Abstract
Asthma is associated with airway remodeling. Evidence of platelet recruitment to the lungs of asthmatics after allergen exposure suggests platelets participate in various aspects of asthma; although their importance is unknown in the context of airway remodeling, their involvement in atherosclerosis is established. Studies from our laboratory have shown a requirement for platelets in pulmonary leukocyte recruitment in a murine model of allergic lung inflammation. Presently, the effects of platelet depletion and corticosteroid administration on airway remodeling and lung function were examined. Ovalbumin (OVA)–sensitized mice, exposed to aerosolized OVA for 8 weeks, demonstrated epithelial and smooth muscle thickening, and subepithelial reticular fiber deposition in the distal airways. The depletion of platelets via an immunologic (antiplatelet antisera) or nonimmunologic (busulfan) method, markedly reduced airway remodeling. In contrast, dexamethasone administration did not affect epithelial thickening or subepithelial fibrosis, despite significantly inhibiting leukocyte recruitment. Thus, pathways leading to certain aspects of airway remodeling may not depend on leukocyte recruitment, whereas platelet activation is obligatory. OVA-sensitized mice exhibited airway hyperresponsiveness (AHR) compared with shamsensitized mice following chronic OVA exposure. Neither platelet depletion nor dexamethasone administration inhibited chronic AHR; thus, mechanisms other than inflammation and airway remodeling may be involved in the pathogenesis of chronic AHR.
Introduction
One consequence of persistent, chronic inflammation is alteration to tissue structure and function. In bronchial asthma, chronic inflammation may contribute to changes in airway architecture observed in this disease, referred to as airway remodeling. Airway remodeling in asthmatics is manifested as hypertrophy and hyperplasia of airway smooth-muscle, epithelial hyperplasia, and thickening of the epithelial basement membrane associated with the deposition of immunoglobulins, fibronectin, and collagen types I and III.1-3 The mechanisms underlying airway remodeling remain unknown and little is known about the functional consequences of these architectural changes in the lung.
It has been suggested that platelet activation may contribute to airway remodeling in asthma,4 since platelets contribute to tissue repair and remodeling in other organs.5 Recent studies emphasize a role for platelets in the initiation of atherosclerotic lesions,6 whereas platelet adherence and activation induce vascular smooth muscle proliferation after vascular injury,7 an event that is suppressed by the administration of antibodies inhibiting platelet glycoprotein Ib–von Willebrand factor interaction.8 Indeed, the accumulation of platelets at sites of coronary angioplasty enhanced neointimal proliferation, an event suppressed by the inhibition of platelet activation.9 Likewise, inhibition of platelet contact to smooth-muscle cells effectively suppressed proliferation in vitro, with implications for restenosis.10 Evidence points to an involvement of platelets in asthma, since large numbers of pulmonary megakaryocytes have been noted in the lungs obtained at autopsy from patients who have died from status asthmaticus.11 Additionally, aggregates of platelets with “fibrillary” material (fibrin) have been observed on the damaged airway lumenal surface in patients with asthma.11,12 Platelet activation has also been reported in both bronchoalveolar lavage (BAL) fluid13 and blood14,15 obtained from allergic patients with asthma following allergen challenge, and platelets are necessary for leukocyte recruitment to the lungs in experimental models of allergic inflammation.16,17 However, it has not yet been shown whether platelets contribute to airway remodeling during chronic allergic inflammation, even though platelets remain one of the best sources of mitogens for many of the structural elements found in the lung.17
Airway remodeling may contribute to an alteration in lung function since the mechanical processes involved in inspiration and exhalation are affected by changes to the airway wall, reducing the airway caliber, thus increasing airflow resistance, whereas smooth-muscle hyperplasia may contribute to an increased contractile reactivity and force of contraction.2,18 Remodeling processes are only marginally reversed by chronic anti-inflammatory glucocorticosteroid administration, as subepithelial fibrosis and collagen deposition remain, even when the presence of inflammatory cells has been reduced.19-21 Thus, airway remodeling may contribute to an irreversible component of airway hyperresponsiveness (AHR) that characterizes chronic asthma, since it is known that chronic treatment with glucocorticosteroids, while reducing inflammatory cell infiltration, does not reverse AHR either clinically22 or experimentally,23 suggesting that chronic AHR may not be dependent on airway inflammation.
In the present study, we have assessed the involvement of platelets in remodeling events in a murine model of chronic airway inflammation and hyperresponsiveness, comparing the effects of platelet depletion to that of the anti-inflammatory actions of long-term glucocorticosteroid administration.
Materials and methods
Sensitization of mice to ovalbumin
Male C57BL/6 mice (20-25 g; Charles River, Kent, United Kingdom) were immunized with chicken egg albumin (0.5 mg/kg, intraperitoneally) as previously described.16 Mice were subsequently exposed to aerosolized ovalbumin (OVA) (10 mg/mL) for 15-minute periods (De Villbis Ultraneb 90 nebulizer; DeVillbis Healthcare, Heston, United Kingdom) 3 times a day for 3 days a week for 8 weeks. Twenty-four hours after the last challenge in week 8, mice were used in the studies outlined below. All studies were carried out under the United Kindom's Animals (Scientific Procedures) Act of 1986 and local ethical approval from King's College London.
Bronchoalveolar lavage
Twenty-four hours after the last day of aerosol challenge, mice were anesthetized with 25% urethane (2 g/kg, intraperitoneally). The trachea was cannulated and lavages were performed, with total and differential leukocyte counts enumerated as previously described.16 Cytospin sections were also stained for the platelet-specific surface antigen CD41 (αIIb) using a specific goat antimouse polyclonal immunoglobulin G (IgG) antibody (sc-6604; Santa Cruz Biotechnology, Santa Cruz, CA) via a horseradish peroxidase (HRP)–streptavidin complex (sc-2053; Santa Cruz Biotechnology). Leukocytes within the sections were background stained using Gill formulation no. 2 hemotoxylin. Platelet presence was quantified as the number of platelet aggregates (typically consisting of 10-100 platelets) on each slide (150 μL BAL fluid).
Blood sampling for platelet, leukocyte, and serum IgE profiles
Blood (90 μL) was collected twice before the challenge from mice via the retro orbital route using glass pipettes dipped in 3.8% sodium citrate solution (Sigma, St Louis, MO) for platelet and leukocyte enumeration. Blood was smeared onto microscope slides for differential leukocyte counts using the DiffQuik method. Platelets were enumerated using the Brecher and Cronkite method.24 Blood was taken from mice via cardiac bleed at the end of the experiment (24 hours after the week-10 allergen exposure) and analyzed for total IgE in serum using an IgE detection enzyme-linked immunosorbent assay (ELISA) kit (Pharmingen, San Diego, CA).
Tissue processing
Lungs were taken from mice under terminal anesthesia and inflated with 10% formalin and were then immersed in 10% formalin for at least one week prior to tissue processing (lungs sliced along lateral axis) into paraffin wax–embedded blocks, sectioned (5 μm) using a microtome, then stained with Masson trichrome to allow detection of smooth muscle and epithelium. Other sections were stained with Gordon and Sweet silver stain to detect reticulin and fibrotic deposition.
Morphometry of airway tissue
Quantitative image analysis was then carried out on distal airways (small, < 0.75-mm perimeter), using the computer-assisted Leica KS300 program (Leica, Heidelberg, Germany). The length of basement membrane (in mm) was measured using a × 10 objective, and the area of epithelium and smooth muscle was measured (μm2) using a × 40 objective. This value was then divided by the length of basement membrane to give a uniform thickness per unit length of airway. Due to fibrotic areas surrounding the cellular components of the airway being laid as a network, the reticulum stain was subsequently quantified as a percentage of area staining black to a depth of 15 μm below the epithelial edge of the basement membrane. The investigator was unaware of the treatment groups throughout the analysis. Two to 3 airways were selected from each animal that had been cut perpendicular (to eliminate distorted measurements due to oblique cuts) to the face of the airway, from any of the 4 sections cut. Distal airways were additionally selected from areas peripheral to the central core of the lung.
Respiratory lung mechanics
Sham- and ovalbumin-immunized mice were anesthetized with urethane (2 g/kg, intraperitoneally) 24 hours after the last antigen challenge on week 8. Mice were laid supine and a tracheotomy was performed to permit the insertion of a cannula (white luer) into the trachea. After surgery, the mice were placed in a plethysmograph and the tracheostomy tube was connected to a 4-way manifold with one port attached to a differential pressure transducer (± 20 cm H20; Validyne Engineering, Northridge, CA) for the measurement of mouth pressure (Pao). Two ports were connected to the inspiratory and expiratory ports of a volume-cycled ventilator (SAR-830A, model 963217; CWE, Norfolk, United Kingdom). Mice were ventilated at 150 breaths per minute with a tidal volume of 0.15 mL to 0.2 mL and a positive-end expiratory pressure of between 3 and 5 cm H20.
Changes in flow were determined with a Fleish pneumotachograph (size, 00000) connected to a side port of the chamber and measured with a differential pressure transducer (± 2 cm H20; model MP 45-14-871; Validyne Engineering). The flow was integrated to give a continuous recording of tidal volume. Transpulmonary pressure was estimated as the difference between mouth pressure and the pressure measured at the proximal end of the pneumotachograph to the plethysmograph, inasmuch as the chest wall contributes little to the overall compliance of the respiratory system. Breath-by-breath recording of total airway resistance (RL; cm H20/L/s) was calculated by an online respiratory analyzer on a personal computer (Lung Function Recorder version 6; Mumed, London, United Kingdom). Mice instrumented in this fashion were neuromuscular blocked with pancuronium bromide (0.2 mg/kg; Sigma) prior to administration of methacholine.
Measurement of AHR to methacholine
Aerosols of methacholine (6.25-100 mg/mL) were generated from a DeVilbiss ultrasonic nebulizer (DeVilbiss Healthcare) and administered directly to the lungs via the inspiratory port of the plethysmograph. Mice were exposed to the aerosols for 8 seconds (60 breaths per minute) and to a tidal volume of 0.3 mL to 0.4 mL with the aid of a second ventilator. Total airway resistance (RL) was measured prior to administration of saline (0.9%) and following administration of saline or methacholine. The maximum increase in RL to methacholine is expressed as a percentage of the values of RL obtained after saline challenge.
The concentration of methacholine that induced a 200% increase in RL above postsaline RL was used as a measure of airway sensitivity (PC200). The slope of the line about this value was interpolated from each individual curve and used as a measure of airway reactivity (slope value; % RL – postsaline / log mg/mL).
Protocol for antiplatelet antisera (APAS) production and method for immune-based platelet depletion
Blood was collected from normal mice and centrifuged at 150g for 10 minutes. The platelet-rich plasma obtained was washed by the Mustard method.25 Pure murine platelets were then homogenized in Freund complete adjuvant and injected subcutaneously in rabbits. A second immunization was performed 10 days later by intramuscular injection of pure murine platelets in Freund incomplete adjuvant. Thirty days after the second immunization the rabbits were bled and the antiserum inactivated at 56°C for 60 minutes and stored at –70°C. Nonimmune control serum (CS) was obtained from nonimmunized rabbits. To maintain the thrombocytopenia by APAS, mice were injected intramuscularly twice weekly (24 hours before the start of the first challenge and 30 minutes before the last day of the challenge in each week) with 0.1 mL APAS. Other mice received CS at the same time points.
Nonimmune-based method of platelet depletion
Busulfan (Sigma), a bone marrow precursor cell–specific toxin, was used to deplete platelets as previously described.26 Mice received busulfan or vehicle on days –4, –2, 1, and 8, and once fortnightly over the chronic challenge period (25 mg/kg, intraperitoneally).
Administration of corticosteroids
Experiments were carried out by administering systemic dexamethasone 21-phosphate (2 mg/kg, intraperitoneally; Sigma) 20 minutes before the start of aerosol challenge on each day of allergen exposure. Saline (0.1 mL) was administered in control mice.
Statistical analysis of data
Data were expressed as arithmetic means plus or minus standard error of mean (SEM). BAL (cell counts) and blood samples (platelet and leukocyte counts, IgE titres) were analyzed using one-way analysis of variance (ANOVA), with Bonferroni multiple comparison tests between groups. The effects of platelet depletion and dexamethasone treatment on peripheral blood platelet and leukocyte numbers during chronic allergen exposure were measured as area under the curve (AUC) and analyzed using Student t test.
Data from lung function studies were recorded as a percentage increase in RL compared with saline exposure. PC200 values are recorded with the corresponding 95% confidence interval (CI). Differences in lung function recordings (PC200 and slope value) and measurements of components of the airway wall (mean ± SEM) were analyzed using 2-way ANOVA. Differences were considered significant if P < .05 between groups.
Results
Maintenance of thrombocytopenia
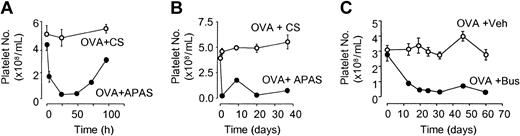
Mice were administered twice-weekly injections of either CS or APAS on alternate upper hind leg regions (24 hours before the start of the first challenge, and 30 minutes before the last day of challenge in each week). This protocol depleted platelets for up to 72 hours (Figure 1A), and maintained platelet depletion via APAS throughout the period of allergen exposure, with platelet numbers enumerated 24 hours after intramuscular injection (Figure 1B).
Platelet levels in peripheral blood of mice after chronic APAS or busulfan administration. (A) APAS or CS was administered intramuscularly (0.1 mL) at 0 hours and blood was collected via retro-orbital bleed or via cardiac puncture (third and final bleed). (B) Platelet depletion recorded over chronic period with APAS or CS administration twice weekly (blood taken 24 hours after any previous injection of APAS or CS). In the case of busulfan (C), mice were exposed to aerosolized OVA (1%) for 8 weeks and busulfan (25 mg/kg, intraperitoneally) was administered once fortnightly throughout the period of exposure. Each point represents the mean and vertical lines the standard error of the mean of 2 to 4 observations.
Platelet levels in peripheral blood of mice after chronic APAS or busulfan administration. (A) APAS or CS was administered intramuscularly (0.1 mL) at 0 hours and blood was collected via retro-orbital bleed or via cardiac puncture (third and final bleed). (B) Platelet depletion recorded over chronic period with APAS or CS administration twice weekly (blood taken 24 hours after any previous injection of APAS or CS). In the case of busulfan (C), mice were exposed to aerosolized OVA (1%) for 8 weeks and busulfan (25 mg/kg, intraperitoneally) was administered once fortnightly throughout the period of exposure. Each point represents the mean and vertical lines the standard error of the mean of 2 to 4 observations.
In separate experiments, mice were depleted of platelets via the nonimmune method of busulfan administration (25 mg/kg, intraperitoneally) once fortnightly (during the morning) throughout the period of allergen exposure. This protocol maintained platelet depletion throughout the period of allergen exposure (Figure 1C). Blood was collected 6 hours after the administration of busulfan when the 2 procedures overlapped.
Effect of platelet depletion and corticosteroid administration on peripheral blood leukocyte numbers
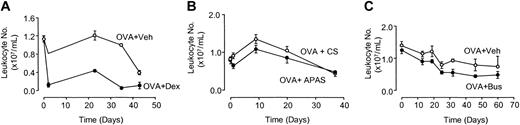
Dexamethasone (Dex) administration over 8 weeks of allergen exposure significantly reduced the number of leukocytes circulating in blood compared with OVA-sensitized controls treated with vehicle (Veh) as measured by AUC (OVA+Veh: 36.9 × 103 ± 3.8 × 103 AUC units versus OVA+Dex: 9.7 × 103 ± 0.5 × 103 AUC units; P < .001; n = 3-5 mice per group; Figure 2A). The reduction in circulating leukocytes was apparent from the first week of allergen exposure and remained suppressed to a similar degree throughout the course of the experiment (Figure 2A). However, the chronic administration of APAS or busulfan (Bus) had no effect on peripheral blood leukocyte numbers compared with OVA-sensitized animals as measured by AUC (OVA+CS: 28.7 × 103 ± 6.5 × 103 AUC units versus OVA+APAS: 23.6 × 103 ± 5.1 × 103 AUC units; n = 4-5 mice per group; Figure 2B; OVA+Veh: 53.4 × 103 ± 4.2 × 103 AUC units versus OVA+Bus: 40.0 × 103 ± 2.0 × 103 AUC units; n = 4 mice per group; Figure 2C). Eosinophil numbers enumerated from blood smears after one week of antigen exposure was observed to decline in animals depleted of platelets with APAS (OVA+CS: 1.61 × 105 ± 0.3 × 105 cells/mL versus OVA+APAS: 0.83 × 105 ± 0.3 × 105 cells/mL; n = 10 mice per group) or busulfan (OVA+Veh: 2.95 × 105 ± 0.6 × 105 cells/mL versus OVA+Bus: 1.59 × 105 ± 0.5 × 105 cells/mL; n = 9-16 mice per group), but this was not significant.
Leukocyte numbers in circulating blood after dexamethasone, APAS, or busulfan administration. Mice were exposed to aerosolized OVA (1%) for 8 weeks and treated with either (A) dexamethasone (2 mg/kg administered intraperitoneally thrice weekly, before each day of exposure), (B) APAS (0.1 mL undiluted, administered intramuscularly twice weekly), or (C) busulfan (25 mg/kg administered intraperitoneally once fortnightly) throughout the period of exposure. Each point represents the mean and vertical lines the standard error of the mean of 3 to 5 observations.
Leukocyte numbers in circulating blood after dexamethasone, APAS, or busulfan administration. Mice were exposed to aerosolized OVA (1%) for 8 weeks and treated with either (A) dexamethasone (2 mg/kg administered intraperitoneally thrice weekly, before each day of exposure), (B) APAS (0.1 mL undiluted, administered intramuscularly twice weekly), or (C) busulfan (25 mg/kg administered intraperitoneally once fortnightly) throughout the period of exposure. Each point represents the mean and vertical lines the standard error of the mean of 3 to 5 observations.
Effect of chronic period of allergen exposure on pulmonary leukocyte recruitment
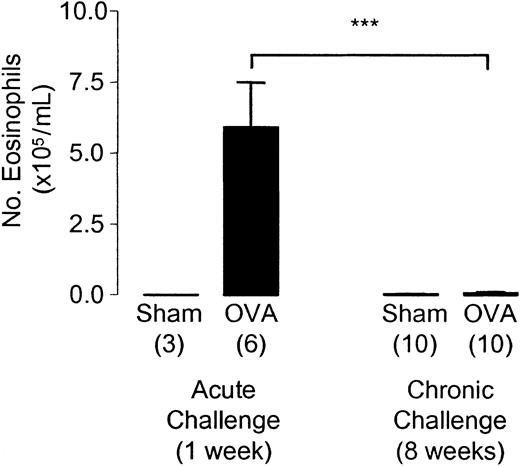
The eosinophilia observed after one week of allergen exposure was no longer evident after 8 weeks of aerosolized OVA exposure (Figure 3). Sensitized animals chronically challenged with OVA were indistinguishable from sham-sensitized animals in the level of eosinophilia observed in BAL fluid. The total number of leukocytes retrieved from the BAL fluid was 2.1 × 105 ± 1.1 × 105 cells/mL in sham-sensitized animals compared with 2.5 × 105 ± 1.1 × 105 cells/mL in OVA-sensitized mice after 8 weeks of aerosol exposure.
Pulmonary leukocyte recruitment after chronic allergen challenge. BAL fluid was taken 24 hours after the start of the last day of challenge in week 1 and week 8 of allergen exposure. Each bar represents the mean and vertical lines the standard error of the mean. Values in parentheses indicate the number of animals per group. ***P < .001 between selected groups.
Pulmonary leukocyte recruitment after chronic allergen challenge. BAL fluid was taken 24 hours after the start of the last day of challenge in week 1 and week 8 of allergen exposure. Each bar represents the mean and vertical lines the standard error of the mean. Values in parentheses indicate the number of animals per group. ***P < .001 between selected groups.
Interestingly, the levels of circulating leukocytes decreased in OVA-sensitized animals as the chronicity of allergen exposure developed, compared with levels of circulating leukocytes before exposure to allergen in studies using dexamethasone (day 0: 1.1 × 107 ± 0.1 × 107 cells/mL versus day 43: 0.4 × 107 ± 0.05 × 107 cells/mL; P < .001; n = 3-5 mice per group; Figure 2A), APAS (day 0: 0.83 × 107 ± 0.05 × 107 cells/mL versus day 37: 0.43 × 107 ± 0.07 × 107 cells/mL; P < .001; n = 4-5 mice per group; Figure 2B), and busulfan, although in this last group the reduction in leukocyte numbers was not significant (day 0: 1.4 × 107 ± 0.13 × 107 cells/mL versus day 60: 0.7 × 107 ± 0.3 × 107 cells/mL; n = 4 mice per group; Figure 2C).
Blood taken 24 hours after the start of the last OVA challenge revealed the levels of total serum IgE were not reduced in mice rendered thrombocytopenic via busulfan (OVA+Veh: 250.5 ± 23.8 ng/mL versus OVA+Bus: 615.3 ± 174.9 ng/mL; n = 6-9 mice per group) or APAS (OVA+CS: 363.7 ± 172.3 ng/mL versus OVA+ APAS: 942.8 ± 175.9 ng/mL; P < .05; n = 6 mice per group).
Pulmonary platelet recruitment after an acute period of allergen exposure
BAL performed on mice after one week of allergen exposure revealed the significant presence of platelets in the lavage fluid of OVA-immunized compared with sham-immunized mice (OVA: 253.1 ± 85.5 aggregates/mL; Sham: 10.0 ± 4.3 aggregates/mL; P < .01; n = 4). No, or very few, red blood cells were found to contaminate the samples. Representative photographs of cytospins typically show an absence of platelets in sham-immunized mice (Figure 4A), with positive staining for the platelet antigen CD41 in OVA-immunized mice (Figure 4B). Figure 4C reveals an absence of red blood cells in BAL samples used in the analysis for the presence of platelets.
Histologic analysis of murine peripheral airways. (A-C) Bronchoalveolar lavage cytospins obtained from sham-immunized (A) and OVA-immunized (B-C) mice were stained with CD41 immunoperoxidase and Gill hemotoxylin (A-B), or hemotoxylin and eosin (C). Note the presence of platelet aggregates (p; arrow) in cytospin from OVA-immunized mice (B). (D-I) Sections from distal airways of sham-immunized (D,G) and OVA-immunized (E,H) mice and OVA-immunized mice rendered thromobcytopenic following treatment with busulfan (F,I). Sections were stained with either Masson trichrome (D-F) or Gordon and Sweet silver stain (G-I). Red arrows in panel E indicate airway smooth muscle. Blue arrows in panels D-F indicate epithelium. White circles in panels G-I represent regions of subepithelial fiber deposition. Horizontal white bars represent 20.0 μm. Original magnifications: A,C-F, × 40 objective; B,G-I, × 100 objective.
Histologic analysis of murine peripheral airways. (A-C) Bronchoalveolar lavage cytospins obtained from sham-immunized (A) and OVA-immunized (B-C) mice were stained with CD41 immunoperoxidase and Gill hemotoxylin (A-B), or hemotoxylin and eosin (C). Note the presence of platelet aggregates (p; arrow) in cytospin from OVA-immunized mice (B). (D-I) Sections from distal airways of sham-immunized (D,G) and OVA-immunized (E,H) mice and OVA-immunized mice rendered thromobcytopenic following treatment with busulfan (F,I). Sections were stained with either Masson trichrome (D-F) or Gordon and Sweet silver stain (G-I). Red arrows in panel E indicate airway smooth muscle. Blue arrows in panels D-F indicate epithelium. White circles in panels G-I represent regions of subepithelial fiber deposition. Horizontal white bars represent 20.0 μm. Original magnifications: A,C-F, × 40 objective; B,G-I, × 100 objective.
Effect of a chronic period of allergen exposure on airway remodeling events
Representative photographs of distal airways from murine lungs after 8 weeks of allergen challenge typically show that the epithelium becomes thicker in mice sensitized to OVA, with evidence of epithelial hyperplasia (Figure 4D-E). Smooth muscle, being absent in the distal airways of sham-sensitized mice, is present in mice sensitized to OVA (Figure 4D-E), whereas residual reticular fibers observed under the epithelium in sham-sensitized mice become more pronounced in OVA-sensitized mice, as evidence of increased fibrosis (Figure 4G-H).
Morphometric analysis of lung tissue revealed significant remodeling events after chronic allergen exposure. This was associated with significant increases in the thickness of the epithelium (Figure 5), smooth-muscle layer (Figure 6), and significantly increased subepithelial fiber deposition (Figure 7) in OVA-sensitized animals compared with sham-sensitized animals. The increased bulk of these airway wall components was reflected in an overall thickening of the airway wall (sham-sensitized mice: 18.5 ± 0.7 μm versus OVA-sensitized mice: 26.2 ± 1.1 μm; P < .01; n = 24 mice per group).
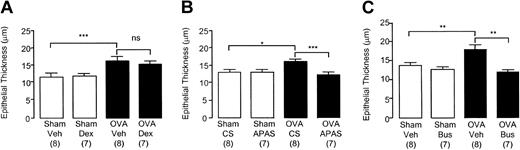
Epithelial thickness in the distal airways of chronically challenged mice. Selected groups of mice were administered (A) dexamethasone (2 mg/kg intraperitoneally thrice weekly before each day of exposure), (B) APAS (0.1 mL undiluted, intramuscularly twice weekly), or (C) busulfan (25 mg/kg intraperitoneally once fortnightly). Lungs were collected 24 hours after the last challenge in week 8. Each bar represents the mean and vertical lines the standard error of the mean. Values in parentheses indicate number of animals per group. *P < .05, **P < .01, and ***P < .001 between selected groups (ns indicates no significance).
Epithelial thickness in the distal airways of chronically challenged mice. Selected groups of mice were administered (A) dexamethasone (2 mg/kg intraperitoneally thrice weekly before each day of exposure), (B) APAS (0.1 mL undiluted, intramuscularly twice weekly), or (C) busulfan (25 mg/kg intraperitoneally once fortnightly). Lungs were collected 24 hours after the last challenge in week 8. Each bar represents the mean and vertical lines the standard error of the mean. Values in parentheses indicate number of animals per group. *P < .05, **P < .01, and ***P < .001 between selected groups (ns indicates no significance).
Smooth-muscle thickness in the distal airways of chronically challenged mice. Selected groups of mice were administered (A) dexamethasone (2 mg/kg intraperitoneally thrice weekly before each day of exposure), (B) APAS (0.1 mL undiluted, intramuscularly twice weekly), (C) busulfan (25 mg/kg intraperitoneally once fortnightly). Lungs were collected 24 hours after the last challenge in week 8. Each bar represents the mean and vertical lines the standard error of the mean. Values in parentheses indicate number of animals per group. *P < .05, **P < .01, and ***P < .001 between selected groups.
Smooth-muscle thickness in the distal airways of chronically challenged mice. Selected groups of mice were administered (A) dexamethasone (2 mg/kg intraperitoneally thrice weekly before each day of exposure), (B) APAS (0.1 mL undiluted, intramuscularly twice weekly), (C) busulfan (25 mg/kg intraperitoneally once fortnightly). Lungs were collected 24 hours after the last challenge in week 8. Each bar represents the mean and vertical lines the standard error of the mean. Values in parentheses indicate number of animals per group. *P < .05, **P < .01, and ***P < .001 between selected groups.
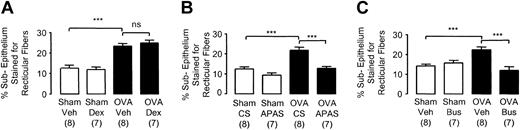
Subepithelial reticular fiber deposition in the distal airways of chronically challenged mice. Selected groups of mice were administered (A) dexamethasone (2 mg/kg intraperitoneally thrice weekly before each day of exposure), (B) APAS (0.1 mL undiluted, intramuscularly thrice weekly), or (C) busulfan (25 mg/kg intraperitoneally once fortnightly). Lungs were collected 24 hours after the last challenge in week 8. Each bar represents the mean and vertical lines the standard error of the mean. Values in parentheses indicate number of animals per group. ***P < .001 between selected groups (ns indicates no significance).
Subepithelial reticular fiber deposition in the distal airways of chronically challenged mice. Selected groups of mice were administered (A) dexamethasone (2 mg/kg intraperitoneally thrice weekly before each day of exposure), (B) APAS (0.1 mL undiluted, intramuscularly thrice weekly), or (C) busulfan (25 mg/kg intraperitoneally once fortnightly). Lungs were collected 24 hours after the last challenge in week 8. Each bar represents the mean and vertical lines the standard error of the mean. Values in parentheses indicate number of animals per group. ***P < .001 between selected groups (ns indicates no significance).
Effect of corticosteroid administration on airway remodeling events
The dose of dexamethasone chosen for the study was sufficient to significantly inhibit eosinophil recruitment to the lungs after one week of challenge in sensitized mice (OVA+Veh: 5.2 × 105 ± 2.3 × 105 cells/mL versus OVA+Dex: 0.3 × 105 ± 0.3 × 105 cells/mL; P < .05; n = 5-9 mice per group). Dexamethasone administration to sensitized mice (OVA+Dex) significantly inhibited thickening of the smooth-muscle layer (Figure 6A) compared with OVA+Veh mice. However, no inhibition of epithelial thickening (Figure 5A) or subepithelial reticular deposition was observed (Figure 7A) after the administration of dexamethasone. Thus, there was a significant yet small inhibition of airway wall thickening in sensitized mice receiving dexamethasone (OVA+Veh: 24.8 ± 2.1 μm versus OVA+Dex: 19.9 ± 1.5 μm; P < .05; n = 7-8 mice per group). The length of basement membrane perimeter used to determine which airways to analyze was identical among these groups, thus excluding bias (Sham+Veh: 0.60 ± 0.03 mm; Sham+Dex: 0.58 ± 0.02 mm; OVA+Veh: 0.56 ± 0.02 mm; OVA+Dex: 0.59 ± 0.03 mm; n = 7-8 mice per group).
Effect of platelet depletion on airway remodeling events
Representative photographs reveal an absence of epithelial hyperplasia in OVA-sensitized mice depleted of platelets after 8 weeks of allergen exposure (Figure 4F), while there is also a lack of smooth-muscle growth and the airway wall remains narrow (Figure 4F). There was also no evidence of subepithelial fibrosis (Figure 4I).
Thus, administration of APAS and busulfan in OVA-sensitized animals significantly inhibited epithelial thickening (Figure 5B-C), smooth-muscle thickening (Figure 6B-C), and subepithelial fiber deposition (Figure 7B-C) compared with OVA-sensitized animals receiving CS or vehicle.
The inhibition of all these remodeling events in mice administered APAS and busulfan resulted in no discernible difference in the thickness of these structural components compared to sham-sensitized controls (Figures 5, 6, 7). No differences were observed between sham-sensitized animals receiving CS or vehicle and those receiving APAS or busulfan (Figures 5, 6, 7). The inhibition of thickening to various components of the airway wall in thrombocytopenic mice was reflected by a significant inhibition of the overall thickening of the airway wall in both APAS (OVA+CS: 25.3 μm ± 1.3 μm versus OVA+APAS: 17.5 ± 1.1 μm; P < .01; n = 7-8 mice per group) and busulfan-treated animals (OVA+Veh: 28.4 ± 2.1 μm versus OVA+Bus: 19.6 ± 0.8 μm; P < .01; n = 7-8 mice per group). There was no difference in airway wall thickness between thrombocytopenic mice sensitized to OVA (OVA+APAS: 17.5 ± 1.1 μm, n = 7 mice per group; OVA+Bus: 19.6 ± 0.8 μm, n = 7 mice per group) and sham control mice (18.5 ± 0.7 μm; n = 16 mice per group). The length of basement membrane perimeter used to determine which airways to analyze was identical among these groups in APAS (Sham+CS: 0.54 ± 0.02 mm; Sham+APAS: 0.58 ± 0.02 mm; OVA+CS: 0.59 ± 0.02 mm; OVA+APAS: 0.61 ± 0.2 mm; n = 7-8 mice per group) and busulfan experiments, thus excluding bias (Sham+Veh: 0.65 ± 0.03 mm; Sham+Bus: 0.61 ± 0.03 mm; OVA+Veh: 0.65 ± 0.03 mm; OVA+Bus: 0.63 ± 0.04 mm; n = 7-8 mice per group).
Effect of corticosteroid administration on lung function
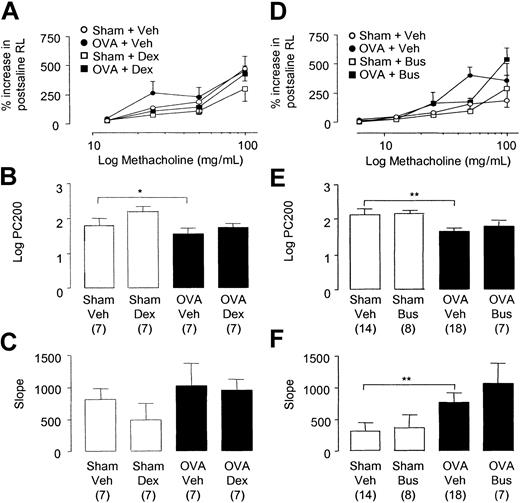
There was no difference in baseline total airway resistance (cm H2O/L/s) between the 4 groups (Sham+Veh: 1663 ± 101.0; Sham+Dex: 1766 ± 121.0; OVA+Veh: 1853 ± 132.0; OVA +Dex: 1757 ± 99.0; n = 7 mice per group). Methacholine induced a dose-dependent increase in baseline RL in sham- and OVA-sensitized mice (Figure 8A). OVA-sensitized C57BL/6 mice displayed a small but significant increase in AHR compared with sham-immunized controls as measured by an increase in sensitivity to methacholine (RL PC200, Sham+Veh: 73.4 mg/mL [95% CI: 17.9-128.8] versus OVA+Veh: 55.3 mg/mL [95% CI: 6.2-104.5]; P < .05; Figure 8B). However, no differences were observed in airway reactivity to methacholine in OVA-sensitized compared with sham-sensitized mice as measured by the slope value (Figure 8C). The administration of dexamethasone to OVA-sensitized animals did not inhibit AHR compared with OVA-sensitized controls as measured by changes to methacholine sensitivity (PC200, Figure 8B) or reactivity (slope value, Figure 8C).
Respiratory pulmonary mechanics in chronically challenged mice. Changes in total lung resistance to increasing doses of aerosolized methacholine expressed as percent increase in RL above postsaline values (A,D). Values for methacholine PC200 (B,E) and slope (C,F) were determined for each dose-response curve. Selected groups of mice were administered (A-C) dexamethasone (2 mg/kg intraperitoneally thrice weekly before each day of exposure) or (D-F) busulfan (25 mg/kg intraperitoneally once fortnightly). Each point or bar represents the mean and vertical lines the standard error of the mean. Values in parentheses indicate number of animals per group. *P < .05 and **P < .01 between selected groups.
Respiratory pulmonary mechanics in chronically challenged mice. Changes in total lung resistance to increasing doses of aerosolized methacholine expressed as percent increase in RL above postsaline values (A,D). Values for methacholine PC200 (B,E) and slope (C,F) were determined for each dose-response curve. Selected groups of mice were administered (A-C) dexamethasone (2 mg/kg intraperitoneally thrice weekly before each day of exposure) or (D-F) busulfan (25 mg/kg intraperitoneally once fortnightly). Each point or bar represents the mean and vertical lines the standard error of the mean. Values in parentheses indicate number of animals per group. *P < .05 and **P < .01 between selected groups.
Effect of platelet depletion via busulfan on lung function
There was no difference in baseline total airway resistance (cm H2O/L/s) among the 4 groups (Sham+Veh: 1589 ± 21.0; Sham+Bus: 1836 ± 144.0; OVA+Veh: 1691 ± 40.0; OVA+Bus: 1711 ± 138.0; n = 7-18 mice per group). Methacholine induced a dose-dependent increase in baseline RL in sham- and OVA-sensitized mice (Figure 8D). OVA-sensitized mice demonstrated AHR to methacholine as reflected by a significant increase in methacholine airway sensitivity (RL PC200, Sham+Veh: 119.7 mg/mL [95% CI: 79.6-159.7] versus OVA+Veh: 65.2 mg/mL [95% CI: 34.6-95.7]; P < .01; Figure 8E) and reactivity compared with sham mice (slope value, Sham+Veh versus OVA+Veh: P < .01; Figure 8F). The administration of busulfan resulted in more than 90% depletion of platelets compared with control animals and significantly inhibited airway remodeling to the lungs. However, the administration of busulfan to OVA-sensitized mice did not inhibit AHR compared with OVA-sensitized controls as measured by changes to methacholine airway sensitivity (PC200, Figure 8E) and reactivity (slope value, Figure 8F). Measurement of lung function was also performed in mice receiving APAS. However, heightened airways responses were observed to methacholine in sham-immunized mice repeatedly treated with APAS. Because of this confounding data in the sham-immunized groups, we did not analyze the data further.
Discussion
The lungs of chronic asthmatic individuals undergo structural changes, for example: cellular hypertrophy and hyperplasia of smooth muscle2,3 ; reticular basement membrane thickening2 ; incorporating fibronectin and collagen types I and III deposition1 ; epithelial hyperplasia and activation2,24 ; and goblet cell hyperplasia.11 In the present study we demonstrate that mice subjected to a chronic period of aerosolized antigen exhibit similar structural changes within the lungs to those of asthmatic individuals, with remodeling events occurring in the distal airways of OVA-sensitized mice, but not in sham-sensitized mice. Changes to airway architecture observed were thickening of epithelium, smooth muscle, and airway wall, and also increased reticular-fiber deposition. Interestingly, there was a notable absence of eosinophilia in the lavage fluid of chronically challenged OVA-sensitized mice, suggesting remodeling events can persist for an extended period of time after inflammatory events have receded.
The depletion of platelets via both APAS and busulfan administration resulted in the virtual abolition of epithelial and smooth-muscle thickening and the deposition of reticular fibers in the extracellular matrix (ECM). Hence, the overall thickness of the airway wall was shown to be not different from that observed in nonsensitized mice, providing evidence for the importance of platelets in these events. The chronic administration of busulfan and APAS resulted in the maintenance of thrombocytopenia above 90% in treated mice for the duration of the 8-week allergen challenge, and these procedures did not significantly alter circulating blood leukocyte numbers, suggesting the protocols used to maintain thrombocytopenia were cell specific. Furthermore, we have recently demonstrated that pulmonary leukocyte recruitment was entirely restored upon the partial reconstitution of platelets in mice made thrombocytopenic,16 consistent with previous studies using either APAS27 or busulfan.26 Additionally, serum IgE levels remained elevated in mice depleted of platelets, suggesting these methods of platelet depletion did not affect the sensitization process. Moreover, it has been shown that APAS has no direct interference with the ability of leukocytes to adhere to damaged arterial walls.27 The nonimmunologic method of platelet depletion via busulfan is complementary to that of the immunlogic method via APAS. Although it is not known which antigens are targeted by APAS, washed, nonactivated platelets from nonimmunized mice were administered to donor rabbits for APAS production. Therefore the antibody would be directed against constitutive platelet markers and not antigens expressed as a result of inflammatory or platelet-activating stimuli, or soluble growth factors that may be present in serum.
The exact nature of the mechanism by which platelets contribute to remodeling, and the influence of other cell types and tissues that have been implicated in these processes is not known. However, it can be hypothesized that platelets may contribute to remodeling either by (1) platelets acting as an essential component of leukocyte recruitment to the lung in the first instance, and therefore indirectly regulating tissue damage; or (2) platelets presenting growth factors, and matrix-metalloproteinases, the role of which is recognized in angiogenesis and vascular injury, and therefore contributing to tissue regrowth independently or parallel to the contribution of inflammatory stimuli. Indeed, we have demonstrated the presence of platelets via specific staining in bronchoalveolar lavage following OVA challenge of OVA- but not sham-immunized mice, suggesting that platelets have the ability to migrate through tissue and thus contribute to remodeling events. This is entirely consistent with the documentation of platelets in the microvasculature of lungs from atopic asthmatics,12 and platelets have been detected in bronchoalveolar lavage fluid and attached to the lumenal side of the epithelium in asthma.13,19 Similarly, recruitment of platelets to the lung has been observed in murine,28 guinea-pig,29 and rabbit13 models of allergic lung inflammation. The absence of red blood cells in lavage fluid from sham- and OVA-immunized mice, and of platelets in lavage fluid from sham-immunized mice, rules out the formation of lesions at the time of the procedure that could account for the presence of platelets in the lungs of immunized mice.
The timing of platelet depletion in this model makes it difficult to dissect out the contribution of inflammatory cells, and we have previously reported an essential requirement of platelets in leukocyte recruitment to the lungs in this murine model.16 Similarly, in human asthma, platelets enhance endothelial attachment of eosinophils.30 Thus, it could be argued that thombocytopenic animals are not subjected to tissue damage in the first instance. Evidence suggests that remodeling processes were totally inhibited in mice depleted of CD4+ T lymphocytes, as was the development of inflammation in a model of chronic lung inflammation.31 However, studies have shown that in the absence of eosinophilia (as occurs in IL-5–deficient mice) or chronic inflammatory cell recruitment to the airways, epithelial hypertrophy and subepithelial fibrosis still occur.32
The requirement of Th2 cytokines in murine models of inflammation has been extensively researched, and the initiation of inflammation for the propagation of remodeling events has been proposed.33 However, we have demonstrated that mice administered dexamethasone, itself sufficient for the abrogation of leukocyte recruitment to the airways, was insufficient in inhibiting all aspects of remodeling, since epithelial hyperplasia/hypertrophy and subepithelial reticular fiber deposition were not affected, changes which obviously contribute to airway wall thickening. These results thus suggest that some of the effects by which platelets affect airway remodeling are not the result of chronic inflammatory pathways, a conclusion strengthened by the observation that airway remodeling persisted way beyond the initial eosinophilia induced by allergen exposure. Platelet depletion provided a far greater protection from airway remodeling than chronic treatment with glucocorticosteroid administration. This suggests the existence of other pathways leading to airway remodeling that are independent of glucocorticosteroid actions but dependent instead on platelet activation. For example, TxA2 generation and release of constituents from platelet granules are not inhibited by glucocorticosteroid treatment,34,35 although it has been reported that the formation of platelet-derived reactive oxygen species is steroid sensitive.36 It therefore appears that platelet function is relatively resistant to inhibition by glucocorticosteroids.
These observations implicate the importance of platelets in tissue regeneration after injury, a phenomenon that has parallels in the vasculature for atherosclerosis and restenosis, where platelets may participate in vascular damage and angiogenesis, facilitating smooth-muscle proliferation after endothelial injury, this being inhibited by antiplatelet therapy.5,8,9,37 Interestingly, platelet P-selectin contributes to atherosclerotic events in vivo, whereby its absence inhibited the migration of smooth-muscle cells into the lesion.38 Platelets are themselves an excellent source of mitogens, matrix metalloproteinases,39 and lysosomal enzymes40 influencing resident structural cells and cells involved in the composition of the ECM in the airway or vasculature. Thus, the role of activated platelets in lesion development may be attributed to platelet P-selectin contact–dependent delivery of platelet-derived mitogens to structural tissue.37,38 In the context of cardiovascular disease, the response to injury to the endothelium is thought to be dependent upon the release of chemotactic factors for circulating structural cells, since a platelet-derived factor was found to be chemotactic for rabbit arterial smooth-muscle cells in culture.41 It has since been demonstrated that the thrombi release platelet-derived growth factor (PDGF), epidermal growth factor (EGF), insulin-like growth factor (IGF), and transforming growth factor beta (TGFβ), providing a matrix for smooth-muscle cell growth.42 Recent studies suggest a critical role for platelet adhesion in the initiation of atherosclerotic lesion formation, since the inhibition of platelet adhesion to the vascular endothelium profoundly attenuated these processes.7 Our results emphasize that tissue remodeling events are dependent on platelet activation. It is of relevance to our observations that PDGF has also been shown to affect human tracheal smooth-muscle mitogenesis, acting as a potent mitogen for airway smooth-muscle cells in culture.43 PDGF also acts as a potent chemoattractant for fibroblasts,44 being implicated in significant pulmonary fibrosis.45,46 Interestingly, PDGF overexpression was observed along with airway smooth-muscle thickening in mice repeatedly exposed to allergen,47 augmenting airway responsiveness through airway remodeling processes. The administration of antibodies against PDGF inhibited both AHR and airway wall thickening, but did not affect inflammatory cell infiltration.47 Platelets are also a source of TGFβ, increasing smooth-muscle cell mitogenesis in culture,48 and may participate in airway obstruction and subepithelial fibrosis, correlating with disease severity.49,50
Although inflammatory cell interactions with epithelial and mesenchymal cells have been implicated in growth factor release, it is apparent that glucocorticosteroid administration in asthmatic patients does not completely reverse changes in lung function or remodeling. One of the noticeable features of persistent changes to the airway architecture is a continuum of fibrosis,19,20 and the epithelium appears to lose normal surface morphology even after 10 years of steroid therapy.51 Parallel pathways governed by epithelial-mesenchymal cell units have thus been implicated in remodeling events,52,53 entering a cycle of continuous repair, stimulated by proinflammatory mediators and fibrogenic growth factors. Thus, the persistent presence of platelet-derived mitogens may cause structurally intact epithelium to sustain chronic inflammatory and remodeling events affecting the underlying lamina propia and lamina muscularis where major structural alterations occur,1 providing a platform for greater smooth-muscle proliferation, a process resistant to corticosteroids in vitro,54,55 although the thickening of airway smooth muscle per se was reduced in the present study by corticosteroid administration.
In mice chronically exposed to aerosolized OVA, an increase in AHR was documented, compared with sham-sensitized animals. Importantly, the degree of inflammatory cell recruitment had waned substantially, and there was a scarcity of inflammatory cells within the tissue when AHR was measured. Similar observations have been made by other investigators and are thought to be due to a process of tolerance by the immune system to repeated allergen exposure,56 while mice display airway dysfunction and remodeling after the resolution of allergen-induced airway inflammation.57 The absence of inflammatory cells may suggest that the observed hypersensitivity to methacholine was the result of the remodeled airway itself as OVA-immunized mice developed a thickening to the airway wall, as well as increases to the components of the airway wall that, theoretically, may contribute to alterations in force of contraction and rigidity. Clinical studies have demonstrated that there is a residual level of AHR in many asthmatics not associated with seasonal exposure to allergens.58 This irreversible component has been attributed to remodeling altering the mechanical processes of lung inspiration and exhalation, contributing significantly to AHR.21,22
However, the depletion of platelets or the administration of dexamethasone abrogated the development of remodeling to varying degrees in OVA-sensitized mice, but without inhibiting the accompanying AHR. Our results suggest, therefore, that remodeling events do not necessarily contribute to AHR in this model. Thus, other undefined factors may contribute to this phenomenon. It has recently been suggested that the major factors governing changes in total lung resistance in the mouse are not dependent on changes in airway smooth-muscle contractility, but to alterations in lung-tissue resistance.59 The mechanism of this change is not known at present, but if true, might offer an explanation for the discordance of platelets inhibiting changes to remodeling but not affecting AHR. It is also possible that the degree of remodeling observed was not sufficient to affect the degree of contractility of the airways observed in our studies, or that remodeling, in the absence of inflammation, is insufficient to affect AHR, since AHR is a composite reflection of airway inflammation and remodeling in asthmatic patients.21 Alternatively, other structural elements such as airway nerves may contribute to the pathogenesis of AHR.60 Such an hypothesis readily explains AHR in the absence of airway inflammation or remodeling.
In summary, our study has revealed that blood platelets are essential for the structural remodeling of the airways induced by chronic exposure to aerosolized allergen in mice, a phenomenon critically dependent upon platelets, but resistant to some extent to glucocorticosteroid treatment. Although AHR was documented in chronically challenged mice, this occurred in the absence of inflammatory cells and it is unclear whether bronchial remodeling contributes to AHR. Whether the suppression of platelet activation, adhesion, or release reaction may influence remodeling events in chronic allergic asthmatics remains to be established.
Prepublished online as Blood First Edition Paper, September 22, 2003; DOI 10.1182/blood-2003-05-1707.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal