Abstract
We report here the identification of angiopoietin-related growth factor (AGF) as a positive mediator for angiogenesis. To investigate the biologic function of AGF in angiogenesis, we analyzed the vasculature in the dermis of transgenic mice expressing AGF in mouse epidermal keratinocytes (K14-AGF). K14-AGF transgenic mice were grossly red, especially in the ears and snout, suggesting that hypervascularization had occurred in their skin. Histologic examination of ear skin from K14-AGF transgenic mice revealed increased numbers of microvessels in the dermis, whereas the expression of several angiogenic factors, such as basic fibroblast growth factor (bFGF), vascular endothelial growth factors (VEGFs), and angiopoietin-1 (Ang-1), was decreased. We showed that AGF is a secreted protein and does not bind to tyrosine kinase with immunoglobulin and EGF-homology domain (Tie1) or Tie2 receptors. An in vitro chamber assay revealed that AGF directly promotes chemotactic activity of vascular endothelial cells. Both mouse corneal and matrigel plug assays showed that AGF induces neovascularization in vivo. Furthermore, we found that plasma leakage occurred after direct injection of AGF into the mouse dermis, suggesting that AGF directly induces a permeability change in the local vasculature. On the basis of these observations, we propose that AGF is a novel angiogenic factor and that handling of its biologic functions could lead to novel therapeutic strategies for control of angiogenesis.
Introduction
Normal tissue function requires an adequate supply of oxygen and nutrition through blood vessels. Therefore, maintenance of vascular system is critical for survival of tissues. In the case of repair or remodeling of tissues after injury, angiogenesis, which is the formation of new blood vessels from pre-existing primary plexus, plays a crucial role in reconstructing the vascular system.1-4 In addition, angiogenesis is involved in responding directly to tissue demands in pathologic conditions such as inflammation, rheumatoid arthritis, diabetic retinopathy, and tumor growth.5 Recently, several growth factors regulating angiogenesis have been reported. Among them, vascular endothelial growth factors (VEGFs), angiopoietins (Angs), and ephrins, all of which are ligands for receptor tyrosine kinases, have been identified to play pivotal roles in angiogenesis in a coordinated manner.6
Angiopoietin-1,-2,-3, and -4 (Ang-1, Ang-2, Ang-3, and Ang-4)7,8 are ligands for the receptor tyrosine kinase TIE2,9,10 which contributes to signaling in angiogenesis.11 Recently, several Angs have been identified as angiopoietin-related proteins (ARPs), which are structurally similar to Angs. Angs and ARPs are characterized structurally by 2 domains, a coiled-coil domain and a fibrinogen-like domain.6,12-18 It is noteworthy that all reported ARPs do not bind to either the TIE1 or TIE2 receptor, suggesting that ARPs have biologic functions different from Angs. Indeed, several recent reports demonstrate that ARPs show pleiotropic effects, not only on vascular cells but on other lineages. For example, angiopoietin-like protein 3 (Angptl3) and fasting-induced adipose factor (FIAF)/proliferator-activated receptor gamma angiopoietin-related (PGAR)/ARP4/Angptl4 may play central roles in lipid/adipocyte metabolism as well as in angiogenesis.13-17
We recently cloned and characterized an ARP designated angiopoietin-related growth factor (AGF) (GenBank accession numbers: AB054065 for mouse; AB054064 for human) that functions as a growth factor for epidermal keratinocytes.18 AGF is identical to an ARP, which was named as an Angptl6/ARP5 (human, AF230330), but its function was unreported. In this study, we have focused our investigation on the role of AGF in endothelial cells, because other ARPs have several functions in angiogenesis.
At first, we investigated vascularization in skin tissues of transgenic mice overexpressing mouse AGF in epidermal basal keratinocytes of the skin (K14-AGF)18 to determine whether AGF promotes in vivo angiogenesis as does Ang-1. K14-AGF transgenic mice show markedly increased numbers of microvessels in the skin, suggesting that AGF promotes angiogenesis. Furthermore, we prepared AGF protein, and our in vitro and in vivo analysis reveals that AGF directly promotes chemotactic activity of endothelial cells and that its biologic function could lead to in vivo neovascularization. On the basis of this finding, we report that AGF is a novel angiogenic-promoting factor.
Materials and methods
Purification of recombinant AGF protein
The coding region of AGF fused at the amino terminus to the FLAG epitope was subcloned into pCEP4 (Invitrogen, Groningen, the Netherlands). HEK293 cells were cultured at 37° C in humidified 5% CO2 air in Dulbecco modified Eagle medium (DMEM) (Sigma, St Louis, MO) supplemented with 10% fetal bovine serum (FBS) (JRH Bioscience, Lenexa, KS) and transfected with pCEP4-AGF with the use of Fugene-6 (Roche Diagnostics, Mannheim, Germany). After transfection of pCEP4-AGF, HEK293 cells were selected with 300 μg/mL Hygromycin B (GIBCO-BRL, Gaithersburg, MD) for 5 days, and the conditioned medium was collected and filtered with a 0.22 μm pore size filter (Millipore, Bedford, MA). To purify AGF protein, the filtered conditioned medium was transferred to an anti-FLAG antibody (M2) affinity gel (Sigma), and only AGF protein was trapped in a gel. After the gel was washed with phosphate-buffered saline (PBS), protein was eluted by adding Gly-HCl (pH 3.0) and immediately neutralized with Tris (tris(hydroxymethyl)aminomethane)–HCl (pH 8.0). The protein was visualized by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) with Coomassie brilliant blue staining (CBB) (Wako, Osaka, Japan) and Western blot analysis with the use of a horseradish peroxidase–conjugated anti-FLAG antibody (M2) (1:200) (Sigma) and an anti-AGF antibody, which was produced by immunizing rabbits with a synthetic peptide corresponding to amino acids 202 through 217 of mouse AGF (NTSRRLDQTPEHQREQ).18
Mice
To analyze whether AGF plays a role in angiogenesis, we examined the vasculature in the dermis of transgenic mice expressing AGF in the epidermis under control of a keratinocyte-specific promoter, K14 (K14-AGF transgenic mice).18 Mice were housed in environmentally controlled rooms of the Laboratory Animal Research Center of Keio University (Tokyo, Japan) under the guidelines of Keio University for animal and recombinant DNA experiments.
Lectin staining for whole mounts of ear skin
Investigation for the density and morphology of microvessels in lectin-stained whole mount of ear skin was performed as described elsewhere.17,19 In brief, after anesthesia with sodium pentobarbital, mice were injected from tail vein with fluorescein-labeled Lycopersicon esculentum lectin (Vector Laboratories, Burlingame, CA), which binds uniformly to the luminal surface of endothelial cells and adherent leukocytes. Removed lectin-stained ears from 5 K14-AGF transgenic mice and their controls were separated from the cartilage. Whole mounts of ear skin were analyzed by a fluorescence microscope (Olympus, Tokyo, Japan). We investigated the vessel length, the number of vessel joints, and the density of vessels to estimate how angiogenesis occurred. The vessel length was measured quantitatively as pixel counts, and the actual number of vessel joints was counted with the Kurabo angiogenesis image analyzer (Kurabo, Osaka, Japan)20 in 4 different fields per each ear.
Mitogenic assay
Mitogenic activity of purified AGF was assessed by the ability to stimulate incorporation of 3H-labeled thymidine into human umbilical vein endothelial cells (HUVECs) (BioWhittaker, Walkersville, MD). HUVECs were plated on collagen-coated 96-well plates (Becton Dickinson Labware, Bedford, MA) at a density of 5 × 103 cells per well in 100 μL endothelial growth media-2 (EGM-2) (Clonetics, San Diego, CA) containing 0.5% FBS. After HUVECs were grown, cells were made quiescent by an 18-hour incubation in M199 medium (GIBCO-BRL) containing 0.1% FBS. Subsequently, cells were washed twice with serum-free medium and incubated in fresh medium containing 0.1% FBS and various concentrations of AGF with or without VEGF (10 ng/mL) (Pepro Tech EC, London, England) for 24 hours. Next, HUVECs were incubated with 3H-labeled thymidine for 4 hours. After an additional 4 hours' incubation, mitogenic activity was assessed by measuring uptake of 3H-labeled thymidine. Medium was removed, and cells were harvested onto 96-well filter plates by means of a sample harvester (FilterMate) (PerkinElmer Life Sciences, Boston, MA). The plates were then dried at 65° C for 15 minutes, sealed after the addition of 40 μL scintillation mixture (Microscint O) (PerkinElmer Life Sciences) per well, and counted on a microplate scintillation counter (Topocount; PerkinElmer Life Sciences). Each experiment was performed in triplicate.
Preparation of cDNA from skin tissues of mice, quantitative RT-PCR, and microarray analyses
DNase-treated total RNA preparations were reverse transcribed by means of the ThermoScript II reverse transcription kit (Invitrogen), followed by real-time polymerase chain reaction (PCR) with an SYBR green PCR Master Mix (Applied Biosystems, Tokyo, Japan). The oligonucleotides used for PCR are listed in Table 1. The levels of PCR products were monitored with an ABI PRISM 7700 sequence detection system and analyzed with ABI PRISM 7700 SDS software (Applied Biosystems). Thermal cycling consisted of denaturation for 10 minutes at 95° C, followed by 50 cycles of 15 seconds at 95° C and 1 minute at 60° C. The adjustment of baseline and threshold was performed according to the manufacturer's instructions. The relative abundance of transcripts was normalized to the constitutive expression level of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) RNA. For the Affymetrix (Santa Clara, CA) microarray analysis, hybridizations were carried out with the use of RNA extracted from ear skins isolated from 2 individual K14-AGF transgenic mice lines and from control mice. Biotin-labeled cRNAwas prepared by means of the Enzo BioArray HighYield RNA Transcript Labeling Kit (Affymetrix), and the unincorporated nucleotides were removed by means of Rneasy columns (Qiagen, Valencia, CA). Mouse U74Av2 arrays were hybridized, washed, stained, and scanned by means of a GeneChip fluidics station 400 (Affymetrix) and a GeneArray scanner (Hewlett-Packard, Palo Alto, CA). The readings from the quantitative scanning were analyzed by the Affymetrix Microarray Suite, version 5.0. For the comparisons, the hybridization intensities were calculated with the use of a global scaling intensity of 100.
Oligonucleotide sequences for PCR
Gene (GenBank accession no.) . | Position, nt . | Primer sequence . |
|---|---|---|
| AGF (AB054065) | 918 to 1089 | 5′-TCGTGTAGTAGCCGTGTGGTGT-3′ |
| 5′-CACCTGATGCACAGGTTCCA-3′ | ||
| bFGF (NM_008006) | 163 to 213 | 5′-AGCGACCCACACGTCAAACT-3′ |
| 5′-CACAACTCCTCTCTCTTCTGCTTG-3′ | ||
| VEGF (NM_009505) | 180 to 230 | 5′-GGAGAGCAGAAGTCCCATGAAGT-3′ |
| 5′-TCGCTGGTAGACGTCCATGA-3′ | ||
| VEGF12021 | 5′-CATAGAGAGAATGAGCTTCCTACAGC-3′ | |
| 5′-CCTTGGCTTGTCACATTTTTC-3′ | ||
| VEGF164 (NM_009505) | 464 to 529 | 5′-ATGTGAATGCAGACCAAAGAAAGA-3′ |
| 5′-CGCTCTGAACAAGGCTCACAG-3′ | ||
| VEGF18821 | 5′-GGTTTAAATCCTGGAGCGTTCA-3′ | |
| 5′-CAGGAACATTTACACGTCTGCG-3′ | ||
| PIGF (NM_008827) | 719 to 809 | 5′-ACGACAAAGGCAGAAAGGAGG-3′ |
| 5′-GGAAATGACATCACGGGTGG-3′ | ||
| Ang-1 (NM_009640) | 587 to 677 | 5′-CTCAGTGGCTGCAAAAACTTGA-3′ |
| 5′-GTGTGGTTTTGAACAGCATTCTG-3′ | ||
| GAPDH (NM_008084) | 119 to 222 | 5′-AAAGTGGAGATTGTTGCCAT-3′ |
| 5′-TTGACTGTGCCGTTGAATT-3′ |
Gene (GenBank accession no.) . | Position, nt . | Primer sequence . |
|---|---|---|
| AGF (AB054065) | 918 to 1089 | 5′-TCGTGTAGTAGCCGTGTGGTGT-3′ |
| 5′-CACCTGATGCACAGGTTCCA-3′ | ||
| bFGF (NM_008006) | 163 to 213 | 5′-AGCGACCCACACGTCAAACT-3′ |
| 5′-CACAACTCCTCTCTCTTCTGCTTG-3′ | ||
| VEGF (NM_009505) | 180 to 230 | 5′-GGAGAGCAGAAGTCCCATGAAGT-3′ |
| 5′-TCGCTGGTAGACGTCCATGA-3′ | ||
| VEGF12021 | 5′-CATAGAGAGAATGAGCTTCCTACAGC-3′ | |
| 5′-CCTTGGCTTGTCACATTTTTC-3′ | ||
| VEGF164 (NM_009505) | 464 to 529 | 5′-ATGTGAATGCAGACCAAAGAAAGA-3′ |
| 5′-CGCTCTGAACAAGGCTCACAG-3′ | ||
| VEGF18821 | 5′-GGTTTAAATCCTGGAGCGTTCA-3′ | |
| 5′-CAGGAACATTTACACGTCTGCG-3′ | ||
| PIGF (NM_008827) | 719 to 809 | 5′-ACGACAAAGGCAGAAAGGAGG-3′ |
| 5′-GGAAATGACATCACGGGTGG-3′ | ||
| Ang-1 (NM_009640) | 587 to 677 | 5′-CTCAGTGGCTGCAAAAACTTGA-3′ |
| 5′-GTGTGGTTTTGAACAGCATTCTG-3′ | ||
| GAPDH (NM_008084) | 119 to 222 | 5′-AAAGTGGAGATTGTTGCCAT-3′ |
| 5′-TTGACTGTGCCGTTGAATT-3′ |
nt indicates nucleotide.
In vivo neovascularization using Matrigel
Preparation, injection, and processing of Matrigel (Becton Dickinson, San Jose, CA) were performed as previously described with some modification.22 Briefly, 8-week-old C57BL/6 mice were injected subcutaneously with 0.4 mL Matrigel containing 40 U/mL heparin (Sigma) and 10 μg/mL AGF. At 5 days later, the mice were killed, and the Matrigel plugs were removed, weighed, and processed for histology or hemoglobin concentration determination. For histologic analysis, plugs were fixed in 4% paraformaldehyde, embedded in polyester wax, and sectioned at 8 μm. The sections were stained with Giemsa or 1:500 diluted fluorescein isothiocyanate (FITC)–conjugated anti–platelet endothelial cell adhesion molecule 1 (anti–PECAM-1) antibody (Pharmingen, San Diego, CA). For hemoglobin determination, plugs were homogenized in 1 mL distilled water. Hemoglobin concentration was determined by a Drabkin reagent kit (Sigma).
Statistics
Data are expressed as means ± standard deviation (SD). Statistical analysis was conducted with the Student t test. Statistical significance was defined as P values less than .05.
Results
K14-AGF mice show marked increases in the number of microvessels
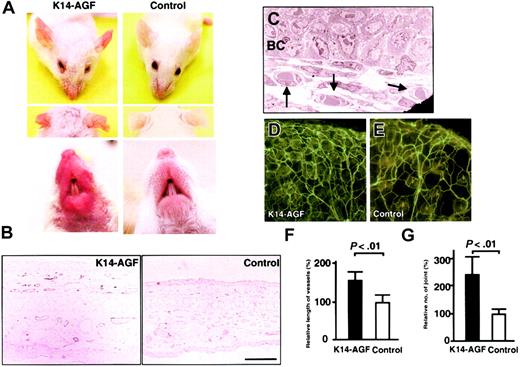
K14-AGF mice exhibited reddish ears, snouts, and soles of feet compared with control mice, suggesting enhanced angiogenesis in these skin tissues (Figure 1A; also data not shown). Immunohistochemical staining with an anti–PECAM-1 antibody directly revealed an increased number of PECAM-1+ endothelial cells in the dermis, indicating enhanced angiogenesis in K14-AGF transgenic mice (Figure 1B). Furthermore, an electron microscopic analysis of ear skin revealed that an increased number of microvessels were capillary-sized vessels with pericytes surrounding endothelial cells (Figure 1C). Lectin staining in K14-AGF transgenic mice also showed significant increases in the density of capillary-like vessels (Figure 1D-E). We analyzed the vessel length and the number of vessel joints as a quantitative estimation for angiogenesis by angiogenesis image analyzer. Both vessel length and the number of vessel joints in K14-AGF mice revealed a significant increase compared with controls, indicating an increase in the density of capillary-like vessels in K14-AGF mice (Figure 1F-G).
Increased number of microvessels in K14-AGF transgenic mice. (A) Gross appearance of the K14-AGF mouse and a control showing that the skin of ears and snout of K14-AGF mice are red compared with controls. (B) Immunohistochemical analysis with anti–PECAM-1 antibody of ear skin from K14-AGF mouse and a control. Increased PECAM-1+ microvessels (purple) are detected in the dermis and subcutaneous layers of K14-AGF mouse. Bar indicates 100 μm. (C) Electron microscopic analysis (original magnification, × 1700) shows that increased vessels are capillary-sized (arrows) in the K14-AGF mouse. BC indicates epidermal basal cells. (D-E) Representative photograph of blood vessels in the ear from the K14-AGF mouse (D) and controls (E) stained with fluorescein-labeled Lycopersicon esculentum lectin. Abundant capillary-sized vessels in the K14-AGF mouse are detected. (F-G) Quantitative analysis for the number of vessels shown in panels D and E. Length of vessels (F) and number of vessel joints (G) in K14-AGF transgenic mice (▪) relative to controls (□) are shown as percentages. Columns represent mean values + SD (n = 5).
Increased number of microvessels in K14-AGF transgenic mice. (A) Gross appearance of the K14-AGF mouse and a control showing that the skin of ears and snout of K14-AGF mice are red compared with controls. (B) Immunohistochemical analysis with anti–PECAM-1 antibody of ear skin from K14-AGF mouse and a control. Increased PECAM-1+ microvessels (purple) are detected in the dermis and subcutaneous layers of K14-AGF mouse. Bar indicates 100 μm. (C) Electron microscopic analysis (original magnification, × 1700) shows that increased vessels are capillary-sized (arrows) in the K14-AGF mouse. BC indicates epidermal basal cells. (D-E) Representative photograph of blood vessels in the ear from the K14-AGF mouse (D) and controls (E) stained with fluorescein-labeled Lycopersicon esculentum lectin. Abundant capillary-sized vessels in the K14-AGF mouse are detected. (F-G) Quantitative analysis for the number of vessels shown in panels D and E. Length of vessels (F) and number of vessel joints (G) in K14-AGF transgenic mice (▪) relative to controls (□) are shown as percentages. Columns represent mean values + SD (n = 5).
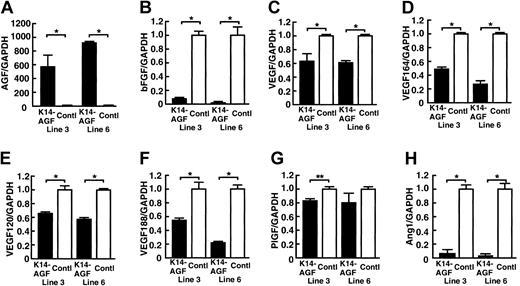
Expression of VEGF and bFGF significantly decrease in K14-AGF mice
As shown in Figure 1B, we observed a markedly thickened epidermal layer in K14-AGF mice compared with controls.18 VEGF and basic fibroblast growth factor (bFGF) are powerful angiogenesis-promoting factors,4,6,23,24 and both are secreted from epidermal keratinocytes.25,26 On the basis of these findings, we speculated that increased numbers of microvessels in skin tissues could have been induced by increased amounts of angiogenic factors, such as VEGF and bFGF derived from thickened epidermis in K14-AGF mice. To investigate this possibility, we examined mRNA levels of several angiogenic factors in skin tissues. Unexpectedly, quantitative reverse-transcription PCR (RT-PCR) analysis revealed a marked decrease in expression of bFGF, total VEGF, VEGF120, VEGF164, VEGF188, and Ang-1 in K14-AGF mice compared with controls (Figure 2). Moreover, to examine the possibility that AGF stimulated keratinocytes to release other angiogenic factors, we compared the expression of angiogenic factors in ear skin tissues of K14-AGF mice and their controls by a microarray analysis. We found that there was no increased angiogenic factor in the expression level in K14-AGF mice (see Table S1; click the Supplemental Data Set link at the top of the online article on the Blood website), suggesting that AGF from epidermis directly affects endothelial cells and promotes angiogenesis in skin tissues of K14-AGF mice.
Quantitative RT-PCR analysis of genes encoding angiogenic factor. Comparison of mRNA encoding AGF (A), bFGF (B), VEGF (C), VEGF164 (D), VEGF 120 (E), VEGF 188 (F), placental-derived growth factor (PlGF) (G), and Ang-1 (H) from ear tissues of two 8-week-old K14-AGF transgenic mice (lines 3 and 6; ▪) and their littermate controls (control; □). Columns represent mean values ± SD (n = 3). The relative abundance of transcripts was normalized to the constitutive expression level of GAPDH mRNA. *P < .001; **P < .01, relative to controls.
Quantitative RT-PCR analysis of genes encoding angiogenic factor. Comparison of mRNA encoding AGF (A), bFGF (B), VEGF (C), VEGF164 (D), VEGF 120 (E), VEGF 188 (F), placental-derived growth factor (PlGF) (G), and Ang-1 (H) from ear tissues of two 8-week-old K14-AGF transgenic mice (lines 3 and 6; ▪) and their littermate controls (control; □). Columns represent mean values ± SD (n = 3). The relative abundance of transcripts was normalized to the constitutive expression level of GAPDH mRNA. *P < .001; **P < .01, relative to controls.
AGF is a secreted protein and does not bind to Tie1 or Tie2 receptors
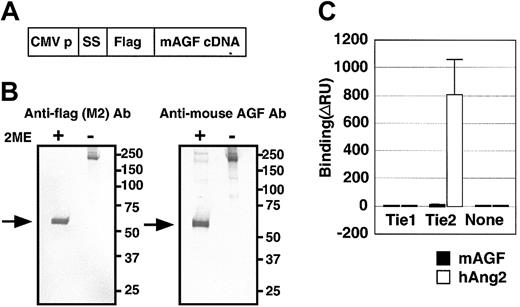
To investigate the biochemical activity of AGF directly, we generated AGF protein fused at the amino terminus to the FLAG epitope (Figure 3A).
AGF and TIE receptors. AGF does not bind to tyrosine kinase with immunoglobulin and EGF-homology domain (Tie) receptors. (A) Schematic representation of the plasmid construction used to generate FLAG-AGF protein. CMV-p and SS indicate the cytomegalovirus promoter and a signal sequence, respectively. (B) After transfection of HEK293 cells with a mouse AGF cDNA with a 5′-terminal extension encoding a FLAG-tag (panel A), mouse AGF-FLAG fusion protein was detected in culture supernatants by Western blot analysis with an anti-FLAG antibody (left) and an antimouse AGF antibody (right), with or without 2-mercaptoethanol (2-ME). Lanes contain approximately 10 ng purified protein. Arrows indicate the monomer of FLAG-AGF protein. (C) Ties are not receptors for AGF. BIAcore binding assay of AGF (200 ng) to the Tie1 and Tie2 receptors. As a positive control, human Ang-2-6xHis-tagged protein (200 ng) specifically bound to the Tie2-Fc protein (460 ng), but not to the Tie1-Fc (460 ng). Error bar represents mean ± SD.
AGF and TIE receptors. AGF does not bind to tyrosine kinase with immunoglobulin and EGF-homology domain (Tie) receptors. (A) Schematic representation of the plasmid construction used to generate FLAG-AGF protein. CMV-p and SS indicate the cytomegalovirus promoter and a signal sequence, respectively. (B) After transfection of HEK293 cells with a mouse AGF cDNA with a 5′-terminal extension encoding a FLAG-tag (panel A), mouse AGF-FLAG fusion protein was detected in culture supernatants by Western blot analysis with an anti-FLAG antibody (left) and an antimouse AGF antibody (right), with or without 2-mercaptoethanol (2-ME). Lanes contain approximately 10 ng purified protein. Arrows indicate the monomer of FLAG-AGF protein. (C) Ties are not receptors for AGF. BIAcore binding assay of AGF (200 ng) to the Tie1 and Tie2 receptors. As a positive control, human Ang-2-6xHis-tagged protein (200 ng) specifically bound to the Tie2-Fc protein (460 ng), but not to the Tie1-Fc (460 ng). Error bar represents mean ± SD.
Hydrophobic sequences characteristic of secretory signal sequences and coiled-coil domains were found in the amino-terminal region of AGF, suggesting that AGF is secreted and oligomerized. SDS-PAGE (data not shown) and Western blot analysis (Figure 3B) of AGF-transfected HEK293 cells revealed a major mouse AGF product larger than 50 kDa detected in the culture medium in reducing conditions (with 2-ME). In contrast, in nonreducing conditions (without 2-ME), AGF oligomerized, as do other proteins containing a coiled-coil motif such as Angs.6,7,8,27 We found that AGF, like other ARPs, does not bind to either Tie1 or Tie2 receptors using a BIAcore system28 (Figure 3C), indicating that AGF acts on endothelial cells through a different receptor.
AGF directly promotes chemotactic activity, but not growth of endothelial cells
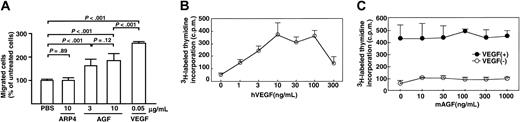
Given the marked increased in number of microvessels seen in K14-AGF mice, we examined whether AGF directly promotes chemotaxis of the mouse brain capillary endothelial cell line bEND3 endothelial cells by a migration assay.17 We found that AGF promoted migration of bEND3 cells through a microchemotaxis membrane, while PBS alone and a member of the ARP family, ARP4, did not (Figure 4A). These in vitro findings show that AGF promotes the chemotactic activity of endothelial cells.
Effect of AGF on growth and migration of endothelial cells. AGF induces endothelial cell migration, but does not affect the growth of endothelial cells. (A) First, 3 or 10 μg/mL AGF, 10 μg/mL ARP4, or 0.05 μg/mL VEGF was added to the lower chamber of the membrane. For each of these, 3 × 104 mouse bEND3 cells per well were inoculated into the upper chamber of a transwell and were incubated for 4 hours at 37° C. The number of endothelial cells migrating from the upper to the lower chamber were counted. Columns represent mean values ± SD (n = 5). (B-C) Incorporation of 3H-labeled thymidine in HUVECs was evaluated as mitogenic activity. In panel B, confluent monolayers of HUVECs were made quiescent for 18 hours and then treated with various concentrations of VEGF. First, 1 μCi (0.037 MBq) 3H-labeled thymidine was added to each well. After a 4-hour incubation, mitogenic activity was assessed by measuring the uptake of 3H-labeled thymidine as counts per minute (cpm). Panel C shows the assessment of mitogenic activity of AGF for endothelial cells. Confluent monolayers of HUVECs were made quiescent for 18 hours and then treated with various concentrations of AGF with (•) or without (○) 10 ng/mL VEGF. The data are presented as mean values ± SD.
Effect of AGF on growth and migration of endothelial cells. AGF induces endothelial cell migration, but does not affect the growth of endothelial cells. (A) First, 3 or 10 μg/mL AGF, 10 μg/mL ARP4, or 0.05 μg/mL VEGF was added to the lower chamber of the membrane. For each of these, 3 × 104 mouse bEND3 cells per well were inoculated into the upper chamber of a transwell and were incubated for 4 hours at 37° C. The number of endothelial cells migrating from the upper to the lower chamber were counted. Columns represent mean values ± SD (n = 5). (B-C) Incorporation of 3H-labeled thymidine in HUVECs was evaluated as mitogenic activity. In panel B, confluent monolayers of HUVECs were made quiescent for 18 hours and then treated with various concentrations of VEGF. First, 1 μCi (0.037 MBq) 3H-labeled thymidine was added to each well. After a 4-hour incubation, mitogenic activity was assessed by measuring the uptake of 3H-labeled thymidine as counts per minute (cpm). Panel C shows the assessment of mitogenic activity of AGF for endothelial cells. Confluent monolayers of HUVECs were made quiescent for 18 hours and then treated with various concentrations of AGF with (•) or without (○) 10 ng/mL VEGF. The data are presented as mean values ± SD.
Next, by evaluating the incorporation of thymidine in endothelial cells, we examined whether AGF affects growth of endothelial cells. We found that AGF did not increase the proliferation of HUVECs (Figure 4C). We found that VEGF, as a positive control, stimulated the proliferation of HUVECs (Figure 4B). In this culture system, we found that the most effective concentration of VEGF to support HUVEC growth was 10 ng/mL. We examined whether AGF affects VEGF-induced proliferation of endothelial cells. AGF had no synergistic effect on VEGF-induced proliferation of endothelial cells (Figure 4C).
AGF induces vascular leakiness
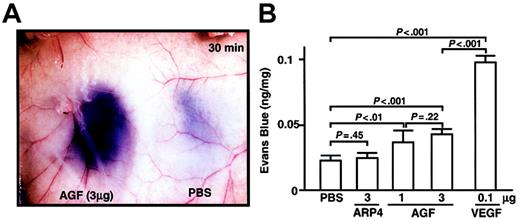
As we found that K14-AGF mice show edematous thickened ears and readily leak plasma from blood vessels,18 we examined, by means of a vascular permeability assay, whether injection of AGF directly induces vascular leakiness.17,19,29 We injected AGF into the dermis of the mice, which had been pretreated with an intravenous injection of Evans Blue dye. Significant leakage of blue dye occurred around the site of the AGF injection (Figure 5A). Quantitative analysis revealed that AGF induces vascular leakiness, but to a lesser extent than with VEGF (Figure 5B). These findings support the idea that AGF directly induces a permeability change in the local vasculature.
AGF induction of vascular leakage. For a vascular permeability assay, male 8-week-old BALB/c mice were anesthetized, and 100 μL 1% Evans blue dye was injected into the tail vein. At 5 minutes after injection of dye, AGF (1 μg or 3 μg), ARP4 (3 μg), VEGF (0.1 μg), or PBS was injected intradermally at adjacent locations on the back skin. (A) A representative result of dye leakage in the back skin 30 minutes after injection of AGF (3 μg, left) and PBS (right) is shown. Original magnification, × 8.4. (B) Vascular leakage is measured spectrophotometrically by Evans blue content of back skin 30 minutes after intradermal injection. Columns represent mean values ± SD (n = 5).
AGF induction of vascular leakage. For a vascular permeability assay, male 8-week-old BALB/c mice were anesthetized, and 100 μL 1% Evans blue dye was injected into the tail vein. At 5 minutes after injection of dye, AGF (1 μg or 3 μg), ARP4 (3 μg), VEGF (0.1 μg), or PBS was injected intradermally at adjacent locations on the back skin. (A) A representative result of dye leakage in the back skin 30 minutes after injection of AGF (3 μg, left) and PBS (right) is shown. Original magnification, × 8.4. (B) Vascular leakage is measured spectrophotometrically by Evans blue content of back skin 30 minutes after intradermal injection. Columns represent mean values ± SD (n = 5).
AGF promotes in vivo neovascularization
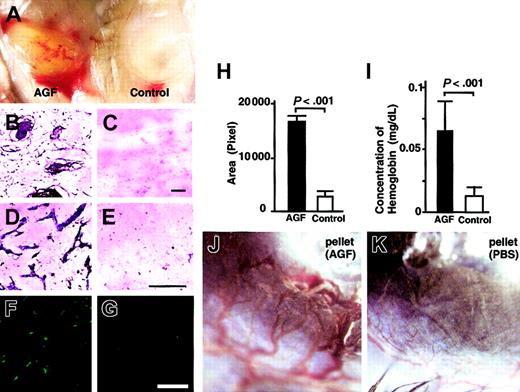
To confirm the hypothesis that AGF induces angiogenesis through promoting chemotactic activity of endothelial cells, we examined whether AGF promotes in vivo neovascularization using a Matrigel plug assay (Figure 6A-I) and a mouse corneal assay (Figure 6J-K). At 5 days after the start of a Matrigel plug assay, Matrigel including 4 μg AGF was grossly yellow, suggesting that it contained many hematopoietic cells (Figure 6A, right). In contrast, Matrigel without factors was white, suggesting the absence of hematopoietic cells (Figure 6A, left). In sections, Giemsa staining confirmed that many microvessels and hematopoietic cells migrated in Matrigel containing AGF, but not in Matrigel without AGF (Figure 6B-E). We showed PECAM-1+ endothelial cells in Matrigel containing AGF (Figure 6F-G). Next, we confirmed the increased number of PECAM-1+ endothelial cells using an angiogenesis image analyzer (Figure 6H). Figure 6I shows the hemoglobin content normalized to the weight of the Matrigel plugs. Matrigel including AGF also contained more hemoglobin compared with Matrigel only. On the basis of these findings, we concluded that AGF induced blood vessels in Matrigel.
AGF induces in vivo neovascularization. AGF induces angiogenesis in an in vivo Matrigel assay (A-I). (A) Matrigel containing AGF (left) or PBS only (right) was subcutaneously injected near the abdominal midline of 8-week-old C57BL/6 mice. Gross appearance of Matrigels on day 5. (B-G) Histologic analysis of sections from Matrigels. Cells in Matrigels were visualized by staining with May-Giemsa (B-E). Endothelial cells were visualized by staining with anti–PECAM-1 antibody (F-G). Scale bars indicate 100 μm. (H) Black (Matrigel containing AGF) and white (Matrigel only) columns represent the area of vessels, which was measured quantitatively by means of an image analyzer. The data are presented as mean values ± SD (n = 5). (I) Values represent the concentration of hemoglobin per Matrigel plug weight ± SD for 5 assays. (J-K) AGF induces angiogenesis in a corneal neovascularization assay (n = 5). Gross appearance of neovascularization in the cornea. Pellets containing AGF (J) induced neovascularization, while pellets containing control buffer (K) did not.
AGF induces in vivo neovascularization. AGF induces angiogenesis in an in vivo Matrigel assay (A-I). (A) Matrigel containing AGF (left) or PBS only (right) was subcutaneously injected near the abdominal midline of 8-week-old C57BL/6 mice. Gross appearance of Matrigels on day 5. (B-G) Histologic analysis of sections from Matrigels. Cells in Matrigels were visualized by staining with May-Giemsa (B-E). Endothelial cells were visualized by staining with anti–PECAM-1 antibody (F-G). Scale bars indicate 100 μm. (H) Black (Matrigel containing AGF) and white (Matrigel only) columns represent the area of vessels, which was measured quantitatively by means of an image analyzer. The data are presented as mean values ± SD (n = 5). (I) Values represent the concentration of hemoglobin per Matrigel plug weight ± SD for 5 assays. (J-K) AGF induces angiogenesis in a corneal neovascularization assay (n = 5). Gross appearance of neovascularization in the cornea. Pellets containing AGF (J) induced neovascularization, while pellets containing control buffer (K) did not.
To confirm the effect of AGF on in vivo vascular formation, we used a corneal neovascularization assay.17,22 In this assay, AGF (3 μg) induced neovascularization in the mouse cornea (Figure 6K), whereas a pellet containing PBS alone did not promote neovascularization (Figure 6L). In the section, we confirmed that a pellet containing AGF induced blood vessels composed of PECAM-1+ endothelial cells in cornea and that a pellet containing PBS alone did not (data not shown).
Discussion
In this report, we show that K14-AGF transgenic mice exhibit increased numbers of microvessels in their skin tissues. Moreover, we found that AGF directly increases chemotactic activity of endothelial cells by in vitro migration assay. Finally, we demonstrated that AGF directly induces in vivo neovascularization. Taking these results together, we propose that AGF is a novel factor promoting angiogenesis.
Recently, we found that AGF is a growth factor for epidermal cells and that K14-AGF transgenic mice show a thickened epidermal layer.18 Epidermal keratinocytes are sources for angiogenic factors, such as VEGF and bFGF. To clarify whether increased number of microvessels in skins of K14-AGF transgenic mice is a direct effect of AGF or is due to other angiogenic factors derived from thickened epidermal layer, we examined the level of expression of growth factors related to angiogenesis in skin tissues from K14-AGF mice. Quantitative RT-PCR analysis revealed that VEGF, bFGF, and Ang-1 were significantly decreased compared with controls, while AGF mRNA levels were markedly increased in skin tissues of K14-AGF transgenic mice. In addition, by microarray analysis, we found that there was no other angiogenic factor in which expression was increased in K14-AGF mice. On the basis of these observations, we speculated that increased angiogenesis in K14-AGF mice was induced by AGF. We did not find increased levels of AGF in the circulation of K14-AGF mice compared with controls. These findings indicate that AGF from keratinocytes induces neovascularization only in skin tissues of K14-AGF mice.
The AGF-mediated effects demonstrated here—namely, chemotactic activity of endothelial cells and vascular leakiness—are similar to effects mediated by VEGF; however, the effect of AGF is not so potent as VEGF. In addition, AGF, like Ang-1, does not induce proliferation of endothelial cells, while VEGF exerts a powerful growth-stimulating activity on endothelial cells.6 In addition, AGF had no effect on VEGF-induced proliferation of endothelial cells. However, it is noteworthy that increased numbers of microvessels in K14-AGF transgenic mice were detected, whereas the expression of several angiogenic factors, such as VEGF, bFGF, and Ang-1, was decreased in skin tissues of K14-AGF transgenic mice. On the basis of these observations, we speculate that AGF promotes in vivo angiogenesis independently. In addition, decreased expression of VEGF, bFGF, and Ang-1 in skin tissues of K14-AGF transgenic mice evoke the speculation that AGF and these angiogenic factors act in angiogenesis with complementarity.
It is demonstrated that VEGF shows pleiotropic effects through the VEGF receptor–2 not only on vascular cells but on cells of other lineages, such as osteoclasts.30,31 VEGF does not stimulate proliferation of osteoclasts, but induces their chemotactic activity, whereas both growth and chemotaxis of endothelial cells are increased by VEGF. We have found that AGF promotes proliferation of epidermal keratinocytes,18 while in endothelial cells only chemotactic activity is affected by AGF. Taken together, these observations indicate that the functions of angiogenesis-promoting factors are dependent on cell type.
Critical to understanding the function of a novel ligand are identification and characterization of its cognate receptor. The fibrinogen-like domain at the C terminus25 of Angs is a binding site for the TIE2 receptor. AGF also contains this domain, suggesting that AGF could be a ligand for either TIE1 or TIE2. However, we show here that AGF, like other ARPs, does not bind to either immobilized TIE1-Fc or TIE2-Fc protein.12,13 A recent report demonstrated that ANGPTL3 binds to integrin αvβ3 on endothelial cells and induces endothelial cell migration and adhesion.13 AGF contains the peptide sequence RGD, which is an adhesive target sequence for many integrins, suggesting the possibility that an integrin may function as an AGF receptor. Definitive identification of an AGF-specific receptor is essential to determine the precise role of AGF.
In this study, we show that AGF has several functions in endothelial cells in addition to its effects on keratinocytes.18 These observations lead us to ask whether AGF acts on vascular cells in physiologic or pathologic conditions or in both. Recently, we found that the expression of AGF was induced in wound-healing sites. Furthermore, rapid repair of wounding in K14-AGF mice was observed.18 Moreover, we observed that AGF is expressed in platelets/megakaryocytes and mast cells, which sequentially arrive at wounding sites. Taken together with previous reports that angiogenesis is a critical component in the wound-healing process,1,2 AGF derived from infiltrated hematopoietic cells, such as platelets or mast cells, affects both vascular cells and keratinocytes, and thereby plays a role in the wound-healing process. AGF is also abundantly expressed in liver and is detected in circulation. Further investigation of the action of circulating AGF on endothelial cells and other cell types is ongoing in loss-of-function studies in the mouse. Those analyses will not only define the function of AGF but could lead to novel therapeutic strategies relating to angiogenesis and regenerative medicine.
Prepublished online as Blood First Edition Paper, February 5, 2004; DOI 10.1182/blood-2003-04-1272.
Supported by Grants-in-Aid for Scientific Research on Priority Areas from the Ministry of Education, Culture, Sports, Science and Technology of Japan; a research grant from the Yamanouchi Foundation for Research on Metabolic Disorders; a grant-in-aid from the Tokyo Biochemical Research Foundation; and a research grant from Human Frontier Science Program Organization.
The online version of the article contains a data supplemement.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Ms Kana Fukushima for experimental assistance.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal