Abstract
Experimental autoimmune encephalomyelitis (EAE), an animal model for human multiple sclerosis (MS), is a typical CD4+ T-cell-mediated autoimmune disease of the central nervous system (CNS) characterized by perivascular inflammation culminating in focal demyelinations. Like MS, EAE induced by proteolipid protein (PLP) usually follows the form of a relapsing-remitting disease. We have previously described an immunotherapy model in which infusion of autologous B cells expressing the PLP encephalitogenic determinant induced PLP-specific unresponsiveness and protected mice from induction of EAE. Here we show that the same treatment when initiated after disease onset, which resembles the clinical situation presented in MS, completely protects all treated animals from further relapses. We also show that protected animals were unresponsive to PLP as measured by delayed-type hypersensitivity (DTH). This represents a novel immunotherapeutic approach that can be exploited to develop treatments for human MS and other T-cell-mediated autoimmune diseases. (Blood. 2004;103:4616-4618)
Introduction
Experimental autoimmune encephalomyelitis (EAE) is an inflammatory disease of the central nervous system (CNS) induced experimentally by immunization with various myelin proteins defined as encephalitogenic proteins. The disease is mediated by encephalitogen-specific CD4+ T cells that cross the blood-brain barrier and induce perivascular inflammation resulting in focal demyelination, which presents as characteristic white matter plaques.1,2 Histologically, EAE closely resembles multiple sclerosis (MS) in humans and is therefore considered an experimental model for the human disease.1,3 Clinically, the remitting-relapsing form of the disease characterized by cycles of spontaneous remissions followed by spontaneous relapses is the most common form in humans4 and in several mouse models of EAE, including the one employed in this study.
We have previously shown that autologous (syngeneic) B cells expressing the highly encephalitogenic proteolipid protein (PLP) determinant can effectively protect naive mice from induction of EAE and that treated animals become specifically unresponsive to PLP.5 Expression of PLP in syngeneic B cells was achieved by a retroviral-based gene transfer system using normal B cells as target cells. The rationale behind this treatment protocol is that antigens expressed in resting B cells in conjunction with major histocompatibility complex (MHC) class II but in the absence of costimulatory molecules (such as CD80/CD86) induce specific T-cell unresponsiveness rather than T-cell activation.6,7 In this study, the efficacy of the same treatment was tested in mice recovering from the initial episode of the disease. This treatment protocol is a better paradigm for human MS where treatment can only begin after the first clinical episode. We show that this treatment completely protects animals from disease progression, and none of the treated mice developed any relapses for at least 6 months. As was the case with naive mice, treated animals were found to be unresponsive to an immunogenic challenge with PLP as measured by delayed-type hypersensitivity (DTH).
Study design
Mice, vectors, and EAE induction
Female (BALB/c × SJL) F1 mice purchased from the Jackson Laboratory (Bar Harbor, ME) were used between 8 and 14 weeks of age. The generation of the MPL and MML cassettes5 containing the PLP and myelin basic protein (MBP) encephalitogenic determinants, respectively, and the MPL-PURO and MML-PURO viral producer cells were described previously.5 EAE was induced by direct immunization with 200 μg PLP p139-151.5
B-cell preparations and retroviral-mediated gene transfer
B cells expressing the PLP encephalitogenic determinant were prepared as previously described,5 and 30 × 106 to 35 × 106 cells/mouse were injected intravenously into syngeneic (BALB/c × SJL) F1 mice in remission from EAE within 2 weeks. Expression of the exogenous chimeric gene in treated animals was verified by reverse transcriptase-polymerase chain reaction (RT-PCR).5
DTH measurement
PLP-specific DTH responses were measured 7 days after B-cell transfer. Mice were challenged in one of the front footpads with PLP p139-151-coupled sheep red blood cells (SRBCs; Bis-diazotized benzidin coupling method).8 DTH was measured as footpad swelling 24 and 48 hours after antigenic challenge.
Results and discussion
PLP is the most potent encephalitogenic determinant in the mouse, and over 90% of SJL and (BALB/C × SJL) F1 mice will develop severe EAE 10 to 15 days after immunization with peptide emulsified in complete Freund adjuvant (CFA) and pertussigen. Most animals recover from the initial episode of disease and enter a state of remission. Of these, about 50% “spontaneously” relapse within 6 weeks and develop severe clinical signs of EAE and then enter again into remission usually within 10 days. To test the efficacy of transferring B cells expressing the PLP encephalitogenic determinant on the progression of EAE, mice in remission were adoptively transferred with 30 × 106 to 35 × 106 genetically modified B cells and followed for 6 months for clinical signs of relapse. As shown in Table 1, all 21 treated animals were completely protected from relapses and none have developed any clinical signs for at least 6 months. In contrast, over 50% of the untreated control mice or mice injected with B cells expressing the unrelated encephalitogenic determinant of MBP developed at least one relapse within 6 weeks. Twenty percent of these mice developed a second relapse (Table 1). B cells expressing the MPL chimeric gene spanning the PLP encephalitogenic determinant were present in the all protected mice, and B cells expressing the MBP determinant were present in all relevant control animals as verified by RT-PCR (data not shown).
Transfer of B cells expressing MPL-PURO protects from relapsing EAE
Vector . | First relapse (%) . | Second relapse (%) . |
|---|---|---|
| MPL-PURO | 0/21 (0) | 0/0 (0) |
| MML-PURO | 5/9 (56) | 1/5 (20) |
| No vector controls | 17/30 (57) | 5/17 (29) |
Vector . | First relapse (%) . | Second relapse (%) . |
|---|---|---|
| MPL-PURO | 0/21 (0) | 0/0 (0) |
| MML-PURO | 5/9 (56) | 1/5 (20) |
| No vector controls | 17/30 (57) | 5/17 (29) |
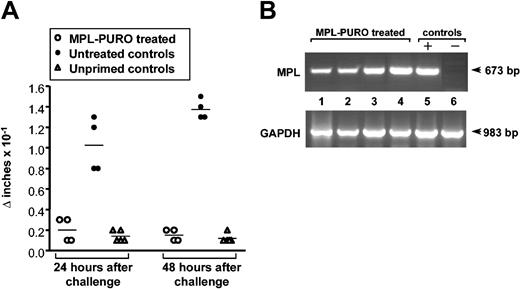
To test whether the treated mice were tolerized to PLP, the specific T-cell response against PLP was assessed by DTH one week after the adoptive transfer of B cells. DTH was measured (as footpad swelling) 24 and 48 hours after challenge with p139-151 PLP-SRBCs8 in the front right footpad. Mice in remission that were not adoptively transferred with modified B cells served as control mice. Responses in normal, unprimed animals were used as a baseline response that was invariably negligible (< 0.1 × 10-1 in). As shown in Figure 1A, all mice that received B cells expressing the relevant PLP determinant were unresponsive by DTH to an antigenic challenge, whereas all control animals responded well. Expression of MPL chimeric gene spanning the PLP encephalitogenic determinant was verified in all unresponsive mice by RT-PCR (Figure 1B).
Absence of PLP-specific DTH responses in mice adoptively transferred with MPL-PURO-expressing B cells. (A) Treated and untreated mice in remission were injected with PLP-coupled SRBCs into the front right footpad. The swelling of the injected footpad was compared with the uninjected left footpad 24 and 48 hours later. Each symbol represents one mouse and the horizontal bars represent the mean response of each group. Baseline responses to the antigen challenge alone were measured in unprimed normal control mice. (B) RT-PCR was performed on total RNA extracted from the spleens of mice treated with MPL-PURO-infected B cells and employed in the DTH assays (lanes 1-4). Lanes 6 and 7 are positive and negative control samples prepared from MPL-PURO-infected WEHI-231 B cells and from the spleen of an untreated mouse, respectively. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was amplified in all samples indicating equal level of total RNA usage.
Absence of PLP-specific DTH responses in mice adoptively transferred with MPL-PURO-expressing B cells. (A) Treated and untreated mice in remission were injected with PLP-coupled SRBCs into the front right footpad. The swelling of the injected footpad was compared with the uninjected left footpad 24 and 48 hours later. Each symbol represents one mouse and the horizontal bars represent the mean response of each group. Baseline responses to the antigen challenge alone were measured in unprimed normal control mice. (B) RT-PCR was performed on total RNA extracted from the spleens of mice treated with MPL-PURO-infected B cells and employed in the DTH assays (lanes 1-4). Lanes 6 and 7 are positive and negative control samples prepared from MPL-PURO-infected WEHI-231 B cells and from the spleen of an untreated mouse, respectively. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was amplified in all samples indicating equal level of total RNA usage.
The results presented here demonstrate that a single transfer of syngeneic B cells genetically modified to express the encephalitogenic determinant of PLP into hosts during the first remission period can completely block the progression of EAE for at least 6 months. The tolerizing effect was even more pronounced than the effect on preventing disease induction in naive animals,5 as all treated mice were completely protected. The form of the in vivo-induced unresponsiveness (also referred to as in vivo clonal anergy or adaptive tolerance) described in this study is typically a consequence of incomplete T-cell activation due to the absence of costimulation that is readily induced in memory cells (reviewed in Schwartz7 ). This form of unresponsiveness was first demonstrated in vivo in an adoptive transfer system where spleen cells expressing the viral Mtv-7 (Mls)9 or the enterotoxin B (SEB)10 superantigens were transferred into MHC-identical mice that did not express these superantigens. Following an initial proliferative response, donor T cells could no longer respond to the host's superantigen and were actually tolerized against it. The same phenomenon was later demonstrated for regular antigens. T-cell receptor (TCR) transgenic T cells become specifically unresponsive upon transfer into mice that constitutively express the antigen recognized by the transgenic TCR.11,12 More relevant to this study, it was demonstrated that antigens presented exclusively on resting B cells led to antigen-specific T-cell unresponsiveness in vitro13,14 as well as in vivo.15,16
The maintenance of clonal anergy was shown to be dependent on the presence of the tolerizing antigen.11,12,17 In our model, PLP-specific mRNA can be readily detected in all protected animals up to 6 months in this set of experiments, and in previous work it was shown that expression can be detected for at least a year.5 The amount of the tolerizing antigen was shown in various systems to be less critical.17,18 In our model, the exact level of expression is hard to determine but it is easily detected by PCR for up to a year,5 and proviral DNA can also be detected as a faint band in most mice in a standard Southern blot analysis (data not shown). We therefore believe that a similar treatment could be developed for the treatment of MS in humans. B cells can be readily isolated in large numbers from peripheral blood and easily stimulated to proliferate. Amphotropic retroviral vectors (that can infect human cells) and various other options were developed by many laboratories including ours, and infection of mature cells circumvents many of the problems associated with the infection of stem cells and other precursors.
Perhaps the main argument against the applicability of this model to human MS is the fact that the exact autoantigens were never defined in humans and, even when they are, chances are that different epitopes will be responsible for the disease in different people. On the other hand, studies in many mammalian species demonstrated that only 2 to 3 myelin-associated proteins contain encephalitogenic determinants and there is no reason to expect that humans will have additional ones. In this case, 3 different constructs expressing the 3 major encephalitogenic proteins could be used to infect each patient's autologous B cells.
Two other models of B-cell mediated tolerance were shown to be effective in blocking EAE induction. In the first, tolerance to MBP was induced by coupling MBP to anti-immunoglobulin D (anti-IgD) antibodies that directed the autoantigen to B cells.15 In this model, surface expression in B cells is transient and is dependent on the presence of the MBP-anti-IgD conjugate in the circulation, which would require continuous injections. It is also unclear whether the fusion protein will eventually induce a specific immune response against itself. In the second model, an IgG-Fc-MBP fusion protein was introduced into B cells,19 which are claimed to both secrete the fusion protein and express the encephalitogenic determinant of MBP on the cell surface. Two different tolerizing mechanisms were implicated. The first is based on the claim that soluble IgG and IgG conjugates are highly tolerogenic,20 although the mechanism for this phenomenon remains unclear. The second mechanism is clonal anergy induced by incomplete T-cell activation by resting B cells as described in the introduction. However, in this model, it is not clear how the fusion protein, which is made inside the cell, is processed for expression in conjunction with MHC class II, since no lysosomal targeting sequences were used. Longevity of expression of the exogenous protein was not assessed in that report. The long-term clinical efficacy is also hard to assess since mice were only followed for up to 40 days and disease severity in control animals was rather low.19 We therefore believe that the model presented in this study is more suitable for application to human MS.
Prepublished online as Blood First Edition Paper, February 26, 2004; DOI 10.1182/blood-2004-01-0091.
Supported in part by National Institute of Health grants 5 R01-NS38272 (Y.R.) and R01-CA50777 (J.P.D.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal