotide, whereas adding a scrambled nucleotide had no effect (Figure 4C). These results indicated that BAFF and APRIL were able to further activate NF-κB. Moreover, p65 and p50 (not shown) increases were found, indicating that the complexes were likely composed of p50/p65 heterodimers, functionally active as transcriptional factors.
BAFF and APRIL stimulate NF-κ B activation in B-CLL cells. Leukemic B cells were incubated for various times with the indicated concentrations of BAFF or APRIL. Extracts were then estimated for the activation of NF-κ B using an ELISA test (TransAm NF-κB) with a specific anti-p65 antibody. (A) BAFF and APRIL dose response after 4 hours of treatment. (B) Time response. (C) Specificity of the binding (16 hours). (D) Reversal of BAFF and APRIL protective effect on spontaneous apoptosis (measured by cytoplasm nucleosome enrichment) by adding 10 μM DETC, an inhibitor of NF-κB activation. Results are expressed as cytoplasm nucleosome enrichment in comparison with untreated cells taken as 1.
BAFF and APRIL stimulate NF-κ B activation in B-CLL cells. Leukemic B cells were incubated for various times with the indicated concentrations of BAFF or APRIL. Extracts were then estimated for the activation of NF-κ B using an ELISA test (TransAm NF-κB) with a specific anti-p65 antibody. (A) BAFF and APRIL dose response after 4 hours of treatment. (B) Time response. (C) Specificity of the binding (16 hours). (D) Reversal of BAFF and APRIL protective effect on spontaneous apoptosis (measured by cytoplasm nucleosome enrichment) by adding 10 μM DETC, an inhibitor of NF-κB activation. Results are expressed as cytoplasm nucleosome enrichment in comparison with untreated cells taken as 1.
Adding 10 μM DETC, an inhibitor of NF-κB activation, was found to revert the protective effects of BAFF and APRIL on spontaneous apoptosis, as detected by the nucleosome-enrichment assay (Figure 4D). These data indicate that NF-κB is a likely target in the protective effects of BAFF and APRIL.
Blocking the interaction of BAFF and APRIL with their receptor(s) restores apoptotic death in B-CLL cells
We tried to prevent the interaction of BAFF and APRIL with their receptors to estimate the consequences on the apoptotic pathway. B-CLL cells were incubated with an excess of BCMA-Fc, a soluble recombinant form of the common receptor, BCMA. In the 6 patients tested, this resulted in an augmentation of apoptosis as estimated by the marked increase, from 24% to 325%, in cytoplasm nucleosome enrichment (Table 5). When BAFF or APRIL was added to BCMA-Fc, reversal was observed, partially for BAFF (3 of 6 patients) and in every case for APRIL. This difference is probably due to a stronger affinity of BCMA for APRIL than for BAFF. In contrast, adding BCMA-Fc to normal purified CD19+ B cells did not elicit any increase in the release of nucleosomes in the cytoplasm (not shown).
BCMA soluble form restores apoptotic death in B-CLL cells
. | Patients . | . | . | . | . | . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | 8B . | 10B . | 15C . | 19 . | 20 . | 21 . | Mean . | |||||
| Control | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |||||
| BCMA-Fc | 1.93 | 1.59 | 3.97 | 1.24 | 4.25 | 1.3 | 2.38* | |||||
| BCMA-Fc + BAFF | 1.74 | 1.94 | 4.16 | 0.3 | 1.83 | 1.41 | 1.9 | |||||
| BCMA-Fc + APRIL | 1.49 | 0.92 | 0.59 | 0.15 | 2.1 | 0.87 | 1.02† | |||||
. | Patients . | . | . | . | . | . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | 8B . | 10B . | 15C . | 19 . | 20 . | 21 . | Mean . | |||||
| Control | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |||||
| BCMA-Fc | 1.93 | 1.59 | 3.97 | 1.24 | 4.25 | 1.3 | 2.38* | |||||
| BCMA-Fc + BAFF | 1.74 | 1.94 | 4.16 | 0.3 | 1.83 | 1.41 | 1.9 | |||||
| BCMA-Fc + APRIL | 1.49 | 0.92 | 0.59 | 0.15 | 2.1 | 0.87 | 1.02† | |||||
Leukemic B cells from 6 B-CLL patients were cultured for 72 hours in the presence of 50 μg/mL BCMA-Fc alone or with 0.25 μg/mL soluble BAFF or APRIL. Results are expressed as the cytoplasm nucleosome enrichment in comparison with untreated cells taken as 1.
Probability (.01 ≤ P < .05) is expressed in comparison with untreated (*) or BCMA-Fc—treated (†) cells.
Similar experiments were performed with specific goat antibodies against BAFF and APRIL and with control goat IgG (Table 6). A proapoptotic effect was observed for most B-CLL leukemic cells after their incubation with the specific anti-BAFF and anti-APRIL antibodies. In 4 of 6 patients, goat anti-BAFF and anti-APRIL induced an increase in nucleosome enrichment from 47.2% to 318.6% and from 33.1% to 437.2%, respectively. In another patient, anti-BAFF treatment increased the enrichment by 20.2%, and anti-APRIL had no effect. In the last case tested, neither antibody had an effect. Comparable and even better results were obtained using a rabbit anti-BAFF polyclonal antibody and rabbit IgG as controls. In 6 of 6 patients, an increase in nucleosome enrichment from 58% to 374.6% was observed (Table 6). These experiments indicated that adding leukemic B cells of molecules that neutralize or prevent, or both, the binding of BAFF and APRIL to their receptors resulted in reinducing the apoptotic process, which reinforced the existence of an autocrine loop of cell survival.
Anti-BAFF and anti-APRIL antibodies restore apoptotic death in B-CLL cells
. | Patients . | . | . | . | . | . | . | . | . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | 8B . | . | 15C . | . | 18B . | 19 . | 20 . | 21 . | Mean . | |||||||
| Antibody, μg/mL | 0.2 | 2 | 0.2 | 2 | 2 | 2 | 2 | 2 | — | |||||||
| Goat | ||||||||||||||||
| IgG | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |||||||
| Anti-BAFF | 1.57 | 1.25 | 4.19 | 0.98 | 1.2 | 1.31 | 1.47 | 1.08 | 1.22* | |||||||
| Anti-APRIL | 1.45 | 1.38 | 5.37 | 0.92 | 0.95 | 1.67 | 1.33 | 0.99 | 1.21 | |||||||
| Rabbit | ||||||||||||||||
| IgG | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |||||||
| Anti-BAFF | 1.58 | 1.1 | 2.13 | 1.25 | 1.59 | 1.41 | 4.75 | 2.11 | 2.04* | |||||||
. | Patients . | . | . | . | . | . | . | . | . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | 8B . | . | 15C . | . | 18B . | 19 . | 20 . | 21 . | Mean . | |||||||
| Antibody, μg/mL | 0.2 | 2 | 0.2 | 2 | 2 | 2 | 2 | 2 | — | |||||||
| Goat | ||||||||||||||||
| IgG | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |||||||
| Anti-BAFF | 1.57 | 1.25 | 4.19 | 0.98 | 1.2 | 1.31 | 1.47 | 1.08 | 1.22* | |||||||
| Anti-APRIL | 1.45 | 1.38 | 5.37 | 0.92 | 0.95 | 1.67 | 1.33 | 0.99 | 1.21 | |||||||
| Rabbit | ||||||||||||||||
| IgG | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |||||||
| Anti-BAFF | 1.58 | 1.1 | 2.13 | 1.25 | 1.59 | 1.41 | 4.75 | 2.11 | 2.04* | |||||||
Leukemic B cells from 6 B-CLL patients were cultured for 72 hours in the presence of 0.2 or 2 μg/mL goat anti-BAFF and anti-APRIL antibodies or rabbit anti-BAFF antibody or their respective isotype controls (goat IgG or rabbit IgG). Results are expressed as the cytoplasm nucleosome enrichment, in comparison with the respective goat or rabbit isotype controls taken as 1.
Probability (.01 ≤ P < .05, for antibody concentration of 2 μg/mL) is expressed in comparison with the respective isotype controls.
Detection of BAFF in the serum of B-CLL patients by SELDI-TOF MS
The presence of BAFF at the surfaces of B-CLL cells led us to hypothesize that a soluble form of this molecule might be found in the sera of B-CLL patients. Western blotting techniques failed to detect such a soluble form, even after immunoprecipitation with an anti-BAFF antibody. We then turned to a very sensitive and specific technique, SELDI-TOF MS.
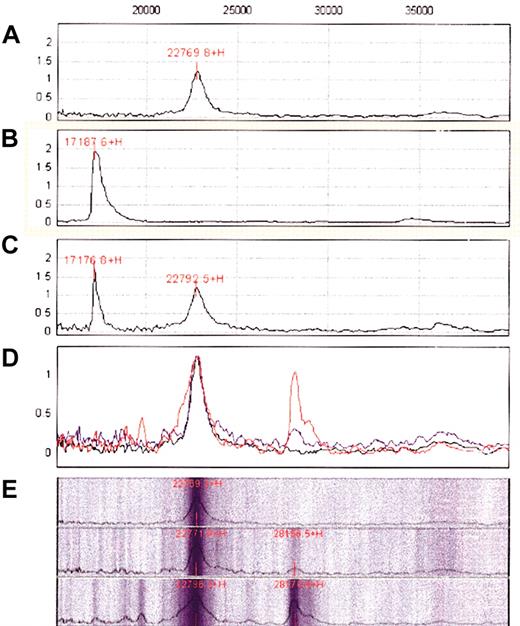
First, the purity of recombinant soluble BAFF was confirmed by the detection of a single peak of the expected size (17 kDa) on a “neutral” golden chip (Figure 5B). BAFF is specifically recognized by the rabbit anti-BAFF antibody fixed onto the PS20 G-coupled ProteinChip, as demonstrated by the detection of a peak of the same size (Figure 5A,C). Then anti-BAFF–coated chips were incubated with sera from healthy blood donors or B-CLL patients. Comparative analysis revealed a peak of high intensity in the B-CLL sera, with a molecular mass of 28 161 Da, that was barely detectable in the sera of healthy donors (Figure 5D-E).
Detection of a soluble form of BAFF in the sera of B-CLL patients by SELDI-TOF MS. (A) Analysis of the protein G chip after affinity binding of the rabbit anti-BAFF antibody. The expected peak of 155 kDa and the different “echoes” corresponding to increasing degrees of ionization are not shown because only proteins in the range of 15 000-40 000 Da are presented for clarity. The 22-kDa peak corresponds to a contaminating protein in the IgG preparation. (B) Analysis of a recombinant soluble form of BAFF (17 kDa; BioVision) on a golden chip. (C) Analysis of the protein G chip after affinity binding of the anti-BAFF antibody and washing and incubation with the recombinant soluble form of BAFF and washing. (D) Analysis of the protein G chip after affinity binding of the anti-BAFF antibody and washing and incubation without (black) or with (blue) normal sera or B-CLL sera (red) and washing. Recordings corresponding to these 3 analyses have been superimposed. (E) Pseudo gel representation of the previous data with separate records for the control (top), normal sera (middle), and B-CLL sera (bottom).
Detection of a soluble form of BAFF in the sera of B-CLL patients by SELDI-TOF MS. (A) Analysis of the protein G chip after affinity binding of the rabbit anti-BAFF antibody. The expected peak of 155 kDa and the different “echoes” corresponding to increasing degrees of ionization are not shown because only proteins in the range of 15 000-40 000 Da are presented for clarity. The 22-kDa peak corresponds to a contaminating protein in the IgG preparation. (B) Analysis of a recombinant soluble form of BAFF (17 kDa; BioVision) on a golden chip. (C) Analysis of the protein G chip after affinity binding of the anti-BAFF antibody and washing and incubation with the recombinant soluble form of BAFF and washing. (D) Analysis of the protein G chip after affinity binding of the anti-BAFF antibody and washing and incubation without (black) or with (blue) normal sera or B-CLL sera (red) and washing. Recordings corresponding to these 3 analyses have been superimposed. (E) Pseudo gel representation of the previous data with separate records for the control (top), normal sera (middle), and B-CLL sera (bottom).
The classical soluble form resulting from the cleavage of BAFF by furinlike convertase displays a 17-kDa molecular weight. To confirm that the 28 161-Da molecule detected by SELDI-TOF MS was effectively BAFF, experiments were repeated with the goat anti-BAFF antibody directly coated to the ProteinChip and yielded identical results. In addition, the 28 161-Da peak disappeared when this antibody was incubated, before its fixation to the chip, with the specific blocking peptide. Adding the blocking peptide also displaced the 28 161-Da molecule, once bound to the antibody (not shown). Together, these experiments indicate that a soluble form of BAFF can be detected in the sera of B-CLL patients but not of healthy donors.
Discussion
The BAFF molecule was initially considered to be restricted to APCs. Except for one report describing its expression in a subpopulation of T cells,6 it was not detected in T or B lymphocytes.47 APRIL was detected in various tumor cells, but virtually not in normal tissues.11 However, in our experiments, the presence of BAFF and APRIL mRNAs was evidenced in B lymphocytes isolated from healthy donors (blood and tonsils) and B-CLL patients. By Western blot, roughly similar amounts of BAFF and APRIL proteins were detected in lysates from purified normal and leukemic B cells. These results are probably not attributed to contamination by residual monocytes because the latter population never exceeded 2%.40 Moreover, further purification of the leukemic cells by CD19+ selection gave identical results.
Using flow cytometry, however, a significant percentage of leukemic B cells displayed membranous BAFF and APRIL, in marked contrast to normal blood-derived CD19+ B cells. Nonspecific binding was excluded by the use of FcγR blocking reagents, and these results cannot be attributed to contaminating monocytes because our leukemic cell suspensions were composed of 92% or more B cells.
Our data are in agreement with those of Novak et al,45 who reported a similar “aberrant” presence of BAFF and APRIL at the mRNA level and membranous expression by a subset of B-CLL patients. Yet they did not detect the presence of BAFF or APRIL mRNAs in normal B lymphocytes, but the reasons for this discrepancy are unclear. Human neutrophils stimulated with granulocyte–colony-stimulating factor (G-CSF) or interferon-γ (IFN-γ) also displayed BAFF mRNA in the absence of surface expression, suggesting the distinct processing of BAFF in different cell types.48
Although normal and leukemic B cells showed comparable amounts of BAFF and APRIL mRNAs and proteins, only B-CLL cells displayed membranous expression, suggesting the existence of a different transport mechanism. BAFF can be released from myelomonocytic cells as a soluble form resulting from enzymatic cleavage from the surface by furinlike convertase.14 In contrast, APRIL is normally not detectable as a membrane-anchored protein but is processed in the Golgi apparatus by furinlike convertase before secretion.15 Divergence in the membranous expression of BAFF and APRIL between normal and leukemic B cells could, therefore, result from differences in the activity of furinlike convertases or other proteolytic enzymes involved in the cleavage of the soluble forms. No association was observed between clinical features and level of expression of BAFF and APRIL on B-CLL cells, even though the highest BAFF expression was observed in a patient with stage C disease. However, larger numbers of patients must be tested for possible correlations to be found.
Using flow cytometry, B cells from B-CLL patients were found to significantly bind BAFF, though the binding was less than what occurred with normal B cells.49 Novak et al45 reported the presence of the 3 receptors—TACI, BCMA, and BAFF-R—on B-CLL leukemic cells. In accordance with their observations, we detected the presence of TACI and BAFF-R mRNAs in B-CLL leukemic cells. The ratio of the different receptors likely influences the lifespan of B cells.35 In mice, BAFF binding capacity and receptors expression varied with B-cell maturation. A maturation-associated increase in BAFF binding capacity correlated with differential expression patterns of the 3 BAFF receptors. Administering BAFF in vivo favors the survival of late transitional and mature peripheral B cell compartments, though the downstream mediators are different.50
Gene expression profiling revealed that B-CLL cells display a homogeneous phenotype related more to memory than to naive B cells.51 Another group reported that leukemic cells from all B-CLL patients, regardless of their VH gene status, exhibit features of activated and antigen-experienced B lymphocytes but that B-CLL cells that differ in the immunoglobulin VH genotype may have different antigen-encounter histories.52 This heterogeneity might explain the variations observed in the responses to BAFF and APRIL according to patient. Alternatively, these variations might be associated with a polymorphism of these factors, as reported for APRIL with regard to SLE patients.53
Adding an exogenous, soluble form of BAFF resulted in a protection of B-CLL cells toward spontaneous and flavopiridol-induced apoptosis, in agreement with the findings of Novak et al.45 In addition, we show that soluble APRIL displays the same protective capacity. Some patients responded better to one factor or the other, and for some patients the presence of both factors led to added protection. This suggests that different receptors may be implicated, in keeping with previous indications of a heterogeneous distribution of these receptors among the patients tested.
BAFF and APRIL also elicited a slight but significant and reproductive stimulation of NF-κB in leukemic B cells. Both molecules have been reported to trigger such activation in normal B lymphocytes, in conjunction with their ability to promote viability and long-term survival. The modest increase observed in leukemic B cells was not unexpected given that the basal level of NF-κB activation in these cells is already high and contributes to the resistance to apoptosis.54 Through 2-hybrid screening, TRAF3 was found to interact with BAFF-R; this interaction was stimulated by BAFF. Overexpression of TRAF3 inhibited BAFF-R–mediated NF-κB activation and IL-10 production, suggesting that TRAF3 is a negative regulator of BAFF-R–mediated NF-κB activation and IL-10 production.55 The slight increase in NF-κB activation elicited by BAFF in B-CLL cells could be attributed to such a mechanism. The involvement of NF-κB in the protective effect of BAFF and APRIL was nevertheless assessed by its reversal in the presence of DETC, an inhibitor of NF-κB activation. Our results show that the receptors for BAFF and APRIL expressed on B-CLL cells are functional transducing units because exogenously provided ligands induce protection against cell death and further stimulate the NF-κB pathway.
Adding soluble BCMA-Fc, which binds BAFF and APRIL and prevents these molecules from interacting with their receptors, to leukemic B cells markedly enhanced the apoptotic process, as assessed by the enrichment in cytoplasmic nucleosomes and the increase in the percentage of annexin-V–labeled cells. In contrast, adding it to normal B cells did not trigger apoptosis, indicating the absence of an autocrine survival loop in these cells. Similar results were obtained when B-CLL cells were incubated with anti-BAFF and anti-APRIL antibodies instead of BCMA-Fc to antagonize the effects of the corresponding cytokines. Preliminary data also indicate that anti-TACI and anti-BCMA antibodies induce apoptosis in leukemic B cells, albeit to a lesser degree, in accordance with the finding that, in these conditions, BAFF-R is still able to provide a survival signal.56
The expression of BAFF at the surfaces of B-CLL B cells but not of normal B cells led us to search for soluble forms of this molecule in the sera of B-CLL patients. In agreement with a previous preliminary report,57 serum levels of BAFF, as estimated by Western blot, were almost undetectable in B-CLL patients, suggesting that the circulating BAFF might be overconsumed by leukemic cells. However, using a sensitive technique, SELDI-TOF, that can detect femtomoles of proteins, we could detect a soluble form of BAFF in the sera of B-CLL patients but not of healthy blood donors. Detection specificity was assessed by 2 different anti-BAFF antibodies and by signal extinction with the specific immunizing peptide. This 28-kDa soluble form of BAFF differs from the classical 17-kDa fragment that results from its cleavage by a furinlike convertase. The calculated mass of BAFF integral protein is 31 222 Da, suggesting that in B-CLL cells other proteases are responsible for cleavage or alternative mechanisms of release are involved, or both. Studies examining these possibilities are under way. In an unrelated SELDI-TOF study performed under different experimental conditions with another type of ProteinChip, a 28-kDa peak was evidenced more frequently in the sera of B-CLL patients than of healthy donors. We are checking that the latter peak effectively corresponds to a soluble form of BAFF and are analyzing possible correlations between soluble BAFF level and clinical parameters.
Overexpression of BAFF protein can result in an SLE-like disease in mice. Circulating levels of BAFF protein are elevated in humans with SLE. Preventing the action of BAFF with an antagonist ameliorates disease progression in mice.58 The importance of interactions between BAFF and its receptors in the development of autoimmune diseases has been underscored by the detection of elevated serum levels of soluble BAFF in a significant percentage of patients with rheumatoid diseases of autoimmune origin, in correlation with autoantibody levels, suggesting a role for BAFF in the onset of these diseases.59 The serum BAFF molecule was identical to the natural soluble form and stimulated B-cell activation in vivo, suggesting that it plays a role in the development of autoimmune B cells directed against self-antigens during lupus pathogenesis.60 In a mouse model of collagen-induced rheumatoid arthritis, TACI-immunoglobulin treatment also inhibited the production of collagen-specific antibodies and the progression of disease.61 These data emphasize that inhibiting BAFF and APRIL interactions with their receptors may be of therapeutic value, and this approach is being tested for use in patients with different autoimmune rheumatoid diseases.62,63
In the latter diseases, BAFF signal is supposed to be provided by the APCs (through membranous or released soluble form), acting on B lymphocytes in a paracrine fashion. Our results and those of Novak et al45 suggest that in B-CLL patients, BAFF and APRIL (membranous and soluble forms) and their corresponding receptors BCMA, TACI, and BAFF-R play crucial roles in the survival of leukemic cells through autocrine rather than paracrine pathways, but the contribution of both BAFF and APRIL may be possible.
Whatever the mechanism, it is hoped that preventing BAFF and APRIL from exerting their actions in vivo will be of therapeutic interest for the treatment of B-CLL patients. Several ways to achieve this have been proposed, such as injecting neutralizing antibodies or soluble forms of their receptors. In addition, specific targeting of leukemic cells with BAFF and APRIL siRNA may be possible. Fuller knowledge of the transduction pathways elicited by these molecules, culminating in selective survival, may lead to new therapeutic approaches.
Prepublished online as Blood First Edition Paper, September 22, 2003; DOI 10.1182/blood-2003-02-0540.
Supported by INSERM and by a grant from Fondation contre la Leucémie.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal