Abstract
We previously demonstrated that yttrium-90 (Y-90) ibritumomab tiuxetan (Zevalin) radioimmunotherapy (RIT) was safe and effective for relapsed or refractory CD20+, B-cell, non-Hodgkin lymphoma (NHL). We now provide long-term follow-up data in responding patients based on International Workshop Response Criteria. Complete (CR), CR unconfirmed (CRu), and partial response (PR) rates were 29%, 22%, and 22%, respectively (overall response rate 73%, 51% in CR/CRu). Mean time to progression (TTP) and duration of response (DR) in responders were 12.6 months and 11.7 months, respectively. At the maximum tolerated dose (0.4 mCi/kg [14.8 MBq/kg]), TTP and DR in complete responders (CR/CRu) were 28.3 and 27.5 months, respectively. Nine patients (24% of responding patients) had a TTP of more than 3 years. Long-term responders (> 5 years) have been identified. Ibritumomab tiuxetan produces durable responses in patients with indolent and diffuse large B-cell lymphoma. (Blood. 2004;103:4429-4431)
Introduction
In February 2002, yttrium-90 (Y-90) ibritumomab tiuxetan radioimmunotherapy (RIT) was approved by the U.S. Food and Drug Administration (FDA) for the treatment of patients with relapsed or refractory low-grade, follicular, or CD20+ transformed B-cell non-Hodgkin lymphoma (NHL), and rituximab-refractory follicular NHL. Ibritumomab, a murine immunoglobulin G1 (IgG1) kappa monoclonal antibody, targets the same epitope on the CD20 antigen as its chimeric counterpart, rituximab (Rituxan). Tiuxetan (MX-DTPA; 1,4-methyl-benzyl isothiocyanate diethylenetriamine pentaacetic acid), a second-generation chelator, is covalently bound to ibritumomab, allowing for formation of a stable radioimmunoconjugate (RIC) by chelating Y-90 for therapy and Indium-111 (In-111) for imaging.
Sequential clinical trials were performed prior to FDA approval.1-5 The phase 3 randomized trial demonstrated a statistically superior overall response rate (ORR) and complete response (CR) rate in patients treated with ibritumomab tiuxetan compared with control subjects treated with rituximab (80% versus 56%, P = .002 and 34% versus 20%, P = .04, respectively).3 Patients refractory to rituximab had a 74% ORR, and thrombocytopenic patients treated with 0.3 mCi/kg (11.1 MBq/kg) had an 83% ORR. More than 500 patients have been treated.6
Patients were evaluated for response to ibritumomab tiuxetan with use of protocol-designated criteria. Subsequently, an international workshop defined uniform standards of response.7 Herein, we report long-term follow-up results of this phase 1/2 clinical trial2 with updated time to progression (TTP) and duration of response (DR) data and updated response rates based on these International Workshop Response Criteria (IWRC).
Study design
A complete description of this phase 1/2, single-arm, dose escalation study has been reported previously.2 Enrollment began in June 1996 and ended in December 1997. Patients with histologically confirmed, relapsed or refractory low-grade or follicular B-cell NHL who had failed 2 prior regimens or 1 anthracycline-containing regimen were eligible. Patients with aggressive NHL in first or subsequent relapse were included if the bone marrow demonstrated less than 25% involvement with NHL, hemoglobin at least 8 g/dL (80 g/L), absolute neutrophil count at least 1.5 × 109/L, and platelets at least 100 × 109/L. Institutional review board approval and written informed consent from all patients were obtained. The primary efficacy endpoint was ORR; secondary efficacy endpoints were TTP and DR. TTP was defined in all patients as the interval from the date of first dose to the date of progressive disease. DR was defined in responding patients as the interval from the date of the first observation of objective response to the date of progressive disease. Restaging evaluation was performed no later than 2 months after treatment.
Results and discussion
Fifty-one patients were enrolled. Five patients were treated at 0.2 mCi/kg (7.4 MBq/kg), 15 at 0.3 mCi/kg (11.1 MBq/kg), 30 at 0.4 mCi/kg (14.8 MBq/kg), and 1 was not treated. The dose was capped at 32 mCi (1184 MBq/kg). The maximum tolerated dose was 0.4 mCi/kg (14.8 MBq/kg) for patients with normal baseline platelet counts. Tumor histology was follicular (FL) in 65%, diffuse large B-cell (DLCL) in 24%, and nonfollicular low-grade and mantle cell lymphoma in 6% each. Median time from diagnosis to treatment was 3.8 years (range, 0.7-33.1 years). All 51 patients had received prior chemotherapy with a median of 2 prior regimens (range, 1-7 prior regimens), and 47 (92%) had received an anthracycline. Ten patients (20%) were resistant to a prior chemotherapy regimen and 9 (18%) were resistant to the last chemotherapy regimen. Seventeen (33%) patients had received prior external beam radiation therapy. Fourteen (27%) patients had at least 2 extranodal sites at diagnosis. At study entry, 59% of patients had at least one mass 5 cm or greater, and 37% had one mass at least 7 cm; 43% had bone marrow involvement with lymphoma.
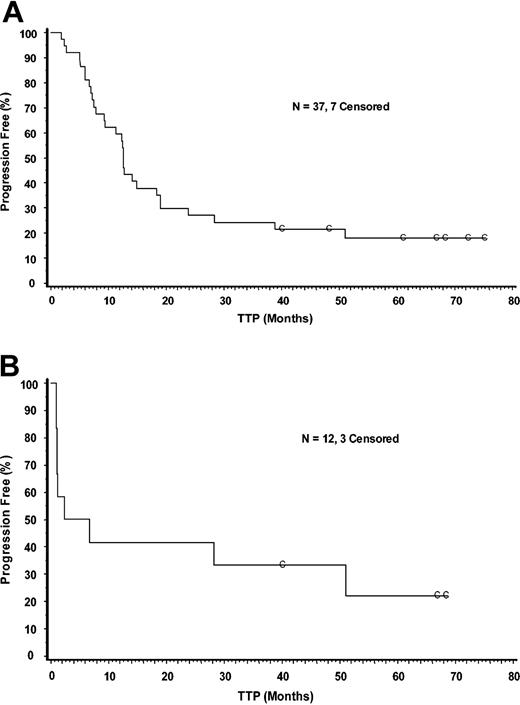
ORR in all patients was 73% (51% CR/CRu, 22% PR) (Table 1). The ORR in patients with FL was 85% and 58% in patients with DLCL. Median TTP in responders was 12.6 months (Figure 1A, Table 2) and median DR was 11.7 months (Table 2). Median TTP and DR for CR/CRu patients were 13.4 and 12.4 months, respectively. Median TTP and DR for CR/CRu patients treated at the 0.4 mCi/kg (14.8 MBq/kg) dose were 28.3 and 27.5 months, respectively (Table 2). Median TTP and DR for patients in CR who were treated at the 0.4 mCi/kg (14.8 MBq/kg) dose was 45.0 and 44.0 months, respectively. Median TTP in DLCL patients was 4.6 months in all 12 patients (Figure 1B, Table 2), including 7 responders and 5 nonresponders. Median DR in the 7 responders was 49.8 months. The reason for the large difference between TTP and DR in the DLCL group is that the rapid progression in the nonresponders (expected in DLCL) lowered the median TTP. This is reflected in the first part of the curve in Figure 1B. Median follow-up in this study is 28.5 months for all patients and 63 months for ongoing responders.
Overall response rate using IWRC criteria
Population . | No. responders . | Overall response rate, % . | CR, % . | CRu, % . | PR, % . |
|---|---|---|---|---|---|
| All patients (n = 51) | 37 | 73 | 29 | 22 | 22 |
| Follicular patients (n = 33) | 28 | 85 | 33 | 24 | 27 |
| DLCL patients (n = 12) | 7 | 58 | 33 | 17 | 8 |
Population . | No. responders . | Overall response rate, % . | CR, % . | CRu, % . | PR, % . |
|---|---|---|---|---|---|
| All patients (n = 51) | 37 | 73 | 29 | 22 | 22 |
| Follicular patients (n = 33) | 28 | 85 | 33 | 24 | 27 |
| DLCL patients (n = 12) | 7 | 58 | 33 | 17 | 8 |
Kaplan-Meier analysis of time-to-progression in intent-to-treat responders and in patients with DLCL. Panel A shows responders; Panel B shows all patients with DLCL. C indicates censored.
Kaplan-Meier analysis of time-to-progression in intent-to-treat responders and in patients with DLCL. Panel A shows responders; Panel B shows all patients with DLCL. C indicates censored.
Median duration of response and time to progression
Population . | No. . | Duration of response, mos. (range) . | Time to progression, mos. (range) . |
|---|---|---|---|
| All patients | 51 | 11.7 (0.7-74.3+) | 9.3 (0.9-75.5+) |
| Responders | 37 | 11.7 (0.7-74.3+) | 12.6 (1.8-75.5+) |
| CR/CRu patients | 26 | 12.4 (0.7-74.3+) | 13.4 (1.8-75.5+) |
| CR/CRu patients (0.4 mCi/kg [14.8 MBq/kg]) | 13 | 27.5 (0.7-74.3+) | 28.3 (1.8-75.5+) |
| CR patients (0.4 mCi/kg [14.8 MBq/L]) | 8 | 44.0 (6.4-74.3+) | 45.0 (7.5-75.5+) |
| All DLCL patients | 12 | 49.8 (1.3-67.6+) | 4.6 (0.9-68.6+) |
Population . | No. . | Duration of response, mos. (range) . | Time to progression, mos. (range) . |
|---|---|---|---|
| All patients | 51 | 11.7 (0.7-74.3+) | 9.3 (0.9-75.5+) |
| Responders | 37 | 11.7 (0.7-74.3+) | 12.6 (1.8-75.5+) |
| CR/CRu patients | 26 | 12.4 (0.7-74.3+) | 13.4 (1.8-75.5+) |
| CR/CRu patients (0.4 mCi/kg [14.8 MBq/kg]) | 13 | 27.5 (0.7-74.3+) | 28.3 (1.8-75.5+) |
| CR patients (0.4 mCi/kg [14.8 MBq/L]) | 8 | 44.0 (6.4-74.3+) | 45.0 (7.5-75.5+) |
| All DLCL patients | 12 | 49.8 (1.3-67.6+) | 4.6 (0.9-68.6+) |
Nine (24%) of 37 responders had a TTP of more than 3 years. Five of these responders had follicular lymphoma (FL) and 4 had DLCL. Among these 9 patients, baseline tumor size was at least 7 cm in 1 patient, between 5 and 7 cm in 3 patients, and less than 5 cm in the remaining 5 patients. The 4 patients with DLCL had failed between 1 and 4 regimens, including at least one anthracycline-containing regimen. Five of the 9 patients (14% [5 of 37] of all responders) are still in remission (Figure 1A): 3 patients with FL and 2 with DLCL. Duration of response for these 5 patients ranges from 60 to more than 74 months.
The safety of ibritumomab tiuxetan in this population has been recently described.6 Adverse events were primarily hematologic and transient. In the present study, 2 (4%) of the 51 patients developed myelodysplastic syndrome 2 to 3 years after ibritumomab tiuxetan. These patients were heavily pretreated (4 or 5 chemotherapy regimens). The incidence of human antimouse antibody (HAMA)/antichimeric antibody (HACA) was 2% of 46 patients assayed.
In this phase 1/2 trial, 50 patients with B-cell NHL were treated with Y-90 ibritumomab tiuxetan. Seventy-three percent responded and 51% achieved a CR/CRu. The response rate in patients with FL and DLCL was 85% and 58%, respectively. For patients treated at the 0.4 mCi/kg (14.8 MBq/kg) dose, the TTP was 28.3 months in patients achieving CR/CRu and 45 months in patients achieving CR. Seven years after trial initiation, 5 patients remain in remission, so responses are durable. It appears that those patients in remission for at least 2 years have a lower likelihood of relapse (Figure 1), similar to what has been observed in more aggressive lymphoma. Notably, 2 of 5 patients with DLCL remain in remission beyond 5 years.
The favorable safety profile seen in this early trial has been confirmed by additional large-scale studies. The primary toxicity is hematologic and is reversible. No increased incidence of secondary myelodysplastic syndrome (MDS) or acute myelogenous leukemia has been observed to date, with an annualized incidence rate of 0.2% from time of diagnosis and 0.6% from time of RIT as reported in a large study of 770 patients treated with ibritumomab tiuxetan.8 Human antimouse antibody (HAMA) formation has been observed in 1% of patients (3 of 211) and human antichimeric antibody (HACA) in 0.05% (1 of 211).6
In summary, long-term follow-up of this phase 1/2 trial demonstrates that Y-90 ibritumomab tiuxetan radioimmunotherapy produces high response rates and durable remissions in various subtypes of B-cell NHL, particularly FL. Our results indicate that Y-90 ibritumomab tiuxetan is a novel, effective treatment option for patients with B-cell NHL who have failed prior chemotherapy, including patients with refractory and/or bulky disease.
Prepublished online as Blood First Edition Paper, March 11, 2004; DOI 10.1182/blood-2003-11-3883.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Judy Berlfein for editorial assistance and Tim Wright and Jessica Olson for technical assistance.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal