Abstract
Chemokines released by the endothelium have proaggregatory properties on platelets. Fractalkine, a recently discovered membrane-bound chemokine with a transmembrane domain, is expressed in vascular injury; however, the effects of fractalkine on platelets have not yet been investigated. Blood was taken from healthy Wistar-Kyoto rats and the expression of the fractalkine receptor on platelets was demonstrated. The modulation of surface expression of P-selectin was assessed by flow cytometry. P-selectin expression was significantly enhanced by in vitro stimulation with recombinant rat fractalkine compared with baseline levels. Selectively inhibiting the function of recombinant fractalkine by an antagonizing antibody or the disruption of the G-protein–coupled intracellular signaling cascade of the fractalkine receptor by pertussis toxin (PTX) completely prevented fractalkine-mediated platelet activation. Preincubation with apyrase significantly attenuated the fractalkine-induced degranulation. In a flow chamber model of platelet adhesion, stimulation with fractalkine significantly enhanced platelet adhesion to collagen and fibrinogen. Similar to P-selectin expression, enhanced adhesion could be prevented by the antagonizing antibody or preincubation of platelets with PTX. Fractalkine, which is overexpressed in atherosclerosis and vascular injury, contributes to platelet activation and adhesion and hence is likely to play a pathophysiologically important role for increased thrombogenesis in vascular diseases.
Introduction
Chemokines (chemotactic cytokines) are a large family of small proteins that induce the chemotaxis of cells and direct the trafficking of white blood cells in immune surveillance. They are distinguished by a cysteine signature motif based principally on the relative position of the first 2 highly conserved cysteine residues: CX3C (fractalkine), CXC, CC, and C, where C is a cysteine and X any amino acid residue.1 The unique difference between fractalkine and the other chemokines is a polypeptide chain that carries the chemokine domain on top of an extended mucinelike stalk, which allows the molecule to exist either as a membrane-anchored or a soluble glycoprotein.1 The fractalkine receptor (CX3CR1) is expressed in several cells including, predominantly, leukocytes.2,3 Interaction between membrane-bound fractalkine and its receptor on white blood cells not only mediates chemoattraction but also leukocyte adhesion.4 Fractalkine is constitutively expressed in a variety of nonhematopoietic tissues such as brain, heart, kidney, and lung,1,5,6 and its expression in cardiac endothelial cells is increased by proinflammatory agents.5 In vitro, fractalkine displays multiple activities including signal transduction through the pertussis toxin (PTX)–sensitive G-protein–coupled receptor CX3CR1,7 the only described fractalkine receptor, and leukocyte adhesion in static binding assays.1,7 Immobilized forms of fractalkine mediate firm adhesion of cells carrying the CX3CR1 receptor, and this adhesion does not require integrins, calcium, or an opposing cell membrane.7,8 Fractalkine-dependent firm adhesion of leukocytes can occur under flow conditions.9,10 Moreover, fractalkine is expressed by macrophages within atherosclerotic plaques,11 and a polymorphism in the fractalkine receptor gene has been associated with a reduced risk for coronary artery disease.12 CC and CXC chemokines induce platelet activation,13-17 and their respective receptors (CCR1, CCR3, CCR4, and CXCR4) have been shown on platelets.13,16-21 Initially, modulation of platelet aggregation by stimulation of chemokine receptors was believed to depend on the presence of low concentrations of conventional agonists such as adenosine diphosphate (ADP) or thrombin,14,15,18,22 whereas recently stronger and costimulation-independent effects were suggested.13,16 The role of fractalkine for platelet activation, however, is unknown. Therefore, in the present study, we investigated whether a functional fractalkine receptor is present on platelets and might be involved in platelet activation and adhesion.
Materials and methods
Flow cytometry
Surface expression of CX3CR1 and P-selectin was assessed using a polyclonal rabbit antirat CX3CR1 antibody (TP-501P; 1 mg/mL; Torrey Pines Biolabs, San Diego, CA) and a polyclonal rabbit anti–P-selectin antibody (anti-CD62P; Becton Dickinson, Heidelberg, Germany). Blood (40 μL; anticoagulated by 3.8% sodium citrate) was diluted with 50 μL phosphate buffered saline (PBS; free of Ca2+ and Mg2+ and enriched with D-glucose [5.5 mM] and 0.5% bovine albumin) and stained with 10 μL of the respective antibody. After 10 minutes of incubation at room temperature, this was followed by incubation with a fluorescein isothiocyanate (FITC)–labeled goat antirabbit immunoglobulin G (IgG) antibody (Sigma, Deisenhofen, Germany). Staining of the samples was also performed only with the FITC-conjugated secondary antibody in the absence of the primary antibody to arbitrarily adjust the unspecific binding to a mean fluorescence of 10, which was used as the negative control for CX3CR1 expression. Platelets were fixed with methanol-free formaldehyde (1.5% final concentration) for 5 minutes. Subsequently, all samples were analyzed in a Becton Dickinson FACSCalibur (Becton Dickinson) at a low flow rate. The platelet population was identified on its forward- and side-scatter distribution, and 20 000 events were analyzed for mean fluorescence using CELLQuest software, version 3.1f.23
In vitro stimulation
Platelets were incubated with recombinant rat fractalkine (rrCX3C; final concentration 1 μg/mL; R&D Diagnostics, Minneapolis, MN) for 3 minutes prior to induction of the standard protocol as described above (the presence of rrCX3C had no effect on unspecific fluorescence). To assess the signaling pathways involved, blood samples were either incubated with sodium nitroprusside (SNP; 100 μM, a dose sufficient enough to completely relax aortic rings from the same animal in vitro), acetyl salicylic acid (ASA; 10 μg/mL, equivalent to plasma levels achieved by the ingestion of 100 mg ASA orally in humans; Bayer AG, Leverkusen, Germany), or apyrase (7.5 U/mL; Sigma). To inhibit the G-protein–mediated signaling the respective samples were incubated with PTX (0.4 nM; Sigma) for 45 minutes. In a further set of experiments the recombinant fractalkine was preincubated with a selectively antagonizing antifractalkine antibody (AF 537; 100 μg/mL; R&D Diagnostics) for 30 minutes before stimulation of platelets. Afterward, staining of the samples was performed as described in “Flow cytometry.”
Flow chamber and adhesion
Blood was donated by healthy humans who had not taken any medication known to interfere with platelet adhesion and activation for at least 10 days before the experiment. Blood was collected into acid citrate dextrose (ACD: citric acid [3.8 mM] and dextrose [125 mM]; 1 mL ACD/4 mL blood). Platelet-rich plasma was obtained by centrifugation at 430g for 20 minutes, and HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid)–modified Tyrode solution buffer 1 (NaCl, 132 mM; KCl, 4 mM; NaHCO3, 11.9 mM; NaH2PO4, 0.36 mM; glucose, 10 mM; pH 6.5; 1 mL PRP/3.5 mL of Buffer 1) was added. Plasma-free platelet suspensions were obtained by centrifugation (900g, 10 minutes) of the platelet-rich plasma; and the resulting pellet was resuspended in HEPES-modified Tyrode solution buffer 2 (buffer 1; pH 7.4; CaCl2, 1 mM; MgCl2, 1 mM) to achieve a final platelet density of 1 × 105/mL. Thereafter, 5 mL washed platelet suspension was collected into a 50-mL syringe (for usage in an automated syringe pump) and prewarmed in a water bath at 37°C for 15 minutes. After prewarming of the platelets, the syringe was placed into an automated pump without delay and platelets were perfused through the chamber at different shear rates (500 per second and 2000 per second). While measuring platelet adhesion, syringes containing washed platelet suspension for subsequent experiments were prewarmed in constant intervals. To avoid platelet activation and exhaustion with time, the duration of each experiment was limited to one hour. In preliminary experiments, this time frame was found to give reproducible results.
Coverslips were coated with monolayers of various adhesive proteins (collagen type-1, fibrinogen) as described previously.24 The concentration chosen for the coating is the concentration of the different proteins that gives optimal platelet adhesion.
Perfusion studies on various surfaces were carried out in a parallel plate perfusion chamber (Oligene, Berlin, Germany), which allows well-defined rheologic conditions. Perfusion was performed using defined shear rates of 500 per second (further referred to as low shear) and 2000 per second (high shear) that appear in healthy blood vessels.25 Preincubation with AF537, PTX, and apyrase was performed as described in “In vitro stimulation.” For stimulation of human platelets, recombinant human fractalkine (rhCX3C; 1 μg/mL; R&D Diagnostics) was used. For blockade of glycoprotein IIb/IIIa, platelets were preincubated with the monoclonal antibody c7E3 (4 μg/mL; Lilly Deutschland, Bad Homburg, Germany) for at least 15 minutes. Platelet interaction with the substrates was studied by using a flow chamber mounted on the stage of an Axiovert 100 (Zeiss, Jena, Germany) microscope. All images were videotaped and evaluated offline using a computer-assisted image analysis program (Cap Image 7.1; Ingenieurbüro Dr Zeintl, Heidelberg, Germany). At the beginning of each experiment the setting was taped prior to perfusion with platelets to obtain the corresponding background image. The number of firmly adherent platelets was assessed by counting the cells that did not move or detach from the surface within 20 seconds.26
Western blot analysis
Washed platelets were prepared as described in the previous section. The platelet number was adjusted to 2 × 109 platelets/mL. Afterward, the washed platelets were kept in a water bath at 37°C for 15 minutes before adding 50 μL of a 3× sodium dodecyl sulfate (SDS)–Stop buffer (consisting of Tris/HCl 200 mM [pH 6.7], 15% (vol/vol) glycerol, 6% (wt/vol) SDS) per 100 μL of washed platelet suspension. Samples were boiled for 5 minutes at 95°C before storage at –20°C.
Platelet samples (10 μg [lane 1], 20 μg [lane 2]) were mixed with sample loading buffer and were separated under reducing conditions on 10% SDS–polyacrylamide gel. Positive controls were human peripheral blood mononuclear cells (PBMCs; 184 μg per lane) and human spleen tissue lysate (ab7921, abcam; 10 μg per lane). Proteins were electrotransferred onto polyvinylidene difluoride (PVDF) membrane (0.2 μm; Immun-Blot, Bio-Rad, Hercules, CA). After transfer, the membranes were blocked in blotting solution (Tris-HCl 20 mM, NaCl 150 mM, 0.05% Tween 20, pH 7.6) with 5% blocking agent (Amersham, Little Chalfont, United Kingdom) overnight at 4°C, followed by incubation with primary antibody (1:1000, ab8021, abcam) in blotting solution with 0.5% blocking agent for 2 hours. After washing, the blots were incubated with horseradish peroxidase–labeled goat antirabbit IgG antibody (1:10 000; Amersham) for one hour. The bands were detected using the enhanced chemiluminescence assay (ECL-Plus kit; Amersham). After autoradiography (Kodak Biomax; Kodak, Rochester, NY), densitometric analysis was performed using National Institutes of Health Image computer software. Molecular weights were determined using prestained SDS-PAGE standard and precision protein standards (Bio-Rad). All reagents for electrophoresis were purchased from Sigma.
Statistics
Data are presented as means ± SEM and analyzed using one-way analysis of variance (ANOVA) with a Tukey post-hoc test. A P value less than .05 was considered statistically significant.
Results
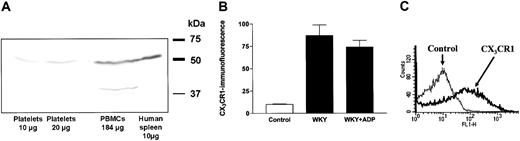
The presence of the fractalkine receptor CX3CR1 on resting platelets was demonstrated by both flow cytometry and immunoblotting. By Western blot analysis using a polyclonal rabbit CX3CR1 antibody, a 50-kDa protein was detected in washed human platelets and was comparable with PBMCs and human spleen lysate, which are well known to express CX3CR1 (Figure 1A). In whole blood, secondary labeling with an FITC-conjugated antirabbit secondary antibody showed a marked increase in fluorescence within the platelet population in flow cytometry analysis compared with blood samples incubated only with the secondary antibody (Figure 1B-C).
Expression of the fractalkine receptor (CX3CR1) on platelets demonstrated by Western blot (human platelets) or flow cytometry analysis (rat platelets). (A) Western blot demonstrating CX3CR1 expression on human washed platelets compared with peripheral blood mononuclear cells (PBMCs) and human spleen lysate as a positive control for CX3CR1. (B) The expression of CX3CR1 was similar in platelets from healthy Wistar-Kyoto rats (WKY) in the absence or presence of stimulation with adenosine diphosphate (ADP). The flow cytometry data are expressed as mean fluorescence ± SEM from 6 separate experiments. (C) A typical flow cytometry histogram with background (fine) and CX3CR1 expression (bold) on platelets from Wistar-Kyoto rats.
Expression of the fractalkine receptor (CX3CR1) on platelets demonstrated by Western blot (human platelets) or flow cytometry analysis (rat platelets). (A) Western blot demonstrating CX3CR1 expression on human washed platelets compared with peripheral blood mononuclear cells (PBMCs) and human spleen lysate as a positive control for CX3CR1. (B) The expression of CX3CR1 was similar in platelets from healthy Wistar-Kyoto rats (WKY) in the absence or presence of stimulation with adenosine diphosphate (ADP). The flow cytometry data are expressed as mean fluorescence ± SEM from 6 separate experiments. (C) A typical flow cytometry histogram with background (fine) and CX3CR1 expression (bold) on platelets from Wistar-Kyoto rats.
Platelet surface expression of P-selectin
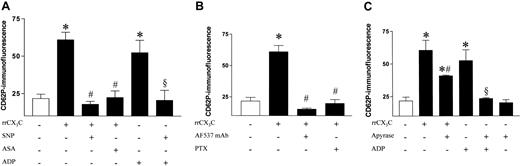
Surface expression of P-selectin on rat platelets was 2.5-fold enhanced by stimulation with recombinant rat fractalkine (rrCX3C) to a comparable extent as following stimulation with ADP (5 μM; Figure 2A). In vitro incubation with either the nitric oxide (NO) donor SNP or the cyclo-oxygenase inhibitor ASA completely prevented this increase (Figure 2A).
Fractalkine-mediated degranulation of rat platelets. (A) P-selectin surface expression in platelets from healthy Wistar-Kyoto rats under basal conditions and following stimulation with recombinant fractalkine (rrCX3C; 1 μg/mL) compared with control samples from the same animals and inhibition by preincubation with either sodium nitroprusside (SNP; 100 μM) or acetyl salicylic acid (ASA; 10 μg/mL) compared with the expression achieved by incubation with ADP (5 μM). (B) Neutralization of recombinant fractalkine by an antagonizing antifractalkine antibody (AF537; 100 μg/mL; 30 min) and inhibition of fractalkine receptor–mediated platelet activation by inhibition of G-protein coupling of the fractalkine-receptor by PTX (0.4 nM; 45 min). (C) P-selectin surface expression in platelets from healthy Wistar-Kyoto rats following stimulation with recombinant fractalkine (rrCX3C; 1 μg/mL) compared with control samples from the same animals in the presence and absence of apyrase (7.5 U/mL). Data are expressed as mean fluorescence ± SEM from 6 separate animals. *P < .01 versus basal; #P < .01 versus rrCX3C; §P < .01 versus ADP.
Fractalkine-mediated degranulation of rat platelets. (A) P-selectin surface expression in platelets from healthy Wistar-Kyoto rats under basal conditions and following stimulation with recombinant fractalkine (rrCX3C; 1 μg/mL) compared with control samples from the same animals and inhibition by preincubation with either sodium nitroprusside (SNP; 100 μM) or acetyl salicylic acid (ASA; 10 μg/mL) compared with the expression achieved by incubation with ADP (5 μM). (B) Neutralization of recombinant fractalkine by an antagonizing antifractalkine antibody (AF537; 100 μg/mL; 30 min) and inhibition of fractalkine receptor–mediated platelet activation by inhibition of G-protein coupling of the fractalkine-receptor by PTX (0.4 nM; 45 min). (C) P-selectin surface expression in platelets from healthy Wistar-Kyoto rats following stimulation with recombinant fractalkine (rrCX3C; 1 μg/mL) compared with control samples from the same animals in the presence and absence of apyrase (7.5 U/mL). Data are expressed as mean fluorescence ± SEM from 6 separate animals. *P < .01 versus basal; #P < .01 versus rrCX3C; §P < .01 versus ADP.
Antagonizing rrCX3C with an antifractalkine antibody directed against the extracellular domain of fractalkine (AF537), which has neither an effect on any other cytokines nor had any direct effect on platelets (data not shown) prior to platelet stimulation, prevented the increase in P-selectin expression, thus demonstrating specific action of rrCX3C. Furthermore, inhibition of G-protein–coupled, receptor-mediated signaling using PTX completely abolished fractalkine-elicited platelet activation (Figure 2B).
When platelets were preincubated with apyrase, surface expression of P-selectin by fractalkine was significantly reduced, suggesting that presence of ADP enhanced the platelet-activating stimulus of fractalkine. Apyrase completely inhibited the ADP-induced expression. The baseline expression in resting platelets remained unaffected by apyrase (Figure 2C).
Flow chamber and adhesion
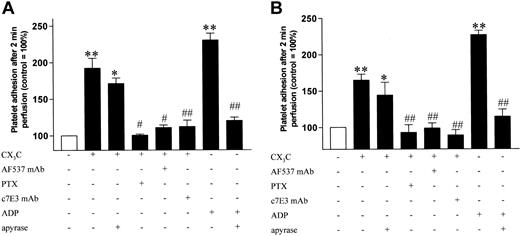
We investigated the effect of stimulation of washed human platelets with rhCX3Con firm platelet adhesion to collagen and fibrinogen at low and high shear rates. Following stimulation with rhCX3C, firm adhesion to collagen (Figure 3) and fibrinogen (Figure 4) was significantly increased under both high (A) and low (B) shear conditions. Pretreatment with either the antagonizing antifractalkine antibody (AF537) or with PTX, which inhibits the fractalkine-induced signaling, prevented the fractalkine-mediated increase in platelet adhesion. The adhesion caused by fractalkine was slightly weaker than the adhesion caused by ADP (5 μM) and was not significantly affected by preincubation of the platelet samples with apyrase, which, however, prevented the ADP-induced platelet adhesion. The differing effect of apyrase on adhesion in contrast to degranulation could be explained by physical shear during the experiments in the flow chamber, which could act as a costimulator itself. To demonstrate that fractalkine-induced adhesion to fibrinogen was specifically mediated by glycoprotein IIb/IIIa, platelet samples were preincubated with the monoclonal glycoprotein IIb/IIIa antagonist c7E3, which completely prevented fractalkine-induced platelet adhesion to fibrinogen (Figure 4). Representative images of platelet adhesion after stimulation with fractalkine and its inhibition by PTX or AF537 are shown in Figure 5.
Effect of fractalkine on platelet adhesion to collagen. Firm adhesion of washed human platelets to collagen was assessed in a flow chamber model under high (A) and low (B) shear following stimulation with rhCX3C (1 μg/mL) or ADP (5 μM) in the presence or absence of apyrase (7.5 U/mL). The proadhesive effect was blocked by preincubation with an antagonizing antibody against fractalkine (AF537; 100 μg/mL; 30 min) as well as by disruption of CX3CR1-mediated signaling with PTX (0.4 nM; 45 min). Data are expressed as the ratio of adhering platelets to the control group (□) ± SEM from at least 5 separate experiments. **P < .01 versus control; ##P < .01 versus stimulation.
Effect of fractalkine on platelet adhesion to collagen. Firm adhesion of washed human platelets to collagen was assessed in a flow chamber model under high (A) and low (B) shear following stimulation with rhCX3C (1 μg/mL) or ADP (5 μM) in the presence or absence of apyrase (7.5 U/mL). The proadhesive effect was blocked by preincubation with an antagonizing antibody against fractalkine (AF537; 100 μg/mL; 30 min) as well as by disruption of CX3CR1-mediated signaling with PTX (0.4 nM; 45 min). Data are expressed as the ratio of adhering platelets to the control group (□) ± SEM from at least 5 separate experiments. **P < .01 versus control; ##P < .01 versus stimulation.
Effect of fractalkine on platelet adhesion to fibrinogen. Firm adhesion of washed human platelets to fibrinogen was assessed in a flow chamber model under high (A) and low (B) shear following stimulation with rhCX3C (1 μg/mL) or ADP (5 μM) in the presence and absence of apyrase (7.5 U/mL). The proadhesive effect was blocked by preincubation with an antagonizing antibody against fractalkine (AF537; 100 g/mL; 30 min) as well as by disruption of CX3CR1-mediated signaling with PTX (0.4 nM; 45 min). Effect of inhibition of glycoprotein IIb/IIIa by preincubation with the monoclonal antibody c7E3 (4 μg/mL). Data are expressed as the ratio of adhering platelets to the control group (□) ± SEM from at least 5 separate experiments, *P < .05 versus control (CO); **P < .01 versus Co; #P < .05 versus stimulation; ##P < .01 versus stimulation.
Effect of fractalkine on platelet adhesion to fibrinogen. Firm adhesion of washed human platelets to fibrinogen was assessed in a flow chamber model under high (A) and low (B) shear following stimulation with rhCX3C (1 μg/mL) or ADP (5 μM) in the presence and absence of apyrase (7.5 U/mL). The proadhesive effect was blocked by preincubation with an antagonizing antibody against fractalkine (AF537; 100 g/mL; 30 min) as well as by disruption of CX3CR1-mediated signaling with PTX (0.4 nM; 45 min). Effect of inhibition of glycoprotein IIb/IIIa by preincubation with the monoclonal antibody c7E3 (4 μg/mL). Data are expressed as the ratio of adhering platelets to the control group (□) ± SEM from at least 5 separate experiments, *P < .05 versus control (CO); **P < .01 versus Co; #P < .05 versus stimulation; ##P < .01 versus stimulation.
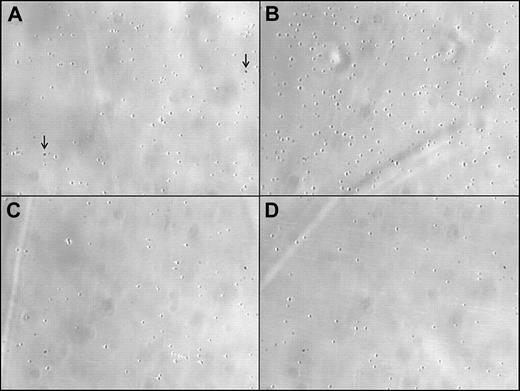
Representative images of firm adhesion of washed human platelets to a fibrinogen-coated membrane in a perfusion flow chamber under high shear. Platelets were either (A) unstimulated (control) or (B) stimulated with recombinant fractalkine (rhCX3C; 1 μg/mL). The proadhesive effect was blocked by either (C) antagonism of rhCX3C (AF537; 100 μg/mL; 30 min) or by (D) disruption of CX3CR1-mediated signaling with PTX (0.4 nM; 45 min). Single spots resemble artifacts due to the setting of the microscope. They are identically existent on every tracing shown (as indicated by the arrow). Original magnification, × 20.
Representative images of firm adhesion of washed human platelets to a fibrinogen-coated membrane in a perfusion flow chamber under high shear. Platelets were either (A) unstimulated (control) or (B) stimulated with recombinant fractalkine (rhCX3C; 1 μg/mL). The proadhesive effect was blocked by either (C) antagonism of rhCX3C (AF537; 100 μg/mL; 30 min) or by (D) disruption of CX3CR1-mediated signaling with PTX (0.4 nM; 45 min). Single spots resemble artifacts due to the setting of the microscope. They are identically existent on every tracing shown (as indicated by the arrow). Original magnification, × 20.
Discussion
In the present study, we demonstrate for the first time the presence of a functional fractalkine receptor (CX3CR1) on human and rat platelets and provide experimental evidence for a functional role of the chemokine fractalkine in platelet activation and adhesion.
Surface expression of P-selectin, which represents an established marker of platelet activation,27 on rat platelets was enhanced by fractalkine and appeared dependent on costimulation with ADP released from platelets or present in plasma as shown by the significant suppression of P-selectin expression in the presence of apyrase. The inhibition of platelet activation following stimulation with fractalkine by PTX suggests a specific interaction of fractalkine with a G-protein–coupled receptor. The only fractalkine receptor described so far, CX3CR1,7 is, like all other currently known chemokine receptors, G-protein coupled. The strongest support for a specific effect of fractalkine on platelet activation is provided by the prevention of P-selectin expression by preincubation with an antifractalkine antibody, which selectively antagonizes the extracellular domain of recombinant fractalkine. Therefore, these data demonstrate that fractalkine, which is overexpressed in vascular injury and atherosclerosis,3,4,12 directly induces platelet activation. A similar platelet activation is induced by the CXC chemokine stroma cell–derived factor-1 (SDF-1) through binding to CXCR4, a G 16 αi-coupled receptor, while the macrophage-derived chemokine (MDC) is believed to activate platelets via CCR4 coupled to the Gαq-protein.15 While some chemokines can exert their platelet activation only in combination with a weak platelet agonist,14,15,22 SDF-1 seems to act as a weak agonist independently from co- or prestimulation to cause platelet activation.16 However, especially the combination of SDF-1 and MDC, including the activation of Gαi by SDF-1 and Gαq by MDC, led to marked platelet activation.14 Gαi causes PI3 kinase–dependent potentiation of dense granule secretion, and Gα induces 2+ q Ca influx and shape change.28 We excluded an activation of Gαq by fractalkine, as neither Ca2+ influx nor shape change were observed in our study (data not shown). A concomitant activation of Gαq and Gαi has been postulated for platelet activation, whereby platelet aggregation could be initiated by 2 different weak agonists separately activating the different G proteins.29 Gαq-deficient mice are unresponsive to a variety of physiologic platelet activators, indicating the importance of this signaling pathway.30 In whole blood, fractalkine exerted platelet activation similar to enhanced surface expression of CD62P and CD40L by ADP under similar conditions.31 As fractalkine predominantly acts via Gαi and pretreatment with ASA completely prevented fractalkine-induced degranulation in our study, an additional effect of thromboxane-mediated costimulation seems to be likely. Furthermore, recent observations demonstrated a calcium-independent activation of PKC by sCD40L inducing the release of alpha and dense granules without causing calcium flux or β3 integrin activation.32 All these findings together suggest that weak agonists such as chemokines or ADP can indeed cause platelet secretion in the absence of aggregation when acting in concert with at least another weak agonist leading to the activation of distinct signaling cascades.
In parallel to the observed platelet activation, platelet adhesion to immobilized collagen and fibrinogen was enhanced following stimulation of platelets with fractalkine. The prevention of increased adhesion by antagonizing the action of recombinant fractalkine using a specific antibody or by disruption of the fractalkine receptor–mediated signaling is consistent with the mechanisms involved in fractalkine-mediated leukocyte adhesion.10 Under the shear conditions described in our experiments, the adhesion following stimulation with fractalkine was similar to the effect of ADP. Platelet adhesion to different surfaces such as collagen and fibrinogen is markedly reduced after inhibition of platelet ADP receptors.33
Increased platelet activation has been reported in several diseases such as hypertension, congestive heart failure, or hypercholesterolemia. All these pathophysiologic states are accompanied by endothelial dysfunction,34-37 and reduced bioavailability of endogenous NO is likely to play a crucial role in modulating platelet activation.36 While fractalkine is not expressed by endothelial cells under physiologic conditions in vivo,38 its expression is enhanced in vascular injury or atherosclerosis. Therefore, fractalkine may substantially contribute to platelet activation in these vascular disorders.4,11 Indeed, in a rat model of cerebrovascular ischemiareperfusion, fractalkine expression was enhanced after transient cerebral ischemia,39 and in a similar model, infarct size was significantly reduced in fractalkine-deficient mice.40 In mice deficient for CX3CR1, progression of atherosclerotic lesion formation is significantly reduced.41,42 The pronounced expression of fractalkine by macrophages within atherosclerotic plaques11,43 further strengthens the pathophysiologic importance of our study. While reduced levels of NO contribute to enhanced susceptibility of platelets to activation,36 fractalkine expression in atherosclerotic lesions could be an additional local proaggregatory and proadhesive stimulus in vivo.
As shown in this study, the membrane-bound chemokine fractalkine induces pronounced platelet activation and enhances platelet adhesion to collagen and fibrinogen. The adhesion to fibrinogen is mediated via glycoprotein IIb/IIIa and initiates proteolytic activity in human endothelial cells.44 In cholesterol-fed ApoE–/– mice representing a well-established model for the development of atherosclerosis in vivo, platelet adhesion to the endothelium increases over time and precedes leukocyte adhesion and development of atherosclerosis. Furthermore, inhibition of platelet activation attenuates the progression of atherosclerosis.26 Platelets do not only respond to chemokines,13,14,16,18 but also trigger the expression of chemokines by endothelial cells45,46 and actively release chemokines themselves.47 Therefore, fractalkine-mediated platelet activation and adhesion might be an important mechanism for hemostatic changes and thrombembolic complications in diseases associated with endothelial/vascular injury by locally activating platelets as well as focusing their adhesion and the release of mediators promoting further vascular damage.
In conclusion, we describe the presence of a functional fractalkine receptor on platelets responsible for a chemokine-mediated activation of platelets leading to increased adhesion. While soluble chemokines may activate platelets rather unspecifically and systemically, membrane-anchored fractalkine may represent a more specific mechanism by focusing activated platelets to the original site of endothelial damage.
Prepublished online as Blood First Edition Paper, September 11, 2003; DOI 10.1182/blood-2002-10-3260.
Supported in part by the Deutsche Forschungsgemeinschaft (SFB 355 B10/C3/C8).
A.S. and C.S. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal