HEMOSTASIS, THROMBOSIS, AND VASCULAR BIOLOGY
FIG1Vascular endothelial growth factors (VEGFs) and their receptors drive key signaling pathways for the generation of new vascular structures in the developing embryo and in various pathologic conditions, including cancer. Although the ligands (VEGF-A, VEGF-B, VEGF-C, VEGF-D, and placenta growth factor) and cell surface receptors (VEGFR-1/fms-related tyrosine kinase [flt], VEGFR-2/fetal liver kinase-1 [flk-1], and VEGFR-3/flt-4; coreceptors neuropilin-1 and -2 [NP-1 and NP-2]) are well known, knowledge about their precise roles in signaling for the complexity of vascular structures is constantly evolving. VEGF-A signals through both receptor tyrosine kinases, VEGFR-1 and VEGFR-2. The latter has historically been seen as the most important for promoting angiogenesis, due to its key role in endothelial cell proliferation and chemotaxis. The role of VEGFR-1 has proven to be more elusive. Adding to the complexity of VEGFR-1 function is the production of an mRNA splice variant that encodes a secreted form of the VEGFR-1 extracellular domain (sflt-1).
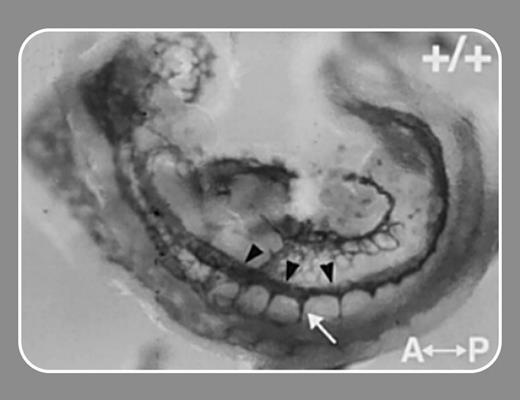
In this issue, Kearney and colleagues (page 4527) use Vegfr-1 null mice and the elegant technique of confocal time-lapse imaging to examine the dynamics of vascular sprout formation in order to test a role for VEGFR-1 in VEGF-induced endothelial cell migration. Their experiments reported here do indeed show decreased sprouts and reduced migration rate in differentiated Vegfr-1-/- vessels. Perhaps the most surprising finding is the capacity of an sflt-1 transgene to rescue the mutant phenotype. The implication is that sflt-1 can bind local VEGF-A and alter the concentration gradient of this growth factor, which drives both sprouting and endothelial cell migration.
This elaborate system of ligand acting in concert with cell-bound receptor and soluble ectodomain could be a paradigm for understanding other signaling systems in which soluble ligand binding domains are involved. One clear example raised by Kearney et al is the Wnt family of receptors and ligands in which both membrane-bound and soluble receptor forms exist. The variants generated by proteolytic cleavage of receptors also add to the puzzle, guaranteeing plenty of work in the future for biochemists and biologists seeking to resolve these issues.
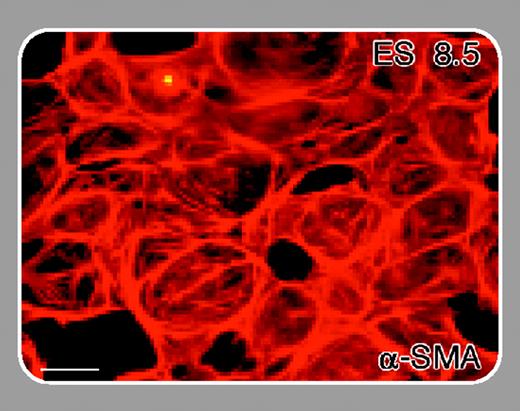
A critical challenge for clarifying the biologic role of different VEGF receptor complexes is to identify the downstream genes influenced by such signaling and to associate these genes with the various steps involved in angiogenesis and vasculogenesis (eg, mitogenesis, chemotaxis, migration, tube formation, and enhancement of vascular permeability). To this end, Zippo and colleagues (page 4536) have used a comparative microarray analysis of Vegfr-2 wild-type (+/+) and -/- embryonic stem (ES) cells to identify downstream transcription targets of VEGFR-2 signaling. The screen has revealed that Pim-1, a gene encoding a serine/threonine kinase, plays a role in the differentiation of ES cells to endothelial cells and smooth muscle cells and may thereby play a role in early vascular development. Furthermore, Pim-1 appears to be required for VEGF-A-dependent proliferation and migration of endothelial cells. Another study recently prepublished in Blood identified a VEGF-A target gene called DSCR1 that regulates the expression of inflammatory markers in endothelial cells.1 FIG2
These studies illustrate just how many layers of the vascular signaling pathways need to be uncovered. While much work remains to be done on ligand/receptor interactions in the extracellular milieu and at the cell surface, studies of events in the nucleus are only just beginning.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal