Abstract
Dendritic cells (DCs) and complement are essential components of the innate immune system. Immature DCs (immDCs) and mature DCs (mDCs) can migrate to lymphoid areas inducing, respectively, tolerance and immune responses. Primary deficiency of complement component C1q (C1q) leads to autoimmunity, suggesting a role in the maintenance of tolerance. In the present study, we investigated the production of C1q by immDCs, mDCs, and macrophages. We demonstrated that monocyte-derived and CD34+-derived interstitial DCs are a rich source of C1q. C1q produced by immDCs is functionally active in complement activation and binding to apoptotic cells. The production of C1q is completely down-regulated upon DC maturation in vitro. Moreover, we found that DC differentiation in the presence of interferon-α (IFN-α) accelerated DC maturation and strongly impaired overall C1q production. Finally, we demonstrated the presence, in significant numbers, of DC-SIGN+/C1q+ cells in T-cell areas of tonsils, next to DC-LAMP+ mDCs lacking C1q. We conclude from these results that immDC, a cell with tolerogenic properties, is a rich source of active C1q in vitro and in vivo, which is down-regulated on maturation. Therefore, immDCs may be considered an additional source of C1q in humans.
Introduction
Dendritic cells and complement are essential components of the innate immune system.1,2 DCs are the most powerful antigen-presenting cells that have a key role in inducing the primary immune response and in tolerance.3 In peripheral tissues, DCs reside in an immature form characterized by a high phagocyte capacity. DCs are able to capture and process foreign material and self-antigens derived from normal tissue turnover. When immDCs encounter apoptotic cells, they are able to engulf and process them and to present self-antigens in association with major histocompatibility complex (MHC) on their surfaces.4,5 Once migrated to draining lymph nodes in the immature or the semimature state, DCs are able to induce tolerance by deleting autoreactive T cells or by inducing regulatory T cells.6,7 In contrast, on encountering proinflammatory signals (bacterial products, cytokines), DCs not only migrate, but also start to mature and acquire a strong T cell–stimulatory capacity to initiate an immune response.8 Specific differences have been described between these 2 activation states of the same cell, including phagocyte activity, cytokine production, surface expression of costimulatory molecules, and cellular distribution of MHC-antigen (MHC-Ag) complexes.9
Complement component C1q (C1q), together with C1r and C1s, is the initiating protein of the classical pathway of complement activation. It has a molecular weight of 460 kDa with 3 polypeptide chains (A, B, and C) linked together to form a triple-helix structure repeated 6 times. C1q is formed by C-terminal globular head regions and N-terminal collagen-like regions,10 allowing C1q to interact with different types of antigens. C1q can bind to self and foreign material, leading to complement activation and to cellular activation through different C1q receptors.11 It has been demonstrated that C1q can bind to apoptotic cells.12,13 Primary C1q deficiency in humans is the strongest susceptibility factor for the development of systemic lupus erythematosus (SLE), the prototype of autoimmune diseases.14,15 An SLE-like disease also occurs in C1q–/– mice with evidence of an impaired clearance of apoptotic cells by macrophages.16,17
In contrast to most other complement components, which are mainly liver derived, macrophages are thought to be the major source of C1q.18,19 Recently, it has been demonstrated that bone marrow transplantation from wild-type mice with sufficient C1q into C1q–/– mice was able to restore the normal serum levels of C1q, suggesting that other weak sources, such as epithelial cells or fibroblasts, do not contribute significantly to the total amount of C1q in serum.19 Recently, it has been suggested that DCs can secrete C1q.20
In view of the involvement of DCs and C1q in the maintenance of tolerance,3,21 the aim of our study was to investigate the production and regulation of C1q by DCs. We generated monocyte-derived DCs and macrophages from monocytes derived from the same donors. Moreover, we analyzed CD34+-derived DCs. We demonstrated that DCs in the immature stage are an important source of C1q, whereas fully mature mDCs are completely devoid of C1q production. In addition, IFN-α was identified as a potent inhibitor of C1q production. Finally, we showed the occurrence of specific DC-SIGN+/C1q+ and DC-LAMP+/C1q– DCs in human tonsils.
Materials and methods
Generation of monocyte-derived DCs and macrophages
Monocytes were isolated from buffy coats obtained from healthy donors using Ficoll-Hypaque (Sigma, St Louis, MO) and Percoll (Pharmacia, Uppsala, Sweden) density gradient centrifugation. After 2 hours of culture in 6-well culture plates (1.1 × 106 cells/mL; Costar, Cambridge, MA), the nonadherent cells were removed by 2 washing steps with phosphate-buffered saline (PBS).
Monocyte-derived DCs were generated as previously described.22 Briefly, monocytes were cultured in RPMI 1640 supplemented with 10% heat-inactivated fetal calf serum (FCS), penicillin/streptomycin (P/S) (all from Gibco/Life Technologies, Breda, The Netherlands), 5 ng/mL granulocyte macrophage–colony stimulating factor (GM-CSF; Sandoz, Uden, The Netherlands), and 10 ng/mL interleukin-4 (IL-4; Peprotech, Rocky Hill, NJ) for 7 days (more than 95% of DCs were CD1a+/CD14–).23 In some experiments, the cells were differentiated in the same medium supplemented with 200 U/mL IFN-α (Intron A; Schering-Plough, Brussels, Belgium). Monocyte-derived macrophages were generated by culturing monocytes in RPMI 1640 with 15% FCS, P/S, and 2 mM l-glutamine for 7 days. These cells have been demonstrated to be strong producers of C1q.24 The medium was changed every 2 to 3 days in each type of cultures.
At day 7, DCs and macrophages were harvested, analyzed for phenotype, and cultured for 48 hours in their respective medium, as described, at a density of 1 × 106 cells/mL. For maturation experiments, day-7 immDCs were harvested and cultured for 48 hours in the presence of different maturation stimuli: 50 ng/mL TNF-α (R&D Systems, Abingdon, United Kingdom), 200 ng/mL lipopolysaccharide (LPS from Salmonella typhosa; Sigma), or CD40L in the DC medium described. CD40L activation was performed using a coculture system with CD40L-transfected L cells (L-CD40L) in a DC/L-CD40L ratio of 4:1.23 Nontransfected L cells were used as control (L cell).
Generation of CD34+-derived DCs
CD34+ progenitor cells were isolated from cord blood samples to generate immDCs as previously described.25 Briefly, CD34+ cells were isolated from the mononuclear fraction through positive selection using anti-CD34–coated microbeads and Midi-Macs separation columns (both from Miltenyi Biotec, Bergish Gladbach, Germany). After cryopreservation, cells were cultured in RPMI 1640 containing 10% FCS, 10 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), 2 mM l-glutamine, 50 mM β2-mercaptoethanol, P/S, 100 ng/mL GM-CSF, 25 ng/mL stem cell factor (R&D Systems), 2.5 ng/mL TNF-α, and 5% AB+ pooled human serum.
After 6 days of culture, cells were collected and labeled with fluorescein isothiocyanate (FITC)–conjugated anti-CD1a (HI149; BD PharMingen, San Diego, CA) and phycoerythrin (PE)–conjugated anti-CD14 (Leu-M3; Becton Dickinson, San Jose, CA). Cells were separated according to CD1a and CD14 expression into CD1a–CD14+ and CD1a+CD14– fractions using a FACStarPlus (BD Biosciences). Sorted cells were further cultured for 7 days in the presence of GM-CSF, P/S, and 10% FCS.
Phenotypic FACS analysis
Cells were harvested and washed twice in PBS containing 1% bovine serum albumin (BSA) and 0.02% NaN3. Fluorescence-activated cell sorter (FACS) analysis was performed using monoclonal antibodies (mAbs) against the following surface markers: CD1a (Leu-6), CD14 (Leu-M3) (both Becton Dickinson), CD86 (IT2.2; PharMingen), CD83 (HB15A; Immunotech, Marseilles, France), and HLA-DR (clone B8.11, 2). For the detection of the membrane-bound form of C1q (mC1q), an mAb directed against the C1q globular head regions (mAb 220426 ; kindly provided by Prof C. E. Hack, Sanquin Research, Amsterdam, The Netherlands) was used. Staining was visualized using PE-conjugated goat-antimouse immunoglobulins (DAKO, Glostrup, Denmark). Cells were assessed for fluorescence using a FACScan (Becton Dickinson). Data obtained were analyzed using WinMDI software (http://facs.scripps.edu).
RNA isolation and RT-PCR analysis
Total RNA was isolated using RNAzol (Campro, Veenendaal, The Netherlands) according to the manufacturer's instructions. After quantification, 1 μg RNA was reverse transcribed into cDNA by oligo (dT) priming using Moloney murine leukemia virus reverse transcriptase (RT; Life Technologies). The amplification of cDNA by polymerase chain reaction (PCR) was performed using primers for the C1q A chain18 (forward, 5′-ATG GTG ACC GAG GAC TTG TG-3′; reverse, 5′-GTC CTT GAT GTT TCC TGG GC-3′) (276-bp product) and for GAPDH (forward, 5′-ACC ACA GTC CAT GCC ATC AC-3′; reverse, 5′-TCC ACC ACC CTG TTG CTG TA-3′) (500-bp product).
C1q isolation
C1q was isolated from human donor plasma.13 In brief, a protein precipitate was made from recalcified human plasma by adding polyethylene glycol 6000 (3% [wt/vol]; Sigma) and incubating for 1 hour on ice. After centrifugation, the precipitate was dissolved in 2.5 × Veronal-buffered saline (VBS: 1.8 mM Na-5,5-diethylbarbital, 0.2 mM 5,5-diethylbarbituric acid, 145 mM NaCl) containing 10 mM EDTA (ethylenediaminetetraacetic acid) and loaded on an affinity column consisting of Sepharose-coupled human immunoglobulin G (IgG) previously incubated with rabbit IgG directed against human IgG. After washing, the column was eluted using 1 M NaCl in PBS/10 mM EDTA. Peak fractions containing C1q, as assessed by a C1q hemolytic assay, were pooled, concentrated, and dialyzed against PBS containing 4.5 mM EDTA. The solution was applied on a cation exchange Biorex 70 column (Bio Rad Laboratories, Hercules, CA). A salt gradient was applied to elute C1q, and the C1q-containing fractions, determined with a hemolytic assay, were pooled and concentrated. The purity of C1q preparation was approximately 99% as assessed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). C1q was stored at –80° C before use.
ELISA
A specific enzyme-linked immunosorbent assay (ELISA) for the detection of human C1q was developed. ELISA was performed using 96-well Maxisorb plates (Nunc, Roskilde, Denmark). The C1q-specific mAb 220426 (0.5 μg/mL) was incubated in coating buffer (100 mM Na2CO3/NaHCO3, pH 9.6) for 2 hours at 37° C. A blocking step was performed using 3% BSA in PBS for 1 hour at 37° C.
Highly purified serum C1q was used as a standard. After adding samples and incubating for 1 hour at 37° C, purified rabbit IgG antihuman C1q (5 μg/mL)27 was used for 1 hour at 37° C, followed by horseradish peroxidase (HRP)–conjugated goat-antirabbit IgG (Jackson ImmunoResearch, West Grove, PA) for 1 hour at 37° C; all these steps were performed in ELISA buffer (PBS, 1% BSA, 0.05% Tween 20). Each step was followed by 3 washes with PBS/0.05% Tween 20. Enzyme activity was assessed by the addition of ABTS (2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt; Sigma) and H2O2. The optical density (OD) at 415 nm was measured using a microplate biokinetics reader (EL312e; Bio-Tek Instruments, Winooski, VT). The sensitivity of the ELISA was 1 ng/mL.
C1q-ELISA specificity was confirmed by measuring C1q in different sera from healthy donors. Moreover, C1q-depleted sera and sera from 2 C1q-deficient donors emitted no signal in the ELISA. As an additional control, we used normal rabbit IgG instead of rabbit IgG from animals immunized with human C1q; this did not result in a detectable signal in the ELISA. For IL-6 detection, the same ELISA was used as described previously.28
C1q hemolytic assay
For hemolytic assays, sheep red blood cells (SRBCs) were sensitized using rabbit anti-SRBC antibodies (antibody-coated erythrocyte [EA]), as described previously.26 Briefly, EA (1 × 108) were mixed with C1q-depleted human serum (diluted 1:75) in a final volume of 100 μL. Subsequently, 100 μL immDC culture medium or purified C1q was added in different dilutions and incubated at 37° C for 60 minutes with shaking. After adding 1.5 mL PBS followed by centrifugation, hemolysis was assessed by measuring OD at 414 nm. The lytic activity of each condition was expressed in Z values.26
Phagocytosis assay
Jurkat cells were washed with PBS and stained with 5 μM carboxyfluorescein diacetate succinamidyl ester (CFSE; Molecular Probes, Leiden, The Netherlands). After staining, the cells were washed with RPMI 1640 containing 10% FCS and were resuspended in serum-free AIMV culture medium (Gibco). Jurkat cells were opsonized by incubation for 18 hours with 30 μg/mL purified human C1q in the presence of 40 μM etoposide to induce apoptosis, as described.13 Labeled apoptotic cells were incubated with day-7 immDCs for 2 hours at 37° C or 4° C in 250 μL AIMV culture medium (DC/apoptotic cell ratio, 1:1). ImmDCs were labeled with anti–HLA-DR and PE-conjugated goat-antimouse immunoglobulin antibodies (DAKO). Uptake was analyzed by flow cytometry using a FACScan. Data from 104 events were acquired.
Immunofluorescence analysis
Human tonsils were analyzed for DCs and C1q through single and double staining. Cryostat sections of 3 μm were treated with PBS containing 0.9% H2O2 and 1% NaN3 for 20 minutes to block endogenous peroxidase activity, washed, and incubated with mouse antihuman DC-SIGN (AZN-D1; kindly provided by Drs Yvette van Kooyk and Teunis B. H. Geijtenbeek, Free University Medical Center, Amsterdam, the Netherlands),29 mouse antihuman DC-LAMP (kindly provided by Dr Serge Lebecque, Schering-Plough, Dardilly, France),30 or rabbit antihuman C1q.27 After washing, mouse IgG was detected using goat–antimouse-HRP (DAKO), and rabbit IgG was detected using goat–antirabbit IgG-HRP (DAKO). The goat–antimouse-HRP– or goat–antirabbit IgG-HRP–treated sections were then incubated with Tyramide-FITC in Tyramide buffer (NEN–Dupont Research Products, Boston, MA). Double-staining experiments were performed detecting rabbit IgG by goat–antirabbit IgG-TRITC (Nordic, Tilburg, The Netherlands). All incubation steps were performed using PBS/BSA 1% as a buffer for 60 minutes in a dark, humid incubator at room temperature.
Statistical analysis
Data were presented as mean ± SD of duplicate or triplicate analysis, representing experiments with up to 7 independent donors, as indicated. Statistical analysis was performed using one-way analysis of variance (ANOVA) with Bonferroni correction for multiple comparison, 2-way ANOVA, and paired Student t test as indicated. The normal distribution of the data was verified by using the Kolmogorov-Smirnov test. Differences were considered statistically significant at a P value less than .05. Data were analyzed using GraphPad Prism (GraphPad, San Diego, CA) software.
Results
Analysis of C1q in immDCs and macrophages derived from the same donor
DCs and macrophages were generated from peripheral blood monocytes. At day 7, RNA was extracted, and RT-PCR analysis showed abundant expression of C1q in immDCs, comparable to the expression in macrophages (Figure 1A). DCs were phenotypically characterized and showed a typical pattern of CD1a+CD14– (Figure 1B). ImmDCs were CD83– with low expression of CD86 and HLA-DR, typical of the immature stage (data not shown). Using mAb 2204 directed against the globular heads of C1q, immDCs and macrophages showed the expression of a membrane-bound form of C1q (mC1q) on their surfaces (Figure 1B), a feature described earlier for macrophages.18,31
Analysis of C1q production in monocyteand CD34+-derived DCs and macrophages. Day-7 immDCs and macrophages were lysed for mRNA extraction (A) or analyzed using FACS (B). For RT-PCR analysis, specific primers for the C1q A chain were used. While histograms indicate isotype control. (C) Detection of C1q purified from serum using a sandwich ELISA. (D) Day-7 macrophages and immDCs were generated from the same monocyte population of 7 independent donors, and C1q production was analyzed in duplicate in 48-hour culture supernatants. Each line represents the mean C1q production in the 2 cell types of 1 donor. The difference between macrophages and DCs was statistically significant (P = .0035, paired Student t test). (E) Freshly isolated CD34+ cord blood hematopoietic progenitors were cultured for 6 days and then FACS-sorted into CD1a+CD14– and CD1a–CD14+ cells. Sorted cells were then cultured independently for 48 hours. Supernatants were collected (day 8) and used in C1q-specific ELISA. Alternatively, sorted cells were cultured until day 13. At day 13, cells were harvested and then cultured for 48 hours. Supernatants were collected (day 15) and used in C1q-specific ELISA. Results are shown as mean ± SD of an experiment performed in triplicate. Similar results were obtained in 2 different donors. *P < .0001 compared with Langerhans cells; 2-way ANOVA of 2 experiments.
Analysis of C1q production in monocyteand CD34+-derived DCs and macrophages. Day-7 immDCs and macrophages were lysed for mRNA extraction (A) or analyzed using FACS (B). For RT-PCR analysis, specific primers for the C1q A chain were used. While histograms indicate isotype control. (C) Detection of C1q purified from serum using a sandwich ELISA. (D) Day-7 macrophages and immDCs were generated from the same monocyte population of 7 independent donors, and C1q production was analyzed in duplicate in 48-hour culture supernatants. Each line represents the mean C1q production in the 2 cell types of 1 donor. The difference between macrophages and DCs was statistically significant (P = .0035, paired Student t test). (E) Freshly isolated CD34+ cord blood hematopoietic progenitors were cultured for 6 days and then FACS-sorted into CD1a+CD14– and CD1a–CD14+ cells. Sorted cells were then cultured independently for 48 hours. Supernatants were collected (day 8) and used in C1q-specific ELISA. Alternatively, sorted cells were cultured until day 13. At day 13, cells were harvested and then cultured for 48 hours. Supernatants were collected (day 15) and used in C1q-specific ELISA. Results are shown as mean ± SD of an experiment performed in triplicate. Similar results were obtained in 2 different donors. *P < .0001 compared with Langerhans cells; 2-way ANOVA of 2 experiments.
To evaluate the production of soluble C1q at the protein level, a specific ELISA for C1q was developed using a sandwich of monoclonal and polyclonal anti-C1q antibodies (“Materials and methods”). Using highly purified serum C1q, the detection limit of this assay was found to be 1 ng/mL (Figure 1C). Culture supernatants (48 hours) of macrophages and DCs, generated in parallel from the same monocytes, were quantified for C1q content in serial dilutions of the supernatants. ImmDCs produced significantly more C1q (mean, 318.4 ng/mL; range, 100-679 ng/mL) compared with macrophages (mean, 64.6 ng/mL; range, 2-148 ng/mL) (Figure 1D). The C1q production by immDCs was 2.5 to 50 times higher than that of macrophages in 7 different donors.
Analysis of C1q production in CD34+-derived DCs
We next investigated C1q production in DCs generated from CD34+ cord blood progenitors cultured in the presence of GM-CSF and TNF-α. During the first week of culture, 2 subsets of DC precursors developed and were subsequently separated according to CD1a and CD14 expression using FACS.25 Culture of sorted cells for 48 hours showed that Langerhans cell precursors (CD1a+) and interstitial DC precursors (CD14+) had the capacity to produce C1q (Figure 1E). The 2 precursor populations were further cultured to give rise to fully differentiated Langerhans cells and interstitial/dermal DCs. Cells were collected at day 13, washed, and cultured for another 48 hours. Analysis of these supernatants showed that at the final differentiation stage, interstitial/dermal DCs had a significant higher capacity to produce C1q than Langerhans cells (Figure 1E).
Functional analysis of C1q produced by immDCs and phagocytosis experiments
Because C1q produced by some cultured cells, such as fibroblasts,32 is unable to induce activation of the complement cascade, we investigated the functional activity of C1q produced by immDCs in a C1q-dependent hemolytic assay. Adding culture supernatants from 48-hour–cultured immDCs to C1q-deficient serum resulted in lysis of SRBCs sensitized with rabbit anti-SRBC antibody (Figure 2A; analysis in 3 donors), indicating activation of the classical pathway. The hemolytic activity of immDC-C1q was lower than that of purified C1q, as previously described for macrophages.24,33
Functional analysis of C1q produced by DCs and phagocytosis experiments. (A) The hemolytic activity of C1q present in DC culture media was investigated using SRBCs and supernatants derived from immDCs in culture (days 7-9). The amount of C1q quantified in ELISA was, respectively, 6.4 ng/mL (purified C1q), 339 ng/mL (DC I), 103.5 ng/mL (DC II), and 50 ng/mL (DC III). Results are shown as mean ± SD of triplicate analysis of DC supernatants from 3 different donors. *P < .001 compared with medium (ANOVA with Bonferroni correction). (B) 48-hour immDC supernatants were incubated for 2 hours with apoptotic Jurkat cells. Purified C1q (5 μg/mL) and fresh DC medium were used as controls. Binding of C1q was detected by flow cytometry using the mAb 2204. (C) CFSE-stained apoptotic cells were opsonized with purified C1q in serum-free medium overnight. After extensive washing, apoptotic cells were coincubated with immDCs for 2 hours each at 37° C and 4° C (DC/apoptotic cell, 1:1 ratio). As control, apoptotic cells cultured in medium alone were used. ImmDCs were stained with anti–HLA-DR. FACS analysis was performed considering the percentage of HLA-DR+/CFSE+ cells. Data are presented as mean ± SD of an experiment performed in triplicate. *P = .0072 compared with nonopsonized apoptotic cells (Student t test). Similar results were obtained in 4 experiments.
Functional analysis of C1q produced by DCs and phagocytosis experiments. (A) The hemolytic activity of C1q present in DC culture media was investigated using SRBCs and supernatants derived from immDCs in culture (days 7-9). The amount of C1q quantified in ELISA was, respectively, 6.4 ng/mL (purified C1q), 339 ng/mL (DC I), 103.5 ng/mL (DC II), and 50 ng/mL (DC III). Results are shown as mean ± SD of triplicate analysis of DC supernatants from 3 different donors. *P < .001 compared with medium (ANOVA with Bonferroni correction). (B) 48-hour immDC supernatants were incubated for 2 hours with apoptotic Jurkat cells. Purified C1q (5 μg/mL) and fresh DC medium were used as controls. Binding of C1q was detected by flow cytometry using the mAb 2204. (C) CFSE-stained apoptotic cells were opsonized with purified C1q in serum-free medium overnight. After extensive washing, apoptotic cells were coincubated with immDCs for 2 hours each at 37° C and 4° C (DC/apoptotic cell, 1:1 ratio). As control, apoptotic cells cultured in medium alone were used. ImmDCs were stained with anti–HLA-DR. FACS analysis was performed considering the percentage of HLA-DR+/CFSE+ cells. Data are presented as mean ± SD of an experiment performed in triplicate. *P = .0072 compared with nonopsonized apoptotic cells (Student t test). Similar results were obtained in 4 experiments.
Biologically active C1q can bind to apoptotic cells12,13 and enhance the phagocytosis of apoptotic cells by macrophages.34 Therefore, apoptotic Jurkat cells were incubated with the supernatant of immDCs, and C1q binding was analyzed using an anti-C1q mAb.13 C1q in the immDC supernatant and purified C1q from serum showed a specific binding to apoptotic Jurkat cells (Figure 2B).
To assess whether the presence of C1q might influence the phagocytic activity of immDCs, we performed phagocytosis experiments using CFSE-labeled apoptotic cells opsonized with highly purified serum C1q. After 2 hours of coincubation, the degree of phagocytosis was analyzed using flow cytometry. The percentage of CFSE+ cells within the HLA-DR+ DC population was defined as the percentage of phagocytosis. Opsonization of apoptotic cells with C1q (Figure 2C, right) resulted in statistically significantly increased phagocytosis by immDCs compared with nonopsonized apoptotic cells (Figure 2C, left). Control conditions at 4° C did not show significant differences. Effective phagocytosis was confirmed using confocal microscopy (not shown).
Modulation of C1q production and expression during DC maturation
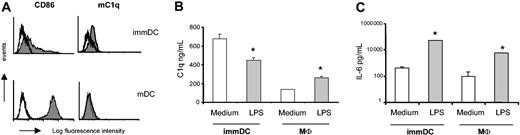
Exposing immDCs to LPS is a known stimulus for DC maturation, as shown by an increased expression of CD86 (Figure 3A), CD83, and HLA-DR (data not shown). Analysis of mC1q showed a decrease in the expression of mC1q on DC maturation (Figure 3A). Activating DCs with LPS also resulted in significantly decreased C1q production in the supernatant (Figure 3B). This decrease was specific for C1q because DCs showed a strong induction of IL-6 production on LPS exposure (Figure 3C). In contrast, activating macrophages with LPS resulted in the increased production of C1q, as described previously (Figure 3B).35
LPS maturation inhibits C1q production and expression in mDCs. Day-7 immDCs and macrophages were cultured for 48 hours in the presence or absence of LPS. (A) Maturation after culture with LPS was confirmed by increased CD86 expression. To detect the mC1q, the mAb 2204 was used. White histograms indicate isotype control. 48-hour supernatants were tested for C1q (B) and IL-6 (C). Data presented (B-C) are mean ± SD of triplicate analysis in 1 donor (representative of 3 donors). *P < .0001 LPS stimulation compared with culture in normal medium (2-way ANOVA of 3 experiments).
LPS maturation inhibits C1q production and expression in mDCs. Day-7 immDCs and macrophages were cultured for 48 hours in the presence or absence of LPS. (A) Maturation after culture with LPS was confirmed by increased CD86 expression. To detect the mC1q, the mAb 2204 was used. White histograms indicate isotype control. 48-hour supernatants were tested for C1q (B) and IL-6 (C). Data presented (B-C) are mean ± SD of triplicate analysis in 1 donor (representative of 3 donors). *P < .0001 LPS stimulation compared with culture in normal medium (2-way ANOVA of 3 experiments).
Next we investigated whether other maturation stimuli, such as TNF-α and CD40L (Figure 4A), were able to modulate C1q expression. As found with LPS, both TNF-α and CD40L down-regulated the expression of mC1q (Figure 4B) (mean reductions of 53%, 46%, and 63%, respectively; n = 7). Finally, we tested the production of C1q by maturing DCs. All 3 stimulatory conditions resulted in marked down-regulations of C1q production of 46%, 36%, and 44% (mean of 7 donors) for LPS, TNF-α, and CD40L, respectively (Figure 4C). Nontransfected L cells, as a control for CD40L, had no effect on C1q production.
Different maturation stimuli inhibit the expression and production of C1q in mDCs. Day 7 immDCs were cultured for 48 hours in DC medium alone or with control L cells or in the presence of stimuli inducing maturation (LPS, TNF-α, or CD40L-transfected L cells). FACS was used to analyze cells for CD86 expression (A). Similar results were obtained for HLA-DR and CD83 expression (not shown). To detect mC1q, mAb 2204 was used (B). Supernatants were harvested (day 9) and tested for C1q using ELISA (C). All data shown are mean ± SD of triplicate analysis from a representative experiment with a single donor. LPS and TNF (*P < .0001) compared with immDCs; CD40L (*P < .0001) compared with L cell–immDCs (2-way ANOVA of 3 independent experiments). (D-E) At the end of 48-hour maturation experiments, mDCs and immDCs were collected, washed, and analyzed using flow cytometry. mDCs derived from culture with LPS, TNF-α (D), or CD40L-expressing cells (E) were then put in a secondary culture in medium only with GM-CSF as a survival factor without maturation stimuli. As a control, immDCs cultured in normal medium or derived from coculture with control L cells were used. Supernatants were harvested at indicated time points and tested for C1q. At the end of 24 hours (D) or 70 hours (E), cell viability was shown to be similar between immDCs and mDCs. Results are representative of at least 3 experiments, performed in duplicate or triplicate, with different donors.
Different maturation stimuli inhibit the expression and production of C1q in mDCs. Day 7 immDCs were cultured for 48 hours in DC medium alone or with control L cells or in the presence of stimuli inducing maturation (LPS, TNF-α, or CD40L-transfected L cells). FACS was used to analyze cells for CD86 expression (A). Similar results were obtained for HLA-DR and CD83 expression (not shown). To detect mC1q, mAb 2204 was used (B). Supernatants were harvested (day 9) and tested for C1q using ELISA (C). All data shown are mean ± SD of triplicate analysis from a representative experiment with a single donor. LPS and TNF (*P < .0001) compared with immDCs; CD40L (*P < .0001) compared with L cell–immDCs (2-way ANOVA of 3 independent experiments). (D-E) At the end of 48-hour maturation experiments, mDCs and immDCs were collected, washed, and analyzed using flow cytometry. mDCs derived from culture with LPS, TNF-α (D), or CD40L-expressing cells (E) were then put in a secondary culture in medium only with GM-CSF as a survival factor without maturation stimuli. As a control, immDCs cultured in normal medium or derived from coculture with control L cells were used. Supernatants were harvested at indicated time points and tested for C1q. At the end of 24 hours (D) or 70 hours (E), cell viability was shown to be similar between immDCs and mDCs. Results are representative of at least 3 experiments, performed in duplicate or triplicate, with different donors.
Fully mature DCs do not produce C1q
Experiments described here showed decreased levels of C1q in supernatants during 48 hours of DC maturation. Therefore, we investigated the capacity of fully mature DCs to produce C1q. DCs were collected 48 hours after the induction of maturation, washed, and put back in culture in fresh medium containing GM-CSF as a survival factor but without maturation stimuli. ImmDCs at day 9 of culture retained the capacity to produce C1q, which became detectable within 2 hours and strongly increased in a 24-hour culture period (Figure 4D). In contrast, mDCs obtained after TNF-α or LPS maturation completely lost the capacity to produce C1q (Figure 4D). Similarly, DCs cultured in the presence of control L cells showed strong C1q production, whereas CD40L-matured DCs did not produce detectable levels of C1q, even after 70 hours of culture (Figure 4E). Lack of C1q production was not the result of a decreased cell viability of mDCs (viability greater than 85% as determined by trypan blue exclusion).
Effect of IFN-α on C1q production by DCs
Recently, it has been demonstrated that proinflammatory factors such as IFN-α can skew monocyte differentiation toward DCs, also inducing DC activation.36,37 Therefore, we investigated the effect of IFN-α on C1q production by DCs. DCs were generated with GM-CSF and IL-4 in the absence or presence of IFN-α. Supernatants collected during the initial culture time (day 5 and day 7) showed a reduced capacity to produce C1q by DCs developing in the presence of IFN-α compared with DCs cultured in normal medium (Figure 5A). At day 7, IFN DCs showed a typical phenotype of mDCs with an increased expression of CD86 and HLA-DR (Figure 5B). Finally, day-7 cells were harvested and, after extensive washing, were put in normal culture medium. DCs generated in the presence of IFN-α were strongly impaired in their capacity to produce C1q, even after 48 hours of culture in normal medium (Figure 5C).
IFN-α inhibits C1q production in DCs inducing DC maturation. DCs were differentiated in regular medium (“Materials and methods”) in the presence or absence of IFN-α. Culture media were collected during culture and tested for C1q (A) (results shown are mean ± SD of 1 of 3 similar experiments [with 3 different donors] performed in triplicate). *P < .0001 compared with immature DCs (2-way ANOVA of 3 experiments). At day 7, DCs were harvested and analyzed using FACS (B). White histograms indicate isotype control. After extensive washing, day-7 DCs differentiated in the absence or presence of IFN-α were cultured in normal DC medium for 48 hours (C). Media were collected at different time points. Results are from triplicate experiments in 1 donor and are representative of those from 3 different donors. Error bars represent SD.
IFN-α inhibits C1q production in DCs inducing DC maturation. DCs were differentiated in regular medium (“Materials and methods”) in the presence or absence of IFN-α. Culture media were collected during culture and tested for C1q (A) (results shown are mean ± SD of 1 of 3 similar experiments [with 3 different donors] performed in triplicate). *P < .0001 compared with immature DCs (2-way ANOVA of 3 experiments). At day 7, DCs were harvested and analyzed using FACS (B). White histograms indicate isotype control. After extensive washing, day-7 DCs differentiated in the absence or presence of IFN-α were cultured in normal DC medium for 48 hours (C). Media were collected at different time points. Results are from triplicate experiments in 1 donor and are representative of those from 3 different donors. Error bars represent SD.
Analysis of DC-SIGN+ and DC-LAMP+ DCs for C1q in situ
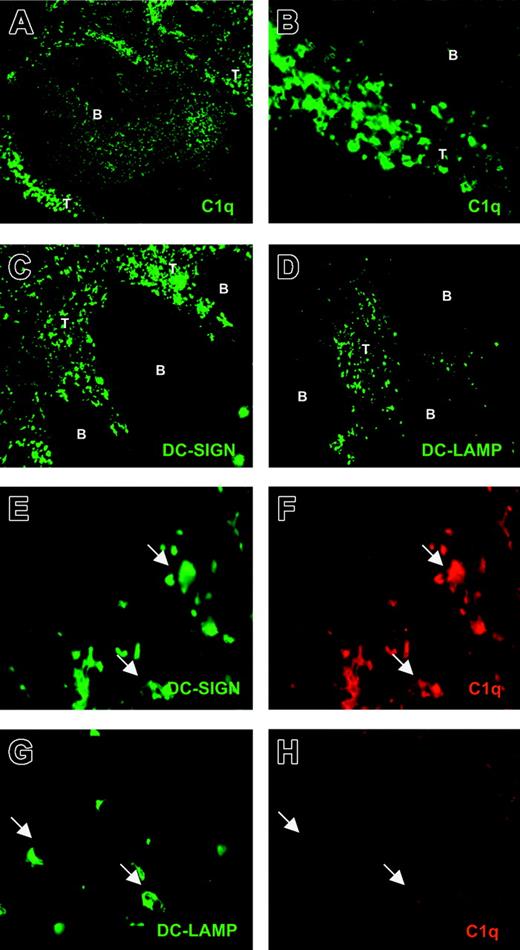
We next investigated whether this dichotomy in C1q expression between immDCs and mDCs also exists in vivo between immature and mature myeloid DCs. Using a rabbit antihuman C1q antibody, we found C1q+ cells localized in the T-cell areas of human tonsil (Figure 6A-B). Moreover, some C1q positivity was observed within the B-cell follicles in a profile resembling the follicular DC network (Figure 6A).38 Two specific DC markers were used to identify DCs in human tonsil sections: DC-SIGN, a C-type lectin expressed in immature and mature myeloid DCs,29,39 and DC-LAMP, a marker of mDCs.30 Although both DC-SIGN+ (Figure 6C) and DC-LAMP+ (Figure 6D) cells were exclusively observed in T-cell areas, as previously demonstrated,29,30 DC-LAMP+ cells were less abundant.
Detection of C1q+ and C1q– DCs in human tonsils. Cryostat tonsil sections were analyzed for DC markers and C1q through single staining. Sections were incubated with rabbit antihuman C1q (A; original magnification, × 100) (B; original magnification, × 400), mouse antihuman DC-SIGN (C; original magnification, × 100), and mouse antihuman DC-LAMP (D; original magnification, × 100), as described in “Materials and methods.” Cryostat tonsil sections were also analyzed through double staining (E-H; original magnifications, × 630). Sections were incubated with mouse antihuman DC-SIGN and rabbit antihuman C1q (E-F, respectively) or mouse antihuman DC-LAMP and rabbit antihuman C1q (G-H, respectively). B indicates B-cell follicle; T, T-cell zone.
Detection of C1q+ and C1q– DCs in human tonsils. Cryostat tonsil sections were analyzed for DC markers and C1q through single staining. Sections were incubated with rabbit antihuman C1q (A; original magnification, × 100) (B; original magnification, × 400), mouse antihuman DC-SIGN (C; original magnification, × 100), and mouse antihuman DC-LAMP (D; original magnification, × 100), as described in “Materials and methods.” Cryostat tonsil sections were also analyzed through double staining (E-H; original magnifications, × 630). Sections were incubated with mouse antihuman DC-SIGN and rabbit antihuman C1q (E-F, respectively) or mouse antihuman DC-LAMP and rabbit antihuman C1q (G-H, respectively). B indicates B-cell follicle; T, T-cell zone.
Double-staining experiments confirmed the occurrence of DC-SIGN+/C1q+ cells within the T-cell area (Figure 6E-F). Moreover, double staining of DC-LAMP and C1q showed the presence of DC-LAMP+ but C1q– DCs (Figure 6G-H), indicating the occurrence of mDCs that lacked C1q.
Discussion
The present study demonstrates that, together with macrophages, human DCs are a rich source of functionally active C1q. Importantly, C1q production is a specific feature of immDCs because fully mature DCs almost completely lack the ability to produce C1q. Moreover, we demonstrated that DC differentiation in the presence of IFN-α leads to the suppression of C1q synthesis by DCs. Finally, we found DC-SIGN+/C1q+ and DC-LAMP+/C1q– DCs in human tonsils.
In contrast to other components of the complement system, which are mainly liver derived, the production of C1q seems to be extrahepatic.32,40 The relevant role of bone marrow–derived cells in C1q synthesis has been shown recently because bone marrow transplantation from C1q+/+ mice into C1q–/– mice was sufficient to restore the normal serum levels of the protein.19 Notably, macrophages have been shown to produce significant amounts of C1q.24,41 It has recently been suggested that immDCs secrete detectable amounts of C1q in supernatants.20 Here we generated DCs and compared them with macrophages derived from the same donors. Our in vitro analysis showed that, on a per cell basis, DCs are more potent than macrophages as C1q producers. These results indicate that immDCs may be considered an additional important source of C1q.
In humans, at least 3 different subsets of DCs can be recognized. These subsets are characterized by different cell surface receptors and functional capacities, and they include interstitial DCs, Langerhans cells, and plasmacytoid DCs.42 Therefore, we have also investigated interstitial DCs and Langerhans cells generated from CD34+ cord blood progenitors.25 At the precursor level, both subsets produced low amounts of C1q. However, we found that in fully differentiated DCs, interstitial DCs produced significantly more C1q than Langerhans cells. Finally, microarray analysis has also shown the presence of C1q mRNA in plasmacytoid DCs, albeit at low abundance compared with myeloid DCs (F. Briere, personal written communication, June 2003). Given that C1q seems to play a role in the uptake of antigen and apoptotic cells,34 this differential expression of C1q in DC subsets might be analogous to the different expression of C-type lectins, present on specific DC subsets, that are also involved in antigen uptake.43
Next we investigated the regulation of C1q during DC activation. Contrary to macrophages, for which LPS induced an increased release of C1q, the production was clearly suppressed in mDCs. Maturation by TNF-α and CD40L had the same effect. Moreover, fully mature DCs were unable to produce C1q, even up to 70 hours after the removal of stimuli. These in vitro findings are in agreement with previously obtained microarray data. Using monocyte-derived DCs, strongly increased expression of the C1q B chain was found in DCs compared with monocytes.44 Similarly, using mouse D1 cells, all 3 chains of C1q were expressed in immature D1 cells and were strongly suppressed on LPS maturation.45 We found a similar down-regulation for the membrane-associated form of C1q on DCs, which has been previously described in macrophages.18 Membrane-bound C1q is thought to be oriented with exposure of the globular heads exhibiting the same binding capacity of released C1q (eg, Fc-binding activity).18,31
Using specific human DC markers, we investigated the occurrence of C1q+ DCs in situ. Previous reports showed that next to follicular DCs, interdigitating cells with a dendritic morphology stained positively for C1q in rat spleen.38 Similar staining was observed in mouse lymphoid tissue.46 However, these studies lacked specific DC markers. Recently, we identified CD11c+, C1q+ DCs in murine kidney (L.A.T., manuscript in preparation). Here we show the presence of DC-SIGN+/C1q+ DCs in human tonsils, indicating the occurrence of DCs with the capacity to produce C1q in vivo. Interestingly, using double staining with the specific maturation molecule DC-LAMP,30 we demonstrated the presence of DC-LAMP+ but C1q– DCs. Therefore, it seems that the in vivo maturation of DCs is also accompanied by the inhibition of C1q production.
Experiments described in this article suggest that under physiologic conditions, immDCs have a role in the production of C1q, a process that might be altered under pathologic conditions. Several factors can skew monocyte differentiation toward DCs or macrophages.37,47 IFN-α, a potent immunostimulatory cytokine, has been shown to differentiate monocytes into activated DCs.36,37 Here we show that IFN-α acts as a potent inhibitory factor of C1q synthesis on differentiating DCs. Therefore, it is tempting to speculate that this activity of IFN-α might contribute to the low levels of C1q frequently observed in SLE,48 a disease in which IFN-α is thought to play a pathogenic role.49-51
As mentioned, C1q–/– mice are characterized by an accumulation of apoptotic cells in tissue, and impaired clearance was demonstrated in peritoneal macrophages.16,17 C1q has been demonstrated to bind apoptotic cells and blebs.12,13,52 In vitro studies have shown the importance of C1q and the calreticulin–CD91 receptor complex for the uptake of apoptotic material.34 This has led to the waste disposal hypothesis.21 According to this hypothesis, as a consequence of impaired clearance by macrophages, apoptotic cells accumulate, become engulfed by DCs, and are presented as autoantigens by DCs to activate autoreactive T cells.21 However, this model is now extended by our findings that immDCs also produce active C1q that increases the uptake of apoptotic cells by immDCs. In recent years, it has become clear that DCs are critical not only for the induction of immunity but, in their immature form, for their tolerogenic properties.3 In addition, activation products of complement, such as iC3b, have been implicated in the increased phagocytosis by DCs and the induction of a tolerogenic phenotype.53,54 Therefore, it is tempting to speculate that the absence of C1q in primary or secondary deficiencies might influence local immDC functions.
In conclusion, we have shown that functionally active C1q can be strongly produced by immDCs. In contrast, mDCs completely lack the capacity to produce C1q. This is reflected in vivo by the presence of DC-SIGN+/C1q+ and DC-LAMP+/C1q– DCs in human tonsils. Finally, we identified IFN-α as a potent inhibitory factor for C1q production by DCs. These results suggest a potential role for C1q in DC biology.
Prepublished online as Blood First Edition Paper, January 15, 2004; DOI 10.1182/blood-2003-09-3046.
Supported by grants from the European Doctorate in Biotechnologies (departmental grant), University of Bari; the Dutch Kidney Foundation (PC95, C98.1763, PC139); the Dutch Organization for Scientific Research (901-12-094); and the European Union (QL61-CT-2002-01215).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.




![Figure 5. IFN-α inhibits C1q production in DCs inducing DC maturation. DCs were differentiated in regular medium (“Materials and methods”) in the presence or absence of IFN-α. Culture media were collected during culture and tested for C1q (A) (results shown are mean ± SD of 1 of 3 similar experiments [with 3 different donors] performed in triplicate). *P < .0001 compared with immature DCs (2-way ANOVA of 3 experiments). At day 7, DCs were harvested and analyzed using FACS (B). White histograms indicate isotype control. After extensive washing, day-7 DCs differentiated in the absence or presence of IFN-α were cultured in normal DC medium for 48 hours (C). Media were collected at different time points. Results are from triplicate experiments in 1 donor and are representative of those from 3 different donors. Error bars represent SD.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/103/10/10.1182_blood-2003-09-3046/6/m_zh80100461560005.jpeg?Expires=1763675577&Signature=RLjkZhDH7G8XL-CZsUQIVJrgoNDw2Brmqntd4xNgWXQgn3nKgeiuExGJzCXVfTG99OEkz~oWql6gdfXUzERn9F-lvtFBbJTBmIy~69daSVWgwwdE7xv45WIQ4ujExbiXSKsPEyCWvfrkp9Kaa2F5Tet8kRhZx3t1~WgANUoOmwefhU9Z3gJFPRXtlebOW4BoHVSnJsAHDwsLRW6ldBFPW~oeCJIl3WXg2wdTDeWbrBp~ecacP813WUyl5gJnJSWWul3CCpKgbgNTqK3ste7UeNxV~jOjcJOKUD4xTQCld3a-YGjHYcv1iD9prO4LOon0FX1Mtkyc1VcIFAjlCpueAg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal