Abstract
In this study we investigated telomere restriction fragment (TRF) length in a panel of mature B-cell lymphoproliferative disorders (MBCLDs) and correlated this parameter with histology and histopathogenesis in relation to the germinal center (GC). We assessed 123 MBCLD samples containing 80% or more tumor cells. TRF length was evaluated by Southern blot analysis using a chemiluminescence-based assay. GC status was assessed through screening for stable and ongoing somatic mutations within the immunoglobulin heavy-chain genes. Median TRF length was 6170 bp (range, 1896-11 200 bp) and did not correlate with patient age or sex. TRF length was greater in diffuse large cell lymphoma, Burkitt lymphoma, and follicular lymphoma (medians: 7789 bp, 9471 bp, and 7383 bp, respectively) than in mantle cell lymphoma and chronic lymphocytic leukemia (medians: 3582 bp and 4346 bp, respectively). GC-derived MBCLDs had the longest telomeres, whereas those arising from GC-inexperienced cells had the shortest (P < 10-9). We conclude that (1) TRF length in MBCLD is highly heterogeneous; (2) GC-derived tumors have long telomeres, suggesting that minimal telomere erosion occurs during GC-derived lymphomagenesis; and (3) the short TRF lengths of GC-inexperienced MBCLDs indicates that these neoplasms are good candidates for treatment with telomerase inhibitors, a class of molecules currently the subject of extensive preclinical evaluation. (Blood. 2004;103:4644-4649)
Introduction
Telomeres are repeated DNA sequences at the ends of chromosomes in eukaryotic cells, and they play a critical role in maintaining chromosomal stability.1-6 In most somatic cells, they shorten at each mitotic division as a result of the inability of DNA polymerase to synthesize open extremities of DNA.2-7 As the number of divisions increases, telomere restriction fragment (TRF) length falls beyond a critical value, leading to senescence, a peculiar phase of cell life mostly characterized by growth inhibition.8-10 Senescence is distinguished by low proliferative activity and is regarded as an important checkpoint in somatic cell life. If the senescence checkpoint is bypassed (eg, through p53 + Rb1 inactivation), cells continue to divide until they reach a second proliferative barrier named crisis. Crisis is associated with genetic instability, chromosomal fusions, and a high tendency toward apoptosis and malignant transformation.11-13
The telomeres of germinal cells are preserved from shortening by an enzymatic RNA-containing complex called telomerase, whose catalytic subunit (h-TERT) has intrinsic reverse transcriptase capability and adds repeated DNA sequences to chromosomal ends.1,14-18 Telomerase is usually not expressed in somatic cells, with few exceptions characterized by high replicative potential.15-18 In addition, telomerase activity (TA) is often turned off during differentiation, even in cell subtypes in which there is significant h-TERT activity during early development.16,17 This is probably related to the limited proliferative potential of terminally differentiated cells. B cells are a notable exception to this rule. Naive B cells have no detectable TA, whereas strong telomerase activation is observed in germinal center (GC)-derived B cells. This is probably related to the high mitotic activity of GC cells.19 In post-GC maturation, increased TA is still observed in some subtypes, but its level is lower than during GC maturation.19 This peculiar aspect of B-cell development is reflected by TRF length in normal B cells. No GC erosion occurs in GC B cells despite proliferative activity that is among the highest ever observed in human cells.19
During tumorigenesis, telomeres usually undergo progressive shortening until TA is restored to allow indefinite cell replication.20,21 This late activation is now regarded as a critical step in tumor development, and telomerase is considered to play a crucial role in sustaining neoplastic cell survival.1,21-23 This explains why most tumors are characterized by short telomeres despite the presence of high TA levels. This peculiar feature of cancer cells has suggested that telomerase inhibition could be a good approach to slow down cancer replication and ultimately to induce selective cancer cell death.1,24-26
In contrast to findings in solid tumors, few data have been accumulated on TRF lengths in mature B-cell lymphoproliferative disorders (MBCLDs). Studies have been primarily restricted to multiple myeloma27 and chronic lymphocytic leukemia,28,29 but TRF lengths in MBCLDs as a whole have not been quantified. The aim of this study was to determine TRF lengths in a large panel of mature B-cell tumors and to assess the link between TRF length and tumor histopathogenesis in relation to the GC. Patients were evaluated for TRF length and GC status (ie, pre-GC, GC-derived, and post-GC) according to the presence of stable or ongoing somatic mutations within the variable regions of immunoglobulin heavy-chain genes (VH).
Patients, materials, and methods
Patient and sample characteristics
One hundred twenty-three tumor samples from 117 patients with MBCLD of 7 subtypes, displaying 80% or more neoplastic infiltration by flow cytometry, were used. Twenty-two were from peripheral blood (PB), 44 from bone marrow (BM), and 57 from lymph nodes (LNs). Samples obtained from different tissues during the same disease phase were available for 6 patients (BM vs PB in 2 patients, BM vs LN in 3 patients, and PB vs LN in 1 patient). Diagnoses was made in accordance with World Health Organization (WHO) classification.30 Our panel consisted of 9 patients with mantle cell lymphoma (MCL), 31 with chronic lymphocytic leukemia (CLL), 31 with follicular lymphoma (FL), 5 with Burkitt lymphoma (BL), 14 with diffuse large-cell lymphoma (DLCL), 9 with marginal zone lymphoma (MZL), and 18 with multiple myeloma (MM) (with PB features of plasma cell leukemia [PCL] in 6). All diagnoses were established by the same hemopathologist, who was unaware of the TRF length results when defining the subtypes. Diagnosis was always supported by histology and flow cytometry. Immunohistochemistry, cytogenetics, and molecular biology analyses were also performed, if required, according to the WHO criteria.30 Samples were taken at diagnosis in 80 patients and after treatment in 37 patients. Samples taken after treatment were obtained at true relapse (occurring after a previous complete or very good partial remission) in 20 patients and during disease progression or stable disease in 7. Ten CLL patients were partial responders still displaying more than 80% tumor contamination in BM or PB (partial response was defined according to Cheson et al31 as a 50% reduction in PB lymphocytes, with total resolution of BM failure if present). Patient demographic and clinical characteristics are shown in Table 1.
Characteristics of samples and patients
. | . | . | . | . | . | . | . | Age . | . | . | . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | No. samples* . | Sample type, no. . | . | . | No. patients* . | Sex, no. . | . | Younger than 30 y . | . | . | Older than 70 y . | Clinical status . | . | ||||
| Histology . | . | LN . | BM . | PB . | . | M . | F . | . | 30-49 y . | 50-69 y . | . | Diagnosis . | After treatment . | ||||
| CLL | 34 | 7 | 10 | 17 | 31 | 20 | 11 | 0 | 5 | 21 | 5 | 13 | 18 | ||||
| MCL | 10 | 9 | 1 | 0 | 9 | 7 | 2 | 0 | 0 | 5 | 4 | 9 | 0 | ||||
| FL | 33 | 19 | 13 | 1 | 31 | 20 | 11 | 1 | 8 | 18 | 4 | 12 | 19 | ||||
| BL | 5 | 2 | 3 | 0 | 5 | 4 | 1 | 1 | 2 | 2 | 0 | 5 | 0 | ||||
| DLCL | 14 | 13 | 1 | 0 | 14 | 7 | 7 | 1 | 2 | 7 | 4 | 14 | 0 | ||||
| MZL | 9 | 7 | 0 | 2 | 9 | 5 | 4 | 0 | 2 | 3 | 4 | 9 | 0 | ||||
| MM | 18 | 0 | 16 | 2 | 18 | 11 | 7 | 0 | 3 | 10 | 5 | 18 | 0 | ||||
| Total | 123 | 57 | 44 | 22 | 117 | 74 | 43 | 3 | 22 | 66 | 26 | 80 | 37 | ||||
. | . | . | . | . | . | . | . | Age . | . | . | . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | No. samples* . | Sample type, no. . | . | . | No. patients* . | Sex, no. . | . | Younger than 30 y . | . | . | Older than 70 y . | Clinical status . | . | ||||
| Histology . | . | LN . | BM . | PB . | . | M . | F . | . | 30-49 y . | 50-69 y . | . | Diagnosis . | After treatment . | ||||
| CLL | 34 | 7 | 10 | 17 | 31 | 20 | 11 | 0 | 5 | 21 | 5 | 13 | 18 | ||||
| MCL | 10 | 9 | 1 | 0 | 9 | 7 | 2 | 0 | 0 | 5 | 4 | 9 | 0 | ||||
| FL | 33 | 19 | 13 | 1 | 31 | 20 | 11 | 1 | 8 | 18 | 4 | 12 | 19 | ||||
| BL | 5 | 2 | 3 | 0 | 5 | 4 | 1 | 1 | 2 | 2 | 0 | 5 | 0 | ||||
| DLCL | 14 | 13 | 1 | 0 | 14 | 7 | 7 | 1 | 2 | 7 | 4 | 14 | 0 | ||||
| MZL | 9 | 7 | 0 | 2 | 9 | 5 | 4 | 0 | 2 | 3 | 4 | 9 | 0 | ||||
| MM | 18 | 0 | 16 | 2 | 18 | 11 | 7 | 0 | 3 | 10 | 5 | 18 | 0 | ||||
| Total | 123 | 57 | 44 | 22 | 117 | 74 | 43 | 3 | 22 | 66 | 26 | 80 | 37 | ||||
For 2 patients with FL, 3 with CLL, and 1 with MCL, 2 different tissues obtained during the same treatment phase were available.
Cell line and DNA extraction
The MM cell line KMS-11 (kindly provided by Massimo Massaia, University of Turin, Italy), which has a TRF length of 3420 bp, was used for dilution tests against PB mononuclear cells from a healthy donor. BM and PB mononuclear cells were separated by Ficoll-Hypaque density gradient centrifugation. Lymph node tissue was always obtained from fresh or frozen samples. Genomic DNA was extracted using the DNAzol reagent (Gibco BRL Life Technologies, Grand Island, NY) according to the manufacturer's recommendations.
Determination of TRF length
TRF length was determined using Southern blot analysis according to the following procedure. Aliquots of 2 μg DNA were digested by mixing HinfI (20 MU) and RsaI (20 MU) (Roche Diagnostics, Mannheim, Germany) for 2 hours at 37°C, according to the manufacturer's recommendations. TRF were then separated by 0.8% agarose gel electrophoresis in 1 × TAE buffer (pH 8.0). Gels were transferred to a positively charged nylon membrane (catalog no. 1 417 240; Roche Diagnostics) and were ultraviolet (UV) light cross-linked for 10 minutes. Hybridization was performed with the TeloTAGG Telomere Length Assay Kit (Roche Diagnostics), which contains all the reagents required. Membranes were prehybridized for 2 hours in the prehybridization solution at 62°C and then hybridized for 3 hours in the same conditions by adding 2 μL of the digoxigenin (DIG)-labeled telomere-specific probe. After hybridization, filters were washed twice at room temperature for 15 minutes in 2 × washing solution and then twice at 39C° in 0.5 × washing solution for 20 minutes. Filters were then incubated with a DIG-specific antibody covalently coupled to alkaline phosphatase (AP). Finally, results were visualized using AP metabolizing CDP-Star, a highly sensitive chemiluminescent substrate. The light signal produced on the site of the hybridized probe was recorded on x-ray film (Lumi-Film Chemiluminescent Detection Film; Roche Diagnostics). Each assay was performed twice. Membranes were then scanned and analyzed with Kodak Digital Science 1D Image Analysis Software (Scientific Imaging Systems, New Haven, CT). Two different parameters were calculated for each telomere smear. The first was mean TRF length, calculated as already described.7,32,33 The second was peak TRF length, the point of maximum signal intensity defining the highest concentration of telomere repeats. These 2 parameters were assessed to minimize any bias associated with contaminating nonneoplastic cells. Percentages were always 20% or less and were thus not expected to influence the peak values, whereas they could have influenced the extremities of the smear, thus potentially biasing calculation of mean TRF length.
VH sequencing analysis
Tumor-specific VH rearrangements were amplified starting from genomic DNA or, for MM and PCL only, from immunoglobulin-specific cDNA as previously described.34-36 Primers and PCR conditions for VH amplification have been previously described.34,37 Briefly, 1 μg genomic DNA or 2 μL cDNA were amplified using 3 sets of consensus forward primers, 2 derived from the framework region 1 (FR1) and 1 from the leader region, together with a J consensus region antisense primer.34,37 The reaction was carried out for 30 to 33 cycles with denaturation at 94°C for 1 minute, annealing at 62°C for 30 seconds, and extension at 72°C for 30 seconds, with a final extension of 10 minutes at 72°C. PCR products were then analyzed by electrophoresis on 2% agarose gels. When an apparently clonal PCR product was observed, the sample of interest was reamplified on a large scale for direct sequencing of the tumor immunoglobulin H (IgH) rearrangement. Purification of polymerase chain reaction (PCR) products, direct sequencing reactions, and sequence analysis were performed as previously described.36,37
VH analysis for somatic mutations
Mutation analysis of the VH region was then performed as follows: nucleotide sequences were aligned to the V-Base sequence directory (MRC Centre for Protein Engineering, Cambridge, United Kingdom) (http://www.mrc-cpe.cam.ac.uk/vbase-ok.php?menu=901). Percentage of homology was calculated by counting the number of mutations between the 5′ end of FR1 and the 3′ end of FR3, as previously described.38 Sequences with 2% or less deviation from any germline VH sequence were considered unmutated and, thus, as belonging to GC-inexperienced cells. Sequences containing more than 2% deviation from any known germline VH were considered mutated and were defined as GC experienced. Patients failing VH sequencing were excluded from further analysis.38
Evaluation of the presence of ongoing SHM
The presence of the ongoing somatic hypermutation (SHM) mechanism was evaluated in all samples, defined as GC-experienced MBCLD to distinguish GC-derived from post-GC-derived MBCLD. Clonal VH rearrangements were amplified with appropriate primers and cloned into the pCR4-TOPO plasmid vector (Invitrogen, Paisley, United Kingdom). Twenty or more randomly chosen bacterial clones were expanded into LB medium overnight. Three milliliters culture medium was purified with Wizard Plus Minipreps DNA Purification System (Promega). Plasmids were then sequenced by IRBM (Servizio Sequenziamento Automatico, Pomezia, Roma, Italy). Only clones with identical or nearly identical CDR3 were considered. Substitution mutations observed in only 1 clone and in more than 1 clone were classed as unconfirmed and confirmed mutations, respectively.39 Only confirmed mutations were considered evidence of ongoing SHM; unconfirmed mutations were disregarded.40-42
Results
Validation of the modified strategy for the calculation of peak TRF length
Because the patient samples were not purified, we had concerns about the potential confounding role of contaminating nonneoplastic cells on calculations of peak TRF length. To validate our strategy, dilution tests with the KMS-11 cell line were performed. Cancer cells were diluted on a background of PB mononuclear cells from a healthy donor (50-year-old man). The dilution test was performed by calculating peak TRF length on samples containing tumor cells in 5% increments from 50% to 100%. The same experiment was performed 3 times. For the purposes of this study, TRF measurements were considered acceptable if those of the contaminated cell samples were 5% or less different from those in the sample containing 100% tumor cells. Peak TRF of samples containing 5%, 10%, 15%, 20%, 25%, 30%, 35%, and 40% contaminating cells showed median increases in peak TRF length, respectively, of 1.5%, 2.0%, 2.3%, 3.0%, 3.8%, 4.6%, 8%, and 16%. Samples containing 45% and 50% contaminating cells showed a bimodal aspect with 2 peaks of similar size, as would be expected from cell populations containing similar amounts of cancer cells and healthy cells. Based on these results, peak TRF values were close enough to the expected rate up to contamination of 30% nonneoplastic cells. In contrast, mean TRF length was acceptable only up to a contamination of 20% (data not shown). Thus, both approaches were appropriate for TRF length calculation in our panel, though peak TRF values were more accurate.
TRF lengths in the whole MBCLD population
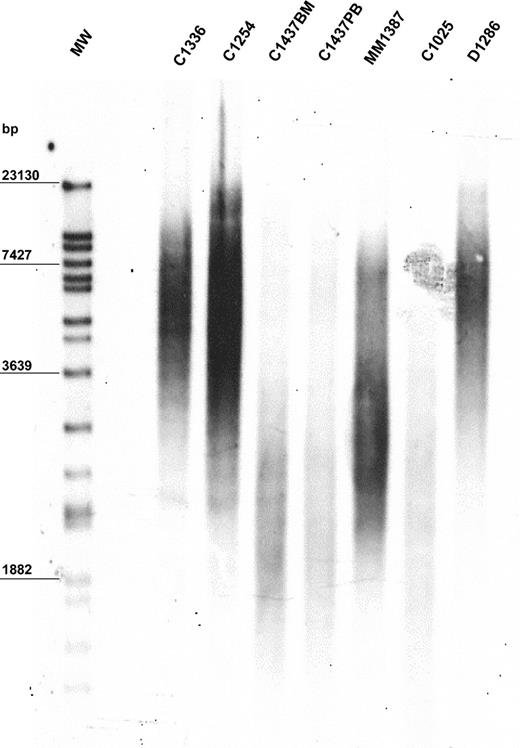
The mean value of peak TRF length in our population was 6170 bp. The population was heterogeneous, with a standard deviation of ± 1985 bp. Median TRF length was 6060 bp (range, 1896-11 200 bp). The 10th, 25th, 75th, and 90th percentiles were 3334, 5100, 7755, and 8725 bp, respectively. A representative example of TRF length analysis is shown in Figure 1. Demographic features were not predictive of TRF length. In particular, TRF length of MBCLD samples was not associated with patient age when the entire population was considered (r2 = 0.0783) (data not shown). In addition, when analysis was performed separately on each disease subtype, we did not observe higher TRF lengths in young patients than in older ones (data not shown). Similarly, sex had no influence on TRF length (data not shown). No significant differences in TRF lengths were apparent between samples taken at diagnosis and those taken after treatment of the 2 disorders (FL and CLL) in which samples from treated patients were available (CLL at diagnosis: median peak TRF length, 5102 bp; range, 2162-8211 bp; CLL after treatment median peak TRF length, 4120 bp; range, 2253-7900 bp; P = .94) (FL at diagnosis: median peak TRF length, 7367 bp; range, 5355-8725 bp; FL after treatment median peak TRF length, 7475 bp; range, 4361-10 030 bp; P = .95). In 6 patients TRF length was assessed on different tissues, but always with identical results (Figure 1; patient C1437).
Representative example of TRF length in 6 MBCLD patients. Peak TRF lengths are expressed as base pairs (bp). MW indicates molecular-weight ladder; BM, bone marrow; PB, peripheral blood. C1336 and C1254 are CLL patients with mutated VH; C1437 and C1025 are CLL patients with unmutated VH (C1437 includes C1437BM and C1437PB); MM1387 is a MM patient; D1286 is a DLCL patient.
Representative example of TRF length in 6 MBCLD patients. Peak TRF lengths are expressed as base pairs (bp). MW indicates molecular-weight ladder; BM, bone marrow; PB, peripheral blood. C1336 and C1254 are CLL patients with mutated VH; C1437 and C1025 are CLL patients with unmutated VH (C1437 includes C1437BM and C1437PB); MM1387 is a MM patient; D1286 is a DLCL patient.
TRF length according to histology
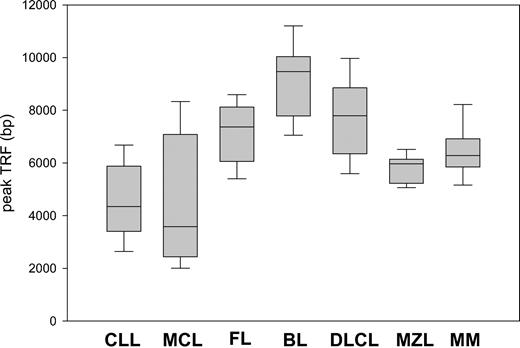
Remarkable differences in peak TRF length were observed between the histologic subtypes (Figure 2). Patients with FL, BL, and DLCL had the highest values, and their median and range values were 7383 bp (range, 4361-10 030 bp), 9471 bp (range, 7052-11 200 bp), and 7789 bp (range, 5244-10 304 bp), respectively. In contrast, MCL and CLL patients had short telomeres, often close to the critical threshold of senescence. Their median and range values were 3582 bp (range, 1896-8961 bp) and 4346 bp (range, 2094-8211 bp), respectively. MZL and MM displayed intermediate TRF lengths: 5969 bp (range, 5058-6681 bp) and 6283 bp (range, 4917-8900 bp). The telomeres of aggressive lymphomas were not shorter than those of indolent subtypes. When indolent lymphomas, namely those with overall survival longer than 3 years when treated conventionally (CLL, FL, MZL), were compared with aggressive subtypes (median survival less than 3 years with conventional treatment) (BL, DLCL, MCL), a modest trend toward greater TRF length was noted for the aggressive subtypes (P = .03) (data not shown).
Peak TRF length according to MBCLD histotype. There are marked differences between the subtypes. Box plots indicate 25th percentile, median value, and 75th percentile (horizontal lines of rectangle from the bottom, respectively) and 10th and 90th percentiles (horizontal lines outside rectangle).
Peak TRF length according to MBCLD histotype. There are marked differences between the subtypes. Box plots indicate 25th percentile, median value, and 75th percentile (horizontal lines of rectangle from the bottom, respectively) and 10th and 90th percentiles (horizontal lines outside rectangle).
TRF length according to GC experience
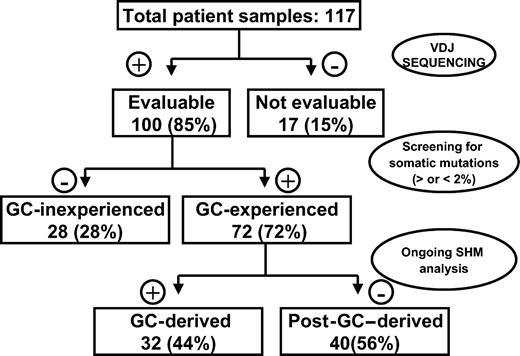
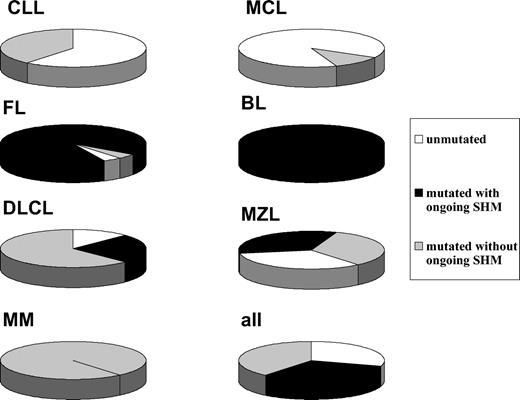
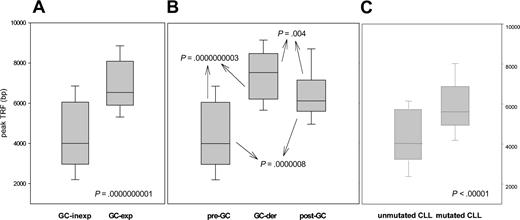
Given that several histologic subtypes (including MCL, CLL, DLCL, and MZL) are highly heterogeneous in terms of GC experience of cell of origin, we looked for associations between GC status and significant differences in TRF length. The presence of stable and ongoing somatic mutations in the VH genes of all patients was determined, as shown in Figure 3. VH sequencing was successful in 100 of the 117 (85%) patients. Patients for whom VH sequencing failed were excluded from further evaluation. Patients showing homology greater than 98% to any known VH sequence were classified as GC inexperienced. Seventy-two (72%) patients defined as GC experienced underwent further cloning to assess the presence of ongoing SHM and were subsequently classified as GC derived (ongoing SHM-positive) or post-GC derived (ongoing SHM-negative). Figure 4 indicates how subtypes were classified according to mutational status. TRF length according to mutational status is represented in Figure 5. GC-inexperienced tumors were compared with GC-experienced tumors (Figure 5A). GC-inexperienced tumors had shorter telomeres than GC-experienced tumors (P = 10-10). GC-experienced tumors were further divided into GC-derived and post-GC-derived tumors (Figure 5B). GC-derived tumors had greater peak TRF lengths than GC-inexperienced (P < 10-9) and post-GC-derived tumors (P = .004). In addition, post-GC-derived tumors appeared to have longer TRFs than pre-GC MBCLD (P < 10-6). As expected, TRF length was also associated with mutational status in CLL patients. Telomeres were significantly longer in the VH-mutated subtype (P < 10-4) (Figure 5C). Of note, the association between TRF length and GC status existed when only patients at diagnosis were considered (GC experienced vs GC inexperienced [P < 10-7]; pre-GC vs GC-derived [P < 10-5]; pre-GC vs post-GC [P < 10-3]; GC-derived vs post-GC-derived [P < .05; data not shown]). The relationship between disease aggressiveness and TRF length was assessed within both subgroups, and no difference was noticed (indolent pre-GC-derived vs aggressive pre-GC-derived; P = .71; indolent GC-derived vs aggressive GC-derived; P = .11; indolent post-GC-derived vs aggressive post-GC-derived; P = .13). Results were similar when mean rather than peak TRF lengths were considered (data not shown).
Experimental strategy for defining histopathogenesis of samples according to GC origin.
Experimental strategy for defining histopathogenesis of samples according to GC origin.
Classification according to GC experience of MBCLD subtypes. White, black, and gray sections represent the percentages of GC-inexperienced tumors, GC-derived tumors, and post-GC-derived tumors, respectively.
Classification according to GC experience of MBCLD subtypes. White, black, and gray sections represent the percentages of GC-inexperienced tumors, GC-derived tumors, and post-GC-derived tumors, respectively.
Peak TRF length according to GC experience. (A) Peak TRF length in GC-experienced and GC-inexperienced tumors, classified according to the presence or absence of somatic hypermutation. (B) GC-experienced tumors are divided into GC derived and post-GC-derived according to the presence or absence of ongoing somatic hypermutation. (C) How TRF length correlates with GC experience in CLL patients. Box plots indicate 25th percentile, median value, and 75th percentile (horizontal lines of rectangle from the bottom, respectively) and 10th and 90th percentiles (horizontal lines outside rectangle).
Peak TRF length according to GC experience. (A) Peak TRF length in GC-experienced and GC-inexperienced tumors, classified according to the presence or absence of somatic hypermutation. (B) GC-experienced tumors are divided into GC derived and post-GC-derived according to the presence or absence of ongoing somatic hypermutation. (C) How TRF length correlates with GC experience in CLL patients. Box plots indicate 25th percentile, median value, and 75th percentile (horizontal lines of rectangle from the bottom, respectively) and 10th and 90th percentiles (horizontal lines outside rectangle).
Discussion
This study is the first comprehensive evaluation of TRF length in all the most frequent histologic MBCLD subtypes. TRF length was found to be highly heterogeneous among different subtypes of MBCLD. FL, DLCL, and BL have long telomeres (median peak TRF length, 7580 bp), whereas MCL and CLL have short telomeres (median peak TRF length, 4116 bp) that are often close to the critical threshold of senescence. Our main finding is that a high peak TRF length is typical of lymphomas arising from the GC as opposed to other MBCLD, particularly those arising from GC-inexperienced cells. This suggests that GC-derived lymphomagenesis occurs in the absence of the marked telomeric loss often associated with neoplastic transformation.
Telomere status has been extensively investigated in solid tumors, but few reports have addressed the same issue in MBCLD, and none have comprehensively investigated it in a large panel of MBCLD, including the most important entities in the WHO lymphoma classification.20-22,30 Some studies have looked at CLL and MM.27-29 Bechter et al28 investigated TRF length in 58 CLL patients and found that long telomeres are associated with favorable clinical features and better survival.28 In MM, Wu et al27 observed that samples displaying reduced TRF lengths have worse outcomes. It has been concluded that short telomeres are mainly a consequence of increased proliferative activity, usually observed in patients with more aggressive disease. However, the association between short telomeres and worse outcomes in CLL is largely explained by the fact that patients with poor-prognosis, VH-unmutated, GC-inexperienced CLL cells have shorter telomeres than patients with VH-mutated CLL, as clearly shown in the recent papers by Damle et al29 and Hultdin et al.43 Our results conclusively demonstrate that the most important feature influencing telomere length in MBCLD is tumor histopathogenesis, with particular respect to GC experience. They also point to a minor role for high-proliferative activity as a basic determinant of TRF length in MBCLD. The peak TRF length of indolent histotypes, in fact, was no greater than that of aggressive histotypes, and the telomeres of highly proliferating MBCLDs, namely BL and DLCL, were longer than those of slowly proliferating disorders, such as CLL. Of note, no difference in TRF length could be associated with disease aggressiveness when MBCLDs characterized by similar histopathogenesis were compared. However, a different proliferative activity may explain TRF differences within a well-defined histopathologic entity, as suggested by Wu et al27 for MM.
The mean TRF length of GC-derived normal B cells is slightly (fewer than 1000 bp in 4 of 5 patients), though consistently, greater than that of naive B cells.19 However, this does not explain the large difference (4000 bp) between their neoplastic counterparts.19,44 Comparing our results with those of Weng et al19 in normal B cells shows that GC lymphomagenesis is associated with an approximate telomeric loss of fewer than 2000 bp. In contrast, lymphomagenesis in GC-inexperienced B cells is associated with a loss of more than 4000 bp and, hence, a TRF length close to the critical senescence threshold. The remarkable telomere preservation in GC-derived tumors should probably be attributed to the presence of high TA in the parent GC B cell, clearly demonstrated by Weng et al.19 However, the role of additional factors might be also important, in particular telomere-binding factors such as TRF1, TRF2, and Pot1.1 Because an effective telomere-preserving machinery is a basic step for neoplastic transformation, it can be speculated that during GC lymphomagenesis, one of the critical checkpoints that plays a protective role against neoplastic transformation is turned off “by default” because of the peculiar biologic features of GC-derived B cells.1,45 This feature, together with the heavy proliferative activity and the genetic instability associated with VH switching and ongoing SHM, may explain why GC-associated MBCLDs form the vast majority of human B-cell neoplasms.
TRF length in post-GC-derived tumors was intermediate between the 2 previously mentioned subgroups. Most samples show similar results of approximately 6000 bp. Post-GC-derived normal B cells have TRF lengths of approximately 8500 bp.19 Our findings indicate that telomeric loss in post-GC lymphomagenesis is approximately 2500 bp. This value is definitely lower than in GC-inexperienced cells. This difference can be explained by the presence of residual TA in parent post-GC cells and by more rapid restoration of TA during lymphomagenesis. The observation that memory B cells have low or no TA suggests that the second hypothesis is more reasonable, at least for CLL. Nevertheless, the remarkable TRF length homogeneity of post-GC MBCLD remains an intriguing finding. This subgroup is the most heterogeneous of the 3 because it includes patients with 6 different subtypes (all but BL) who have little in common biologically and clinically. However, despite such important differences, these tumors undergo a remarkably similar degree of telomere erosion during lymphomagenesis.
We studied 80 samples taken at diagnosis and 37 taken after treatment. We noticed no significant difference in terms of TRF length between the 2 subgroups, in seeming contradiction to a previous report by Remes et al,46 who observed significant telomeric differences when samples obtained at diagnosis were compared with those obtained at relapse. This apparent contradiction can probably be explained by 2 factors. First, in our panel, only a fraction of patients had true relapses, whereas several samples were obtained from partial responders and patients with progressive or stable disease. Second, our panel of treated patients had only indolent neoplasms, whereas the population described by Remes et al46 consisted mostly of patients with aggressive tumors. It is reasonable to speculate that significant variations in TRF length may occur after chemotherapy only in the presence of marked cytoreduction. This might allow the selection of specific tumor populations with TRF lengths different from those observed at diagnosis. Compared with those described by Remes et al,46 our patients underwent minor degrees of cytoreduction because of less chemosensitive disease and milder chemotherapy. This might explain why they displayed TRF lengths similar to those observed at diagnosis in patients with analogous tumors.
In addition to their biologic relevance to MBCLD pathogenesis, our findings are of clinical interest because telomerase is now regarded as one of the most promising targets for anticancer treatment. Antisense oligonucleotides and small molecules such as BIBR-153224-26,47 have been evaluated in vitro as anticancer agents for solid tumors, and they may soon become significant in the treatment of hematologic cancers. Telomerase inhibitors act through several mechanisms, including induction of pharmacologic senescence and triggering of apoptosis.26,47,48 Although the mechanism of telomerase inhibitors is probably not only telomere oriented, theoretical considerations and in vitro experiments indicate that their therapeutic effects will be particularly pronounced in the presence of short telomeres. Our results indicate that these agents may be particularly suitable for the treatment of MCL and VH-unmutated CLL. In contrast, the long telomeres of FCL and DLCL cells might potentially protect cancer cells from pharmacologically induced senescence for a long time. At our laboratory we are performing in vitro cell line studies to verify this hypothesis.
Prepublished online as Blood First Edition Paper, February 26, 2004; DOI 10.1182/blood-2003-12-4412.
Supported in part by Associazione Italiana Ricerca sul Cancro (Milan), Compagnia di San Paolo, (Turin), Consiglio Nazionale delle Ricerche, and Ministero dell' Università e della Ricerca. B.M., M.C. Compagno, and D.D. are or were recipients of fellowships from Fondazione Angela Bossolasco (Turin). I.R. is the recipient of a fellowship from Fondazione Italiana Ricerca sul Cancro (Milan). M.D. is the recipient of a fellowship from Comitato Piemontese Luigi Ghirotti.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal