Abstract
The myeloproliferative disorder of mice lacking the Src homology 2 (SH2)-containing 5′ phosphoinositol phosphatase, SHIP, underscores the need for closely regulating phosphatidylinositol 3-kinase (PI3K) pathway activity, and hence levels of phosphatidylinositol species during hematopoiesis. The role of the 3′ phosphoinositol phosphatase Pten in this process is less clear, as its absence leads to embryonic lethality. Despite Pten heterozygosity being associated with a lymphoproliferative disorder, we found no evidence of a hematopoietic defect in Pten+/- mice. Since SHIP shares the same substrate (PIP3) with Pten, we hypothesized that the former might compensate for Pten haploinsufficiency in the marrow. Thus, we examined the effect of Pten heterozygosity in SHIP-/- mice, predicting that further dysregulation of PIP3 metabolism would exacerbate the pheno-type of the latter. Indeed, compared with SHIP-/- mice, Pten+/-SHIP-/- animals developed a myelodysplastic phenotype characterized by increased hepatosplenomegaly, extramedullary hematopoiesis, anemia, and thrombocytopenia. Consistent with a marrow defect, clonogenic assays demonstrated reductions in committed myeloid and megakaryocytic progenitors in these animals. Providing further evidence of a Pten+/-SHIP-/- progenitor abnormality, reconstitution of irradiated mice with marrows from these mice led to a marked defect in short-term repopulation of peripheral blood by donor cells. These studies suggest that the regulation of the levels and/or ratios of PI3K-derived phosphoinositol species by these 2 phosphatases is critical to normal hematopoiesis. (Blood. 2004;103:4503-4510)
Introduction
The phosphatidylinositol 3-kinase (PI3K) pathway plays a critical role in the regulation of a wide range of important cellular processes including resistance to apoptosis, entry into the cell cycle, differentiation, mobility, metabolic pathways, level of protein synthesis, and cell size (reviewed in Katso et al1 ). These varied effects are brought about by PI3K activation in the course of receptor-mediated responses to many types of extra-cellular stimuli, such as growth factors, interleukins, colony-stimulating factors, chemokines, and antigens. These enable PI3K to phosphorylate plasma membrane PI(4,5)P2-containing lipids yielding the pivotal second messenger PI(3,4,5)P3 (PIP3). As a result, levels of PIP3 that are extremely low at rest become sharply increased leading to the translocation of a wide variety of molecules, including 3-phosphoinositide-dependent protein kinase-1 (PDK1) and protein kinase B (PKB)/Akt, to the inner leaflet of the plasma membrane. This is mediated by the binding of pleckstrin homology (PH) domains in these molecules to the PIP3-containing lipid head groups. This redistribution, which functions to activate and/or juxtapose specific PH domain-containing molecules with their substrates or associated proteins, rapidly triggers the activation of a range of “downstream” signaling pathways (reviewed in Cantley2 ).
An appreciation for the pivotal role of PI3K and specific lipid phosphoinositol moieties in the regulation of proliferation, survival, and differentiation of hematopoietic cells has been attained through considerable lines of research (reviewed in Fruman and Cantley3 ). It has also become evident that the multiplicity of effects stemming from PI3K activity need to be tightly regulated. One of the ways in which proximate downstream control of PI3K activity is achieved is via a 2-pronged attack on the main product of this enzyme, PIP3, by the phosphatase with tensin homology (Pten) and 2 SH2-containing inositol phosphatases, SHIP and SHIP2.
While the 5′ inositol phosphatase SHIP2 is expressed in all tissues, SHIP is found primarily in hematopoietic cells.4,5 Like SHIP2, the tumor suppressor molecule Pten, a 3′ inositol phosphatase, is widely expressed.6 Although both of these molecules act on PIP3, the products that they generate are quite different: Pten activity leads to the formation of PI(4,5)P2, a relatively abundant membrane-bound inositol lipid; while SHIP hydrolyzes PIP3 to generate PI3,4 P2. Interestingly, the latter appears to be second messenger in its own right, as shown by the finding of PH domains that appear to be specific for this molecule.7,8
The different products of these 2 phosphatases, as well as their distinct expression patterns, subcellular localizations, and adaptor functions, suggested that Pten and SHIP might have overlapping as well as distinct roles in cell signaling. This was illustrated by targeted disruption of each of the 2 genes in mice. While SHIP-/- mice are viable, they exhibit a progressive and eventually fatal myeloproliferative disorder whose most striking pathologic feature was the accumulation of macrophages and neutrophils in the lungs.9,10 SHIP-deficient mice of mixed genetic background are occasionally able to survive the lung pathology, only to develop a lupuslike syndrome characterized by antinuclear antibodies and immune complex-mediated glomerulonephritis (C.D.H., unpublished results, February 2000). Although mice lacking Pten demonstrate embryonic lethality,11,12 Pten heterozygotes are predisposed to a wide range of different malignancies, consistent with the role of Pten as a tumor suppressor. Interestingly, with age Pten+/- mice develop a progressive lymphoproliferative/autoimmune disorder characterized by lymphadenopathy, splenomegaly, hypergammaglobulinemia, immune complex-mediated glomerulonephritis, and resistance to Fas-induced apoptosis.12,13 We found that Pten+/- B cells exhibited an increased response to a chemotactic stimulus, further suggesting that partial reduction of Pten levels could dysregulate the PI3K signaling pathway.14 These immune system disturbances most likely result from an alteration in PIP3 metabolism, providing a possible explanation for why the superimposition of SHIP heterozygosity exacerbated the lymphoproliferative/autoimmune disorder of Pten+/- mice.15
The importance of regulating PIP3 levels in hematopoiesis is illustrated by the severe myeloproliferative disorder in mice completely deficient for SHIP.9,10 We therefore hypothesized that haploinsufficiency for Pten might similarly disturb some aspect of hematopoiesis. In addition, as it was possible that by sharing the same substrate, PIP3, SHIP might compensate for a partial deficiency of Pten, we examined the effects of Pten heterozygosity in the context of complete deficiency for SHIP. In this instance it could be hypothesized that PIP3 metabolism would be further dysregulated, perhaps exacerbating the SHIP-/- phenotype. Lastly, given the potent tumor suppressor function of Pten, the myeloproliferative-like syndrome of SHIP-/- mice, and the potential role for SHIP in leukemic transformation,16,17 we hypothesized that the combination of Pten heterozygosity with SHIP deficiency would promote leukemogenesis. Instead, Pten+/-SHIP-/- mice developed a myelodysplastic syndrome.
Materials and methods
Mice
Pten+/- and SHIP-/- mice were generated as previously described9,12 and were back-crossed to the C57BL/6 background (n = 6-7). Pten+/-SHIP-/- mice were generated by intercrosses of Pten+/-SHIP+/- and SHIP+/- mice. Locus-specific polymerase chain reaction (PCR) was used to genotype the mice. The congenic C57BL/6/Ly-Pep3b (Jackson Laboratory, Bar Harbor, ME) strain was used as the transplant recipient. Hematopoietic cells of the 2 strains can be distinguished on the basis of allelic differences at the Ly5 locus, that is, C57BL/6 mice express Ly5.2, whereas Pep3b mice express Ly5.1. Irradiated animals were provided with acidified water (pH 3.0). Strains used in the experiments were maintained at either the University of Calgary barrier facility or at the British Columbia Cancer Research Center vivarium, in accordance with protocols approved by the respective animal care committees at the Universities of Calgary and British Columbia.
Blood analysis
Mice were euthanized by an overdose of the avertin anesthetic, and blood samples obtained by cardiac puncture were transferred to microtainer tubes containing the anticoagulant EDTA (ethylenediaminetetraacetic acid). Samples were analyzed for red blood cell (RBC), white blood cell (WBC), and platelet counts, and hematocrit by Coulter counter (Stack S; Beckman Coulter, Fullerton, CA), and peripheral blood smears were stained with Wright-Giemsa and scored by morphology (100 cells counted/slide).
Clonogenic assays for committed hematopoietic progenitor growth
Single-cell suspensions were obtained by flushing femurs with α-minimal essential medium (MEM) media with 2% fetal calf serum (FCS; Invitrogen Canada, Burlington, ON, Canada), or by pressing spleen or liver segments in α-MEM media, 2% FCS through a wire mesh. Unfractionated bone marrow (BM), spleen, or liver cells were plated in 1.1 mL complete methylcellulose media (in duplicate) supplemented with erythropoietin, interleukin-3 (IL-3), IL-6, and stem cell factor (SCF) (M3434; StemCell Technologies, Vancouver, BC, Canada). Dishes were incubated for 10 to 12 days at 37°C, 5% CO2, and 95% humidity. Growth factor dose responses were performed using 1% methylcellulose media (M3234, StemCell Technologies) supplemented with various doses of either granulocyte macrophage-colony-stimulating factor (GM-CSF), IL-3, or steel factor (SF) (R&D, Minneapolis, MN), and then incubated as above for 10 to 12 days. All colonies of more than 20 cells were scored morphologically using a gridded stage on an inverted microscope.
Megakaryocyte progenitor assays were performed by plating whole bone marrow cells in triplicate in MegaCult serum-free media and collagen solution (StemCell Technologies) supplemented with 50 ng/mL thrombopoietin, 20 ng/mL recombinant human IL-6, 50 ng/mL recombinant human IL-11, and 10 ng/mL recombinant mouse IL-3 (as per Stem Cell Technologies protocols). Double chamber slides (Stem Cell Technologies) were incubated for 10 days at 37°C, 5% CO2, and 95% humidity. Slides were dehydrated, fixed in acetone, and stained with a solution of acetylthiocholiniodide (Sigma, St Louis, MO), sodium citrate (Sigma), copper sulfate (Fisher, Fairlawn, NJ), and potassium ferricyanide (Sigma) according to Stem Cell Technologies protocols. Total megakaryocytic colony-forming units (CFU-Mk's) consisted of the combined numbers of small colonies (3-30 cells), large colonies (> 30 cells), and mixed colonies, whereas the numbers of non-CFU-Mk colonies were tabulated separately.
Fluorescence-activated cell sorting (FACS) analysis and lineage depletion
Single-cell suspensions were obtained by flushing femurs with α-MEM media with 2% FCS. Cells were washed with phosphate-buffered saline (PBS), blocked with 2 μg/mL anti-FcγRIIB antibody (2.4G2; BD Pharmingen, San Jose, CA), and subsequently stained with fluorescein isothiocyanate (FITC), phycoerythrin (PE), or peridinin chlorophyll-alpha protein (PerCP)-conjugated antibodies against CD11b, CD41, NK1.1, and B220 (BD Pharmingen). Ter119, obtained as a biotin-conjugate, was visualized using a streptavidin-FITC antibody (BD Pharmingen). A minimum of 10 000 events were analyzed using a FACScalibur (Becton Dickinson, Mountain View, CA) flow cytometer equipped with CellQuest software (Becton Dickinson), and data were analyzed using FlowJo software (TreeStar, Ashland, OR). Lineage-depleted samples were collected from both femurs per mouse using the StemSep isolation protocol and reagents (StemCell Technologies). The cells in the flow-through were enumerated, blocked with anti-FcRIIB antibody, and then stained with PE- or FITC-conjugated antibodies against c-kit and Sca-1 (Ly-6A) (BD Pharmingen), and analyzed using FlowJo software.
Multilineage repopulation analysis
Lethally irradiated (900 cGy of 137Cs gamma irradiation) Pep3b (Ly5.1+) recipients were injected with 1 × 106 cells per mouse of wild-type, Pten+/-, SHIP-/-, or Pten+/-SHIP-/- bone marrow cells (2-4 independent donors per genotype, 4-8 recipients per donor). Donor-derived contributions were evaluated by monitoring the percentage of Ly5.2+-B lymphoid (B220+), T cell (CD3+), or myeloid cells (Mac1+) in the periphery at 4 weeks after transplantation by flow cytometry using the appropriate monoclonal antibodies (BD Pharmingen).
Statistics
Statistical significance was performed using a one-way analysis of variance (ANOVA) combined with a Tukey test for multiple comparisons in all experiments.
Results
Peripheral blood abnormalities and extramedullary hematopoiesis in Pten+/-SHIP-/- mice
Previously, it was reported that the SHIP-/- mice having a mixed 129J/C57BL/6 background survived approximately 14 weeks, and occasionally up to a year.9 Subsequent back-crossing onto the C57BL/6 background resulted in a more severe phenotype, such that by the n = 6 back-cross, SHIP-/- mice were dying by 6 to 12 weeks of age (Helgason et al18 ; and our unpublished observations, September 2003). Although showing runting and decreased body weight similar to that of SHIP-/- littermates (wild-type 19.2 g ± 0.20 g; Pten+/- 21.7 g ± 0.76 g; SHIP-/- 15.14 g ± 0.60 g; Pten+/-SHIP-/- 13.2 g ± 0.96 g), our colony of Pten+/-SHIP-/- mice rarely survived beyond 5 weeks of age.
Perhaps contributing to their demise, peripheral blood analysis of Pten+/-SHIP-/- mice between the ages of 4 and 5 weeks revealed a marked normocytic anemia as well as thrombocytopenia (Table 1). Interestingly, the SHIP-/- mice also showed a significant anemia and a corresponding decrease in their hemoglobin (Table 1, P < .05). These results contrast with previous data obtained from SHIP-/- mice on a mixed genetic background,9 and may reflect an increased severity of the reported erythroid defect due to back-crossing of this mutation onto the BL/6 background. In contrast to the other genotypes, Pten+/-SHIP-/- mice also exhibited a significant leukocytosis (Table 1) due to increased percentages of circulating granulocytes. While neutrophils accounted for the major fraction of the leukocyte elevation seen in most Pten+/-SHIP-/- peripheral blood smears (Table 2), leukocytosis in one Pten+/-SHIP-/- mouse was due in part to high levels of basophils. Despite the decreased percentage of circulating lymphocytes seen in Pten+/-SHIP-/- mice (Table 2), absolute numbers of circulating lymphocytes were increased (P < .025, compared with all genotypes, extrapolated from the data in Tables 1 and 2), as were circulating monocytes, although the latter was not significant when compared with SHIP-/- mice (P < .05 compared with wild-type and Pten+/- mice alone). No immature forms, such as blast cells, more than the rare metamyelocyte, or excess nucleated erythrocytes, were observed in the peripheral blood of any of the genotypes, and none of the bone marrow samples revealed evidence of leukemia (data not shown). According to recently published guidelines for the classification of nonlymphoid hematopoietic neoplasms in mice,19 the phenotype of Pten+/-SHIP-/- mice meets the criteria for a myelodysplastic syndrome.
Peripheral blood counts in Pten+/−SHIP−/− and control mice
. | Peripheral complete blood count values . | . | . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|
| Genotype . | Red blood cell count, × 1012/L . | Hemoglobin, g/L . | Hematocrit . | White blood cell count, × 109/L . | Platelet count, × 109/L . | ||||
| Wild-type | 8.1 ± 0.2 | 137.7 ± 3.5 | 0.41 ± 0.01 | 3.6 ± 0.3 | 662.2 ± 110.0 | ||||
| Pten+/− | 8.0 ± 0.2 | 137.7 ± 2.7 | 0.42 ± 0.01 | 3.8 ± 0.8 | 745.0 ± 75.6 | ||||
| SHIP−/− | 7.0 ± 0.5* | 115.5 ± 9.5† | 0.35 ± 0.03† | 5.8 ± 1.3 | 492.0 ± 37.7 | ||||
| Pten+/−SHIP−/− | 4.9 ± 0.3‡ | 83.4 ± 5.0‡ | 0.26 ± 0.02‡ | 18.8 ± 3.0‡ | 106.2 ± 27.0‡ | ||||
. | Peripheral complete blood count values . | . | . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|
| Genotype . | Red blood cell count, × 1012/L . | Hemoglobin, g/L . | Hematocrit . | White blood cell count, × 109/L . | Platelet count, × 109/L . | ||||
| Wild-type | 8.1 ± 0.2 | 137.7 ± 3.5 | 0.41 ± 0.01 | 3.6 ± 0.3 | 662.2 ± 110.0 | ||||
| Pten+/− | 8.0 ± 0.2 | 137.7 ± 2.7 | 0.42 ± 0.01 | 3.8 ± 0.8 | 745.0 ± 75.6 | ||||
| SHIP−/− | 7.0 ± 0.5* | 115.5 ± 9.5† | 0.35 ± 0.03† | 5.8 ± 1.3 | 492.0 ± 37.7 | ||||
| Pten+/−SHIP−/− | 4.9 ± 0.3‡ | 83.4 ± 5.0‡ | 0.26 ± 0.02‡ | 18.8 ± 3.0‡ | 106.2 ± 27.0‡ | ||||
Blood samples were analyzed on a Coulter Gen-S counter. Data represent analysis of 4 to 8 mice per genotype ± SEM.
P < .05 compared with wild-type.
P < .05 compared with wild-type and Pten+/−.
P ≤ .005 compared with all genotypes.
Leukocyte differentials in Pten+/−SHIP−/− and control genotypes
. | White blood cell differentials, % . | . | . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|
| Genotype . | Neutrophils . | Lymphocytes . | Monocytes . | Eosinophils . | Basophils . | ||||
| Wild-type | 8.2 ± 1.0 | 82.6 ± 6.4 | 8.5 ± 5.6 | 0.2 ± 0.2 | ND | ||||
| Pten+/− | 9.0 ± 1.2 | 78.0 ± 7.0 | 12.3 ± 5.5 | 0.8 ± 0.5 | 0.2 ± 0.2 | ||||
| SHIP−/− | 9.1 ± 2.9 | 75.9 ± 5.6 | 14.4 ± 5.8 | 1.0 ± 0.5 | 0.2 ± 0.2 | ||||
| Pten+/−SHIP−/− | 26.8 ± 8.5* | 48.2 ± 6.0† | 16.2 ± 4.7 | 0.4 ± 0.2 | 8.0 ± 7.9 | ||||
. | White blood cell differentials, % . | . | . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|
| Genotype . | Neutrophils . | Lymphocytes . | Monocytes . | Eosinophils . | Basophils . | ||||
| Wild-type | 8.2 ± 1.0 | 82.6 ± 6.4 | 8.5 ± 5.6 | 0.2 ± 0.2 | ND | ||||
| Pten+/− | 9.0 ± 1.2 | 78.0 ± 7.0 | 12.3 ± 5.5 | 0.8 ± 0.5 | 0.2 ± 0.2 | ||||
| SHIP−/− | 9.1 ± 2.9 | 75.9 ± 5.6 | 14.4 ± 5.8 | 1.0 ± 0.5 | 0.2 ± 0.2 | ||||
| Pten+/−SHIP−/− | 26.8 ± 8.5* | 48.2 ± 6.0† | 16.2 ± 4.7 | 0.4 ± 0.2 | 8.0 ± 7.9 | ||||
Blood smears were stained with Wright-Giemsa and percentages were determined out of 100 white blood cells scored. Data represent differentials from 6 to 8 mice per genotype ± SEM. ND indicates not detected.
P = .06 compared with all genotypes.
P ≤ .025 compared with all genotypes.
On necropsy, spleens and livers from Pten+/-SHIP-/- mice revealed splenomegaly and hepatomegaly that was significantly increased over that of littermate controls of other genotypes (P ≤ .005 compared with all genotypes; Figure 1A). Lung weights (Figure 1A), as well as pulmonary infiltrates of myeloid cells and disruption of splenic architecture, were comparable to those of aged-matched SHIP-/- littermates (data not shown). SHIP-/- and Pten+/-SHIP-/- mice demonstrated no evidence of overt myelofibrosis on hematoxylin and eosin staining of marrow sections (data not shown). Livers of Pten+/-SHIP-/- mice, however, revealed multiple foci of extramedullary hematopoiesis (Figure 1B-C) that were absent from control livers. Although extramedullary hematopoiesis was reported in SHIP-/- mice,10 it was not evident in our C57BL/6 SHIP-/- mice at this age. Furthermore, the level of liver involvement in Pten+/-SHIP-/- mice increased between 1 and 5 weeks (data not shown), suggesting that the presence of hematopoietic cells in the liver was not due to persistence of fetal hematopoiesis, but instead was the result of progressive migration of progenitor cells to the liver.
Pten+/-SHIP-/- mice demonstrate increased hepatosplenomegaly and evidence of extramedullary hematopoiesis. (A) Organ weights expressed as percentages of body weight ± SEM. **P < .001 compared with wild-type and Pten+/-, but not significantly different between Pten+/-SHIP-/- and SHIP-/-. ***P ≤ .005 compared with all genotypes. (B) Hematoxylin-and-eosin-stained sections of liver reveal multiple foci of extramedullary hematopoiesis in Pten+/-SHIP-/- mice. (C) Sections from age-matched SHIP-/- controls revealing normal liver histology. Original magnification, × 40.
Pten+/-SHIP-/- mice demonstrate increased hepatosplenomegaly and evidence of extramedullary hematopoiesis. (A) Organ weights expressed as percentages of body weight ± SEM. **P < .001 compared with wild-type and Pten+/-, but not significantly different between Pten+/-SHIP-/- and SHIP-/-. ***P ≤ .005 compared with all genotypes. (B) Hematoxylin-and-eosin-stained sections of liver reveal multiple foci of extramedullary hematopoiesis in Pten+/-SHIP-/- mice. (C) Sections from age-matched SHIP-/- controls revealing normal liver histology. Original magnification, × 40.
Reduced numbers of marrow-derived megakaryocytic and myeloid progenitors in Pten+/-SHIP-/- mice
To assess the BM composition of Pten+/-SHIP-/- mice, analyses of whole bone marrow samples by flow cytometry was carried out with antibodies to the following cell surface markers: B220 (B cell), CD11b (myeloid), CD41 (megakaryocytic), NK1.1 (natural killer cell), and Ter119 (erythroid). No significant differences were seen between Pten+/-SHIP-/- mice and SHIP-/- littermates (data not shown). Overall cellularity of Pten+/-SHIP-/- marrow was significantly decreased when compared with wild-type and Pten+/- mice (P ≤ .005), but despite a modest downward trend in the Pten+/-SHIP-/- values (cellularity ×107: wild-type 2.45 ± 0.19; Pten+/- 2.34 ± 0.16; SHIP-/- 1.46 ± 0.13; Pten+/-SHIP-/- 1.20 ± 0.11), it did not differ significantly from that of SHIP-/- mice.
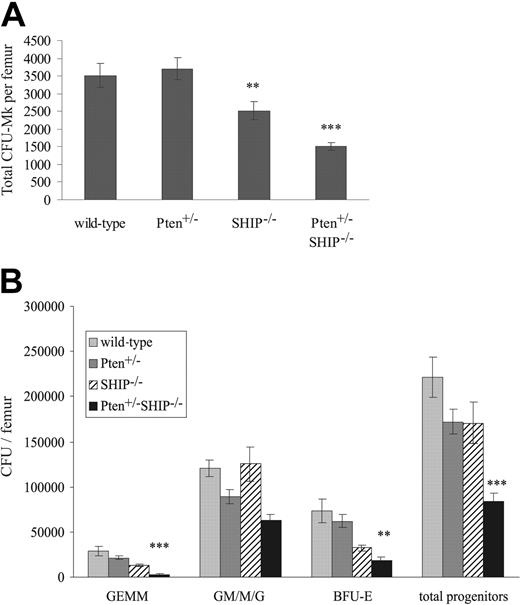
Given the level of thrombocytopenia observed in Pten+/-SHIP-/- mice, despite the lack of a detectable increase in circulating serum thrombopoietin (TPO) levels in these mice (enzyme-linked immunosorbent assay [ELISA] data not shown), we hypothesized that a functional defect might be present at the progenitor level. Thus, colony-forming assays were used to evaluate CFU-Mk numbers in the femurs of these mice. This revealed a significant decrease in the numbers of CFU-Mk in the marrows of SHIP-/- mice as compared with wild-type and Pten+/- samples (Figure 2A, P < .05). In keeping with the observed thrombocytopenia, CFU-Mk numbers in Pten+/-SHIP-/- femurs were further reduced in comparison to the SHIP-/- samples (Figure 2A, P < .05 compared with SHIP-/-, P < .001 compared with wild-type and Pten+/-). Furthermore, it was noted that Mk cultures from both SHIP-/- and Pten+/-SHIP-/- mice contained increased numbers of non-CFU-Mk colonies, primarily of the macrophage lineage. The latter likely were the result of the increased sensitivity of these progenitors to the growth factors in the cultures (data not shown, see Figure 4 and Helgason et al9 ).
Decreases in megakaryocytic and myeloid progenitor numbers in Pten+/-SHIP-/- marrow. (A) Megakaryocyte progenitor cultures were fixed, dried, and stained after 10 days of incubation. The total number of colonies was assessed and expressed as values per femur. Cultures from SHIP-/- marrow show decreased numbers of progenitors per femur, whereas the number of progenitors from Pten+/-SHIP-/- marrow was significantly reduced compared with all other genotypes. (B) BM cells were plated in duplicate in methylcellulose complete with growth factors, and the number and type of colonies was scored 10 to 12 days later. Pten+/-SHIP-/- mice showed decreases in GEMMs, BFU-Es, and total overall progenitor numbers compared with littermate controls. **P < .05 compared with wild-type and Pten+/-alone; ***P < .05 or less compared with all genotypes. Data were compiled from assays performed on 3 mice per genotype plus or minus the standard error of the mean (SEM) of the number of colonies.
Decreases in megakaryocytic and myeloid progenitor numbers in Pten+/-SHIP-/- marrow. (A) Megakaryocyte progenitor cultures were fixed, dried, and stained after 10 days of incubation. The total number of colonies was assessed and expressed as values per femur. Cultures from SHIP-/- marrow show decreased numbers of progenitors per femur, whereas the number of progenitors from Pten+/-SHIP-/- marrow was significantly reduced compared with all other genotypes. (B) BM cells were plated in duplicate in methylcellulose complete with growth factors, and the number and type of colonies was scored 10 to 12 days later. Pten+/-SHIP-/- mice showed decreases in GEMMs, BFU-Es, and total overall progenitor numbers compared with littermate controls. **P < .05 compared with wild-type and Pten+/-alone; ***P < .05 or less compared with all genotypes. Data were compiled from assays performed on 3 mice per genotype plus or minus the standard error of the mean (SEM) of the number of colonies.
Pten+/-SHIP-/- mice show increased sensitivity to GM-CSF. Bone marrow cells plated in methylcellulose in the presence of decreasing amounts of growth factors: GM-CSF (A), IL-3 (B), and SF (C). Total number of colonies growing after 10 days was scored and expressed as the percentage of colonies growing in the presence of the maximal dose of each growth factor. Both Pten+/-SHIP-/- and SHIP-/- showed increased low-dose sensitivity over Pten+/- and wild-type for GM-CSF, IL-3, and SF, and Pten+/-SHIP-/- cells showed significantly increased response over all genotypes at 0.001 ng/mL GM-CSF. These are the combined results of data from 3 to 4 mice per group plated in duplicate and are shown ± SEM. ***P < .05 compared with all genotypes.
Pten+/-SHIP-/- mice show increased sensitivity to GM-CSF. Bone marrow cells plated in methylcellulose in the presence of decreasing amounts of growth factors: GM-CSF (A), IL-3 (B), and SF (C). Total number of colonies growing after 10 days was scored and expressed as the percentage of colonies growing in the presence of the maximal dose of each growth factor. Both Pten+/-SHIP-/- and SHIP-/- showed increased low-dose sensitivity over Pten+/- and wild-type for GM-CSF, IL-3, and SF, and Pten+/-SHIP-/- cells showed significantly increased response over all genotypes at 0.001 ng/mL GM-CSF. These are the combined results of data from 3 to 4 mice per group plated in duplicate and are shown ± SEM. ***P < .05 compared with all genotypes.
Colony-forming assays were also performed to evaluate the numbers of committed myeloid progenitors in marrows of these Pten+/-SHIP-/- mice. On a per femur basis, there was a reduction in the numbers of total myeloid progenitors in the bone marrows of Pten+/-SHIP-/- mice compared with controls (Figure 2B, P < .05 compared with all genotypes). Providing a plausible explanation for the anemia, a decrease in primitive erythroid burst-forming units (BFU-Es) was observed (P ≤ .01 compared with wild-type and Pten+/-, but not significantly reduced compared with SHIP-/-). Similarly, granulocyte-erythrocyte-macrophage-megakaryocyte (GEMM) subpopulations from Pten+/-SHIP-/- marrows were reduced (Figure 2B, P < .05 compared with all genotypes). In summary, these assays revealed a decrease in both megakaryocytic and myeloid progenitor numbers in femurs of Pten+/-SHIP-/- mice, providing a plausible explanation for the peripheral cytopenias we observed.
Pten+/-SHIP-/- mice have increased numbers of myeloid colony-forming units in the liver
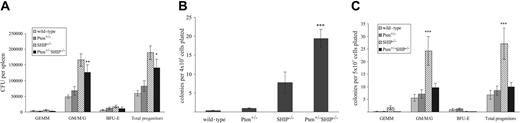
As an increase in colony-forming unit formation in the periphery of SHIP-/- mice had been previously reported,9 we performed colony-forming assays for myeloid progenitors in peripheral tissues of Pten+/-SHIP-/- mice. Splenic CFU numbers from Pten+/-SHIP-/- mice did not differ significantly from those of SHIP-/- controls, but similar to SHIP-/- splenocytes, they were significantly increased over the wild-type and Pten+/-controls (Figure 3A, P < .025). In keeping with the presence of extramedullary hematopoietic activity, colony-forming assays of Pten+/-SHIP-/- liver samples yielded a significantly higher frequency of CFUs (primarily of the granulocytic and monocytic lineages) than did littermate controls of the other genotypes (Figure 3B, P ≤ .01). Furthermore, to assess the potential contribution of peripheral blood-derived progenitor contamination in liver samples, circulating CFUs were quantitated. As shown in Figure 3C, SHIP-/- mice demonstrated a statistically significant increase in granulocyte-macrophage CFU frequency in peripheral blood (P ≤ .005 over all genotypes). Curiously, this phenotype was repressed by Pten heterozygosity, since Pten+/-SHIP-/- CFU frequencies were not significantly different from those of wild-type and Pten+/- peripheral blood samples (Figure 3C). Thus, the increased CFU frequency of Pten+/-SHIP-/- liver samples was attributable to extramedullary hematopoiesis, rather than to contamination of hepatic blood progenitors.
Clonogenic assays of liver and peripheral blood reveal differences between SHIP-/- and Pten+/-SHIP-/- myeloid progenitors. (A) Splenocytes plated in duplicate revealed no differences in splenic CFUs between SHIP-/- and Pten+/-SHIP-/-, but they did show an increase in the granulocyte/macrophage lineages over wild-type and Pten+/-cells. (B) Liver cells demonstrated a significant increase in total CFUs (predominantly granulocytic and macrophage) in cells from Pten+/-SHIP-/- mice. (C) Colony-forming assays performed on peripheral blood revealed a statistically significant increase in SHIP-/- CFUs that was not seen in Pten+/-SHIP-/- peripheral blood samples. ***P < .05 compared with all genotypes; **P < .01 compared with wild-type and Pten+/- only. *P < .025 compared with wild-type alone. Data were compiled from at least 3 mice per genotype plus or minus SEM of the number of colonies.
Clonogenic assays of liver and peripheral blood reveal differences between SHIP-/- and Pten+/-SHIP-/- myeloid progenitors. (A) Splenocytes plated in duplicate revealed no differences in splenic CFUs between SHIP-/- and Pten+/-SHIP-/-, but they did show an increase in the granulocyte/macrophage lineages over wild-type and Pten+/-cells. (B) Liver cells demonstrated a significant increase in total CFUs (predominantly granulocytic and macrophage) in cells from Pten+/-SHIP-/- mice. (C) Colony-forming assays performed on peripheral blood revealed a statistically significant increase in SHIP-/- CFUs that was not seen in Pten+/-SHIP-/- peripheral blood samples. ***P < .05 compared with all genotypes; **P < .01 compared with wild-type and Pten+/- only. *P < .025 compared with wild-type alone. Data were compiled from at least 3 mice per genotype plus or minus SEM of the number of colonies.
Pten+/-SHIP-/- progenitors show a modest increase in sensitivity to GM-CSF
Lack of SHIP was reported to confer increased sensitivity of committed progenitors to growth factor stimulation.9 Hypothesizing that Pten heterozygosity might further deregulate such responses, we assessed the sensitivity of Pten+/-SHIP-/- progenitors to titrations of growth factors. Consistent with previous observations,9 we saw increased sensitivity of both SHIP-/- and Pten+/-SHIP-/- progenitors to SF, IL-3, and GM-CSF as compared with wild-type and Pten+/- controls. We additionally observed a significantly increased sensitivity of Pten+/-SHIP-/- over SHIP-/- at one dose of GM-CSF (Figure 4A, P < .05 over all genotypes). Although there were trends suggestive of increased sensitivity of Pten+/-SHIP-/- over SHIP-/- with IL-3 and SF, except for GM-CSF there was no statistical difference in the response of these 2 genotypes toward the other cytokines tested (Figure 4B-C). Similar to previous findings,9 factor-independent growth was occasionally seen in both SHIP-/- and Pten+/-SHIP-/- cultures (data not shown). This appeared as small clusters of predominately monocytic cells that very rarely met the more-than-20-cell criterion that defined a colony. Although there was modest increase in the sensitivity of Pten+/-SHIP-/- cells to GM-CSF, the physiologic relevance of this result, especially within the context of the complex pathology of the mice, is unclear.
Pten+/-SHIP-/- marrow cells are defective in their ability to produce short-term reconstitution of lethally irradiated hosts
The decreased clonogenic potential of committed Pten+/-SHIP-/- BM progenitors (Figure 3A), especially given an equivalent or even greater sensitivity to growth factors, combined with the finding of peripheral blood cytopenias, was suggestive of a stem cell defect in Pten+/-SHIP-/- mice. Analysis of Lin-Sca1+c-kit+ cells by flow cytometry, however, revealed no significant differences in either the percentages or absolute numbers of this population among the different genotypes (Table 3). Interestingly, however, Pten+/-SHIP-/- mice revealed a downward trend in the absolute numbers of this cell type (Table 3).
Absolute number and percentage of Lin− Sca1+ c-kit+ cells in the bone marrow
. | Lin− cells . | . | Scal+ c-kit+ . | . | ||
|---|---|---|---|---|---|---|
| Genotype . | Percentage of femur . | Absolute number, × 105 . | Percentage of lin− compartment . | Absolute number, × 104 . | ||
| Wild-type | 0.48 ± 0.12 | 2.01 ± 0.50 | 11.58 ± 1.62 | 2.18 ± 0.45 | ||
| Pten+/− | 0.87 ± 0.05 | 4.00 ± 0.80 | 8.37 ± 1.37 | 3.45 ± 1.21 | ||
| SHIP−/− | 0.91 ± 0.24 | 2.48 ± 0.53 | 14.46 ± 3.12 | 3.58 ± 0.97 | ||
| Pten+/−SHIP−/− | 0.58 ± 0.15 | 1.30 ± 0.34 | 13.35 ± 1.17 | 1.65 ± 0.37 | ||
. | Lin− cells . | . | Scal+ c-kit+ . | . | ||
|---|---|---|---|---|---|---|
| Genotype . | Percentage of femur . | Absolute number, × 105 . | Percentage of lin− compartment . | Absolute number, × 104 . | ||
| Wild-type | 0.48 ± 0.12 | 2.01 ± 0.50 | 11.58 ± 1.62 | 2.18 ± 0.45 | ||
| Pten+/− | 0.87 ± 0.05 | 4.00 ± 0.80 | 8.37 ± 1.37 | 3.45 ± 1.21 | ||
| SHIP−/− | 0.91 ± 0.24 | 2.48 ± 0.53 | 14.46 ± 3.12 | 3.58 ± 0.97 | ||
| Pten+/−SHIP−/− | 0.58 ± 0.15 | 1.30 ± 0.34 | 13.35 ± 1.17 | 1.65 ± 0.37 | ||
Whole bone marrow was enumerated, lineage cell depleted, recounted, and then stained for cell-surface Scal and c-kit expression. Values in the chart were extrapolated from femoral cell counts. Results represent pooled data from 3 to 4 mice per genotype ± SEM.
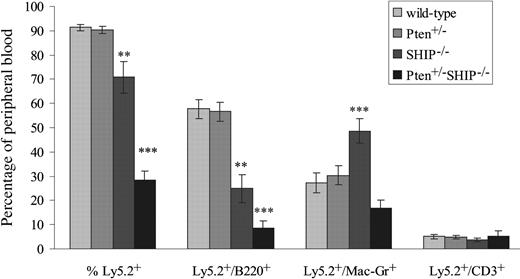
As it was also important to carry out an analysis of BM progenitor function across the different genotypes, we evaluated the capacity of bone marrow cells to reconstitute the peripheral blood of irradiated hosts. In these experiments, 106 unfractionated marrow cells from 4- to 5-week-old donors of each of the genotypes were transplanted into lethally irradiated C57BL/6/Ly-Pep3b recipients (4-8 recipients per donor). Over the course of 4 independent experiments, we found that mice reconstituted with Pten+/-SHIP-/- marrow cells regularly failed to survive beyond 4 weeks after transplantation (data not shown). The percentages of circulating donor marrow-derived Ly5.2+ leukocytes were therefore assessed at 4 weeks after transplantation. As shown in Figure 5, both wild-type and Pten+/- mice contained donor-derived populations of more than 90%, while SHIP-/- marrow cells proved significantly less effective at repopulating the recipients. Thus, percentages of Ly5.2+ cells in the peripheral blood of SHIP-/- recipients were significantly decreased over wild-type and Pten+/- (P ≤ .005), as were the percentages of Ly5.2+/B220+ cells (P ≤ .001, Figure 5). These results, in addition to the significant increases in the percentage of Ly5.2/Mac1+ (over all genotypes, P ≤ .01), were in keeping with recent evidence of a stem cell defect in SHIP-/- mice.18
Ability of Pten+/-SHIP-/- BM cells to repopulate irradiated recipients is severely compromised. Flow cytometry analysis of peripheral blood samples obtained from recipients of BM cells from the various donor genotypes is shown. Cells expressing Ly5.2+, Ly5.2+ and B220+; Ly5.2+ and Mac1+; and Ly5.2+ and CD3+ are represented as a percentage of total peripheral blood leukocyte populations. The data were derived from 2 donors of each genotype; CD3 phenotyping was performed on 5 to 9 recipients/genotype, and B220 and Mac1 analysis on 9 to 12 recipients/genotype ± SEM of percentages. ***P < .05 or less compared with all genotypes, **P ≤ .01 or less compared with wild-type and Pten+/-.
Ability of Pten+/-SHIP-/- BM cells to repopulate irradiated recipients is severely compromised. Flow cytometry analysis of peripheral blood samples obtained from recipients of BM cells from the various donor genotypes is shown. Cells expressing Ly5.2+, Ly5.2+ and B220+; Ly5.2+ and Mac1+; and Ly5.2+ and CD3+ are represented as a percentage of total peripheral blood leukocyte populations. The data were derived from 2 donors of each genotype; CD3 phenotyping was performed on 5 to 9 recipients/genotype, and B220 and Mac1 analysis on 9 to 12 recipients/genotype ± SEM of percentages. ***P < .05 or less compared with all genotypes, **P ≤ .01 or less compared with wild-type and Pten+/-.
Strikingly, peripheral blood samples of Pten+/-SHIP-/- marrow cell recipients revealed a marked defect in repopulation by donor-derived Ly5.2+ cells (P ≤ .001 compared with all genotypes, Figure 5). The percentages of Ly5.2+B220+ cells were significantly reduced over all genotypes (P < .05). In contrast to the increase in donor-derived Mac1+ cells in SHIP-/- cell recipients, the percentage of Ly5.2+/Mac1+ cells was not increased in Pten+/-SHIP-/- recipients compared with recipients of wild-type and Pten+/-cells (Figure 5). Analysis of percentage Ly5.2+/CD3+ cells in the peripheral blood, on the other hand, showed no significant differences among the various genotypes. Taken together, these results indicate that Pten heterozygosity exacerbates the repopulating defect of SHIP-/- BM cells, providing a plausible explanation for the cytopenias seen in Pten+/-SHIP-/- mice.
Discussion
Although they are prone to the development of a spontaneous lymphoproliferative phenotype,13 Pten+/- mice did not reveal abnormalities in any of the hematologic assays we employed. The previous characterization of the SHIP knockout on a 129 and C57BL/6 mixed genetic background9 had noted an increased percentage of neutrophils in the peripheral blood of 4- to 5-week-old mice, however, no leukocytosis, anemia, or thrombocytopenia was observed, even in mice up to 8 to 10 weeks of age. Similar results were obtained for mice from our C57BL/6 SHIP-/- colony, with the exception of a modest but significant anemia (Table 1). The hematologic phenotype of Pten+/-SHIP-/- mice, in contrast, was clearly distinguishable from that of SHIP-/- mice and can be characterized as a novel myelodysplastic syndrome.19
Peripheral blood abnormalities in Pten+/-SHIP-/- mice were of considerable interest, especially the normocytic, normochromic anemia, which was even more remarkable given the likelihood of chronic hypoxia owing to the rapidly progressive (and eventually fatal) lung infiltration and ensuing loss of ventilatory capacity in these mice. Colony-forming assays demonstrated significant decreases in primitive BFU-Es (Figure 2B), suggesting not only that Pten+/-SHIP-/- marrows were incapable of responding appropriately to the anemia (and hypoxia) by increased production of erythroid lineage cells, but also pointing to the possible etiology of the anemia. There is some evidence that deficiency for SHIP alone results in a hyporesponsiveness of erythroid progenitors to erythropoietin (EPO).20 While the mechanism for this is unknown, the exacerbation of the anemia with the introduction of Pten heterozygosity may suggest a link between EPO responsiveness and phosphatidylinositol levels. Interestingly, we also saw significant decreases in the GEMM progenitors in Pten+/-SHIP-/- marrow, a population that ultimately gives rise to granulocyte, erythroid, megakaryocyte, and myeloid lineages, suggesting that defects at this primitive progenitor level may be responsible for the cytopenias observed in these mice. Additionally, and perhaps accounting for the thrombocytopenia, there was a significant decrease in the number of megakaryocytic progenitors in the marrow of Pten+/-SHIP-/- mice (and to a lesser extent, in SHIP-/- marrow samples). To our knowledge this is the first report suggesting a link between lipid phosphatase levels and defects in megakaryocyte progenitor numbers. Hyporesponsiveness to TPO, similar to that reported for erythroid precursors to EPO in SHIP-/- mice, might also play a role in the thrombocytopenia. Other factors such as overproduction of myelosuppressive cytokines, and conceivably hypersplenism in these rather ill mice, might also contribute to this phenotype. With respect to the former, plasma levels of transforming growth factor β (TGF-β) revealed no significant differences between the genotypes (data not shown). Taken together, however, our results are most consistent with a BM insufficiency syndrome in Pten+/-SHIP-/- mice on the basis of a marrow progenitor defect. This notion was clearly reinforced by our short-term reconstitution studies demonstrating that Pten+/-SHIP-/- BM samples were grossly inadequate at repopulating the peripheral blood pools of irradiated wild-type recipients.
In contrast to SHIP-/- mice and the other genotypes, a large increase in peripheral blood granulocytes was seen in Pten+/-SHIP-/- mice. Although this seems paradoxical given the decreased clonogenic potential of Pten+/-SHIP-/- marrow GM/M/G progenitors, it might be accounted for by an increase in survival of the differentiated cells. This is an interesting observation given recent work showing that TGF-β is able to induce apoptosis of hematopoietic cells via up-regulation of SHIP, with the resulting drop in PIP3 levels acting to reduce the antiapoptotic activity of PKB/Akt.21 In keeping with the importance of PIP3 and SHIP in this process, BM-derived macrophages from SHIP-/- mice were resistant to TGF-β-induced apoptosis. Additional experiments on Pten+/-SHIP-/- mice will be required to determine whether the Pten+/- background enhances the survival of SHIP-/- granulocytes. Increased granulocyte counts can also result from the actions of pro-inflammatory cytokines. However, TNF-α, IL-1α, and IL-1β were not detected in serum samples from any genotypes (ELISA data not shown). Similarly, serum IL-6 was not significantly increased in Pten+/-SHIP-/- serum compared with SHIP-/- serum (ELISA data not shown).
Myeloproliferative disorders and cytopenias can precede the development of myeloid leukemias; furthermore, there is evidence that PI3K and/or its negative regulators may have roles in the progression of this disease. 16,17,22-24 However, despite the complex peripheral blood abnormalities in Pten+/-SHIP-/- mice, no evidence of leukemic transformation was seen in these mice, or in the irradiated recipients of Pten+/-SHIP-/- BM cells (data not shown). The infiltration of macrophages into the alveolar walls of mice that received transplants of both SHIP-/- and Pten+/-SHIP-/- cells (not shown), and the presence of extramedullary hematopoiesis in the livers of recipients of Pten+/-SHIP-/- (not shown), demonstrated that these 2 phenotypes were transplantable. If Pten+/-SHIP-/- mice were able to survive for longer periods, and particularly if spontaneous mutations of the remaining wild-type Pten allele occurred, bone marrow malignancies might be seen.
PI3K is activated in response to ligand engagement by a variety of receptors, and the role of SHIP in negatively regulating PI3K signals in cells of hematopoietic origin is well known.25 Not surprisingly then, SHIP-/- progenitor cells demonstrated increased sensitivity to various growth factors.9,10 Our introduction of Pten heterozygosity onto SHIP-deficient cells increased this sensitivity significantly at one concentration of GM-CSF, and only marginally to SF and IL-3. These in vitro results suggested that a further increase in PIP3 levels (due to Pten haploinsufficiency) was augmenting the growth response of progenitor cells. However, Pten+/-SHIP-/- mice exhibited decreased numbers of committed progenitors in the colony-forming assays, raising the possibility of a stem cell defect in these mice.
While these experiments were in progress, a study characterizing the stem cell compartment of SHIP-/- mice was published that revealed a repopulating defect of SHIP-/- cells and suggested an impairment of hematopoietic stem cell (HSC) self-renewal.18 5-fluorouracil (5-FU) treatment and subsequent competitive repopulating unit (CRU) analysis of the stem cell compartment of these mice also indicated that a greater proportion of the SHIP-/- stem cell compartment was cycling compared with wild-type BM. Reduced capacity for serial transplantation has also been described in mice deficient for the p21 cell-cycle inhibitor,26 as well as in mice transplanted with TGF-β receptor type II receptor dominant-negative transduced bone marrow cells.27 The latter also developed an infiltrative disorder reminiscent of SHIP-/- mice. It has been suggested that TGF-β acts as a regulator of HSC growth potentially via its ability to up-regulate cell-cycle inhibitors,28,29 although the inhibitor involved is unclear.30 Our short-term reconstitution study has demonstrated that the defect in multilineage repopulating ability of SHIP-/- BM is worsened by Pten heterozygosity. Given the effects of PI3K in the promotion of the cell cycle,31 it is plausible that loss of negative regulation of this pathway may result in a phenotype akin to that seen in the absence or inhibition of cell-cycle inhibitors. Studies regarding the long-term repopulating ability, renewal capacity, and cycling status of the stem cell compartment of Pten+/-SHIP-/- mice will be the focus of future studies, and may provide some insight into the role of PI3K regulators in stem cell biology.
Complete deletion of SHIP results in increased spleen colony-forming unit (CFU-S) in the peripheral blood,18 and a significant increase in peripheral blood CFUs as seen in the current study. However, Pten heterozygosity appeared to suppress this SHIP-/-- dependent phenomenon, perhaps owing to a global decrease in committed progenitors. Alternatively, there may have been increased migration of these cells into extramedullary sites, such as lung, spleen, and liver. The liver, for example, is a site of stromal cell-derived factor 1 (SDF-1) secretion,32 and using a colony-forming assay we demonstrated a significant increase in the content of progenitor cells in this Pten+/-SHIP-/- tissue. The presence of extramedullary hematopoiesis in liver sections was consistent with this finding. In support of a role for SDF-1, there is evidence that SHIP-/- BM progenitors, B cells, and T cells, as well as Pten+/- B cells, show increased sensitivity to this chemokine.14,33 Indeed, an assessment of the response of whole BM cells to SDF-1 chemotactic gradients revealed a trend toward increased sensitivity of Pten+/-SHIP-/- cells to this chemokine (J.L.M. and F.R.J., unpublished observations, March 2003).
While Pten heterozygosity alone failed to reveal any measurable effects on hematopoiesis using the assays employed herein, introduction of partial Pten deficiency onto the SHIP-/- background clearly modified the hematopoietic phenotype of SHIP-/- mice. The ability of Pten heterozygosity to further disrupt hematopoiesis in SHIP-/- mice argues for the importance of tightly regulating the levels of this key phosphatase, and also suggests that loss of a single allele of Pten might be under selective pressure during leukemogenesis. The results support the idea that Pten and SHIP function cooperatively during early hematopoiesis, and raise the possibility that PIP3 levels in progenitors cells are critical determinants to homeostasis of this compartment.
Supported by a grant from the Canadian Institutes for Health Research (F.R.J.). F.R.J. was supported by Alberta Heritage Foundation for Medical Research (AHFMR) Scientist and Canada Research Chair awards; C.D.H. was the recipient of Canadian Institutes for Health Research New Investigator and Michael Smith Foundation for Health Research Scholar awards; and J.L.M. was supported by an AHFMR Studentship award.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
Prepublished online as Blood First Edition Paper, March 4, 2004; DOI 10.1182/blood-2003-09-3262.
We are grateful to Drs R.K. Humphries, J. Damen, and S. Hadjur for many valuable discussions. We also thank G. Eom and L. Huang for their help in the analysis of the bone marrow recipients, K. Joris who irradiated the recipients, the vivarium staff at the British Columbia Cancer Agency for their technical assistance, and S. Chan and J. Gorday for maintenance of the transgenic mouse colonies at the University of Calgary.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal