Abstract
Recently various genetic defects in immunity mediated by interferon γ (IFN-γ) have been described, including mutations in the IFN-γ receptor 1 (IFN-γR1) and receptor 2 (IFN-γR2), signal transducer and activator of transcription 1 (STAT 1), and interleukin 12 receptor β 1 (IL-12Rβ1), and IL-12 p40 genes. These mutations are associated with the occurrence of severe infections with intracellular pathogens especially nontuberculous mycobacteria and vaccine-associated bacilli Calmette-Guérin (BCG). Here we report data on a previously healthy adult patient primarily presenting with severe infections with Burkholderia cocovenenans and subsequently Mycobacterium cheloneae. We found a strong inhibitory anti–IFN-γ activity in the patient's plasma and identified a highaffinity neutralizing anti–IFN-γ autoantibody. Unfortunately, the patient died due to severe sepsis before we knew the nature of the inhibitory activity. The application of alternative therapeutic approaches such as intravenous immunoglobulin or immunoadsorption may have been beneficial in this case. Screening for neutralizing anti–IFN-γ autoantibodies should supplement testing for IFN-γ and IL-12 pathway defects in patients with recurrent infections with intracellular pathogens, especially with nontuberculous mycobacteria.
Introduction
Interferon γ (IFN-γ) is synthesized by activated T and natural killer (NK) cells and strongly stimulates monocytes/macrophages, increasing their microbicidal activity, antigen presentation function, and production of proinflammatory cytokines on contact with microbial stimuli. Recently, genetic defects in the IFN-γ receptor system have been described in patients with vaccine-associated bacilli Calmette-Guérin (BCG) or nontuberculous mycobacteria (NTM) infections, demonstrating the importance of IFN-γ–mediated immunity in human host defense against intracellular pathogens.1-3 In addition, IFN-γ has been shown to be an obligatory host survival factor in a murine model of Burkholderia pseudomallei infection.4 Therapeutic consequences for patients with IFN-γ receptor defects depend on the kind of defects. Patients with a partial defect may do well with intensified antibiotic treatment and administration of IFN-γ, whereas patients with complete IFN-γ receptor deficiency may profit only from bone marrow transplantion.5,6 Beside IFN-γ receptor defects, abnormalities in signal transducer and activator of transcription 1 (STAT-1), interleukin 12 (IL-12) receptor β1, and IL-12 p40 expression have been reported in some patients with NTM infections supplementing the variety of causes for impaired IFN-γ–mediated immunity.7,8 Here we report data on a patient with an acquired defect of IFN-γ–mediated immunity presenting with severe infections with Burkholderia cocovenenans and NTM.
Study design
Case report
A 25-year-old woman from Thailand was admitted to the hospital due to necrotizing lymphadenitis, pneumonia, and tonsillitis. Three months earlier, she had developed severe community-acquired pneumonia with negative bacteriologic results in another hospital. She had been living in Germany for the last 8 years and had last been in Thailand 4 years ago. Her past medical history and the family history were unremarkable as far as known (father unknown). Diagnostic lymphadenectomy revealed an accumulation of neutrophil granulocytes without signs for malignancy. Microorganisms were not detectable. Following tonsillectomy she developed bilateral pneumonia, splenic and intracerebral abscesses, and osteomyelitis. By this time Burkholderia species were isolated from lymphadenoid tissue, different wound lesions, bronchial secretion, and a fluid-containing pustule at the left thigh. By comparative 16S rDNA sequence analysis the isolate was classified as B cocovenenans (synonym, Burkholderia gladioli). Additional colonizing microorganisms, Candida species and Enterococcus faecalis, were isolated from bronchial secretions. Mycobacteria were not detected and HIV serology was negative. B cocovenenans is a plant pathogen and invasive infections in humans have been described in patients with chronic granulomatous disease and in patients with cystic fibrosis on starting immunosuppressive therapy after lung transplantation.9,10 Analysis of phagocytic function and respiratory burst activity of the patient's peripheral blood phagocytes was normal as was lipopolysaccharide (LPS)–induced monocytic tumor necrosis factor α (TNF-α) production. The flow cytometric analysis of immune cells revealed abnormalities compatible with systemic inflammation and reaction against intracellular antigens. Extensive examinations for any autoimmune disorder revealed negative results. After the patient was stabilized, she was discharged but antibiotics were prescribed for another 6 months, and then all antibiotics were discontinued. Unfortunately, a few months later she again developed malaise, fever, lymphadenitis, and laboratory signs of inflammation. Assuming a relapse of B cocovenenans disease, antibiotic therapy was reapplied but achieved no clinical improvement. Microbiologic diagnostics failed to detect B cocovenenans in clinical specimens but in the course of disease bone marrow aspirates revealed infection with Mycobacterium chelonae. Broad antimycobacterial therapy was immediately initiated but the patient's condition rapidly deteriorated and severe sepsis developed. Empirical therapy with imipenem was added to the antibiotic regimen; nevertheless, the patient died from irreversible septic shock and multiorgan failure. Because no microorganisms other than NTM were detected in blood cultures and postmortem tissue specimens from liver, spleen, and lymph nodes, M chelonae was considered to be the causative agent of fatal septic shock.
Concanavalin A stimulation
To induce IFN-γ synthesis, either whole blood cells or isolated peripheral blood mononuclear cells (PBMCs; 1 × 106 cells/mL in RPMI 1640 medium supplemented with 10% fetal calf serum [FCS] and 2 mM glutamine) were stimulated with 100 (whole blood) or with 10 (PBMCs) μg/mL concanavalin A (Serva, Heidelberg, Germany) over 24 hours and IFN-γ was measured in the supernatant by enzyme-linked immunosorbent assay (ELISA; Biosource, Camarillo, CA).
Plasma spiking with increasing concentrations of IFN-γ
Recombinant human IFN-γ (Boehringer Ingelheim, Ingelheim, Germany) was incubated with the patient's plasma at a ratio of 1:1 and the recovery of IFN-γ was measured by ELISA.
Testing for biologic relevance of the IFN-γ–neutralizing activity
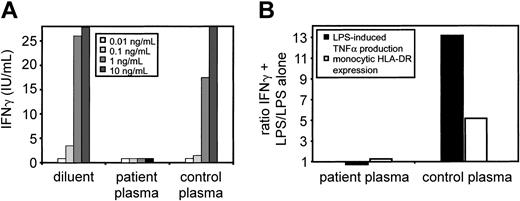
PBMCs (1 × 106 cells/mL) from a healthy proband were incubated with 5% (vol/vol) patient's plasma or control plasma with or without recombinant human IFN-γ (1 ng/mL) for 20 hours followed by stimulation with LPS (50 pg/mL; Sigma, St Louis, MO) for 4 hours. The supernatants were analyzed for TNF-α (Immulite; DPC Biermann, Bad Nauheim, Germany) and monocytic HLA-DR expression was measured by flow cytometry (FACS Calibur, Becton Dickinson, Heidelberg, Germany). Data are shown as ratio of IFN-γ plus LPS/LPS alone.
Antihuman IgG isotype ELISA
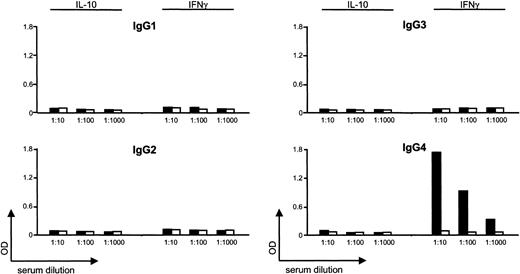
The patient's serum or control serum was added to plates coated either with recombinant human IFN-γ (PeproTech, Heidelberg, Germany) or recombinant human IL-10 (control). Following extensive washing and incubation with peroxidase (POD)–labeled antihuman IgG isotype antibodies, the substrate reaction was measured.
Results and discussion
Whole blood stimulation with concanavalin A revealed no detectable IFN-γ production, whereas IL-4 production was unaffected. Intracellular IFN-γ staining of whole blood cells following stimulation with phorbol myristate acetate (PMA)/ionomycin stimulation was positive, excluding deficient IFN-γ production. Surprisingly, stimulation of PBMCs with concanavalin A showed normal levels of IFN-γ secretion (all data not shown). This suggested an inhibitory IFN-γ activity in the patient's plasma. We tested this hypothesis by adding increasing concentrations of recombinant IFN-γ to the patient's plasma and measuring IFN-γ concentration by ELISA (Figure 1A). In contrast to control plasma, the patient's plasma completely blocked the detection of IFN-γ, indicating an extremely high-affinity anti–IFN-γ activity. This activity was specific for IFN-γ because the detection of recombinant TNF-α, IL-10, IL-5, IL-4, and IL-2 was not affected by the patient's plasma (data not shown). To test the biologic relevance of this finding, PBMCs from a healthy volunteer were incubated with IFN-γ in the presence of either patient's plasma or control plasma and stimulated with LPS. We then measured TNF-α production as well as monocytic HLA-DR expression; both parameters are normally up-regulated by IFN. In contrast to control plasma, the patient's plasma almost completely inhibited TNF-α and HLA-DR upregulation by exogenous IFN-γ (Figure 1B). In the absence of autologous plasma, the patient's PBMCs responded normally to exogenous and endogenous IFN-γ excluding defects in IFN-γ–mediated signaling.
Plasma spiking of increasing concentrations of IFN-γ demonstrates high-affinity IFN-γ–binding and neutralizing activity. (A) Recombinant human IFN-γ was added to patient and control plasma at various concentrations and plasma IFN-γ concentrations were determined by ELISA. (B) IFN-γ–binding activity does neutralize IFN-γ activity. PBMCs from a healthy proband were incubated with patient plasma or control plasma with or without recombinant human IFN-γ for 20 hours followed by stimulation with LPS for 4 hours. The supernatants were analyzed for TNF-α and monocytic HLA-DR expression was measured by flow cytometry. Data are shown as ratio of IFN-γ plus LPS/LPS alone.
Plasma spiking of increasing concentrations of IFN-γ demonstrates high-affinity IFN-γ–binding and neutralizing activity. (A) Recombinant human IFN-γ was added to patient and control plasma at various concentrations and plasma IFN-γ concentrations were determined by ELISA. (B) IFN-γ–binding activity does neutralize IFN-γ activity. PBMCs from a healthy proband were incubated with patient plasma or control plasma with or without recombinant human IFN-γ for 20 hours followed by stimulation with LPS for 4 hours. The supernatants were analyzed for TNF-α and monocytic HLA-DR expression was measured by flow cytometry. Data are shown as ratio of IFN-γ plus LPS/LPS alone.
Further analysis demonstrated that plasma IgG depletion by protein A removed the anti–IFN-γ activity, indicating the existence of an anti–IFN-γ autoantibody (data not shown). The isotype was subsequently identified as IgG4 by anti-IgG isotype-specific ELISA (Figure 2).
High-affinity IFN-γ–binding activity is an anti–IFN-γ IgG4 autoantibody. Patient serum (▪) or control serum (□) was added to plates coated either with recombinant human IFN-γ or recombinant human IL-10. Following extensive washing and incubation with POD-labeled antihuman IgG isotype antibodies, the substrate reaction was measured. OD indicates optical density.
High-affinity IFN-γ–binding activity is an anti–IFN-γ IgG4 autoantibody. Patient serum (▪) or control serum (□) was added to plates coated either with recombinant human IFN-γ or recombinant human IL-10. Following extensive washing and incubation with POD-labeled antihuman IgG isotype antibodies, the substrate reaction was measured. OD indicates optical density.
Preliminary results suggest that this autoantibody recognizes an epitope in the C-terminal region of IFN-γ, but the pathogenesis of autoantibody formation to IFN-γ in this patient remains unclear. Naturally occurring anticytokine antibodies have been described in healthy persons and are believed to be regulatory natural autoantibodies.11 To our knowledge, this is the first report on a natural autoantibody to IFN-γ that exhibits such a powerful neutralizing capacity resulting in severe immunodeficiency. Based on this observation testing for IFN-γ–neutralizing autoantibodies should be included in patients with recurrent infections with intracellular pathogens and especially with NTM. Tragically, the patient died due to severe sepsis before we knew the nature of the inhibitory activity. With earlier knowledge of the character of the inhibitory activity, alternate therapeutic approaches (eg, intravenous immunoglobulin, selective protein immunoadsorption) may have been helpful.
Prepublished online as Blood First Edition Paper, August 28, 2003; DOI 10.1182/blood-2003-04-1065.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal