Abstract
The Stroke Prevention Trial in Sickle Cell Anemia (STOP) was a randomized multicenter controlled trial comparing prophylactic blood transfusion with standard care in sickle cell anemia (SCA) children aged 2 to 16 years selected for high stroke risk by transcranial Doppler (TCD). More than 2000 children were screened with TCD to identify the 130 high-risk children who entered the randomized trial. A total of 5613 TCD studies from 2324 children were evaluated. We also collected information on stroke. We describe the changes in TCD with repeated testing and report the outcome without transfusion in the STOP screened cohort. Risk of stroke was higher with abnormal TCD than with normal or conditional TCD (P < .001) or inadequate TCD (P = .002), and risk with conditional TCD was higher than with normal TCD (P < .001). Repeated TCD in 1215 children showed that the condition of 9.4% of children became abnormal during observation. Younger patients and those with higher initial flow velocities were most likely to convert to abnormal TCDs. Screening in STOP confirmed the predictive value of TCD for stroke. Substantial differences in the probability of conversion to abnormal TCD were observed, with younger children and those with higher velocity more likely to have an abnormal TCD with rescreening.
Introduction
Cerebrovascular disease is one of the most serious complications of sickle cell anemia (SCD).1 Cerebral infarction typically results from occlusion or stenosis of large arteries supplying the brain.2,3 Elevated blood flow velocity measured by transcranial Doppler (TCD) is a powerful predictor of stroke due to SCD.4,5 The stroke risk from SCD goes up in direct proportion to increasing time-averaged mean of the maximum velocity (TAMMvel) in the distal internal carotid artery (dICA) or proximal middle cerebral artery (MCA).4,5 On the basis of TCD risk stratification, we began the randomized Stroke Prevention Trial in Sickle Cell Anemia (STOP) in 1994. This trial confirmed the ability of TCD to predict stroke (the risk of stroke in the standard-care arm was 10% per year over the 30 months, about 15 to 20 times that of unselected children with SCD) and demonstrated that regular blood transfusions reduce first stroke by greater than 90%.6
Approximately 2000 children were screened with TCD at the 14 STOP study centers between 1995 and 1996. More than 200 high-risk patients were identified, of whom 130 were randomized to receive either periodic blood transfusions or standard care. After enrollment was completed and for the duration of the study (1996-2000), we collected data on the occurrence of stroke or death in all screened subjects. Some of these children underwent repeated TCD testing in an ancillary study. The purpose was to examine the stroke risk relative to the TCD findings in this large cohort and determine the rate of conversion from low-risk to high-risk TCD during a defined observation period.
Patients and methods
Subjects
A cohort of children aged 2 to 16 years with confirmed hemoglobin SS or Sβ0 thalassemia was screened with TCD. Individuals shown to have an increased stroke risk with screening TCD were randomly assigned to receive standard medical care or regular blood transfusions designed to keep the sickle hemoglobin (Hb S) below 30% of total hemoglobin. Children with prior clinical stroke were excluded, but those with subclinical lesions on magnetic resonance imaging (MRI) were included. Additional exclusion criteria included other indications or contraindications for chronic transfusion, human immunodeficiency virus infection, treated seizure disorder, pregnancy, or a serum ferritin level greater than 500 ng/mL. The institutional review board at each participating study site approved the study. The trial design is described in detail elsewhere.7
Recruitment and TCD screening for STOP occurred from February 1995 to November 1996, and stroke surveillance continued until June 2000. All randomized patients (except 1) were followed for stroke until June 2000, and we attempted to make yearly contact with all children who had been screened with TCD to assess stroke and vital status. From November 1996 until November 1999, TCD screening was offered as an ancillary study to patients not enrolled in the main trial in order to examine rates of conversion from low-risk to high-risk states over time. All STOP centers participated in this ancillary study and used TCD methods comparable to those of the initial TCD screen. In the TCD ancillary study, investigators were encouraged to enroll and restudy younger SCD patients (we assumed that these children would be more likely to change TCD risk category) and to restudy those with conditional TCD during initial screening (see the following paragraph). However, any screened patient or any new patient who met original STOP screening criteria was also eligible for the ancillary study. The rationale for including new patients was that these children were likely to be no different from those already screened and their inclusion presented an opportunity to expand knowledge of temporal changes in TCD. The ancillary study also provided a way for clinics to screen children who were not available during the screening phase and to get high-quality, consistent TCD data of potential clinical interest on these children while the STOP study was in progress.
TCD ultrasound. Each study center used a TCD standard protocol developed for children with SCD, and examiners were trained for this protocol with identical equipment and software (2-MHz pulsed Doppler; Nicolet EME TC 2000; Nicolet, Madison, WI).6,7 The TCD examiner moved the sample volume depth in 2-mm increments to record the highest TAMMvel and the clearest waveform profile in these arterial segments: from the right and left temporal approaches, the MCA at 3 or more points along the artery separated by 2 mm; the distal ICA; the anterior cerebral artery (ACA) and posterior cerebral artery (PCA); and the basilar artery from the suboccipital approach. Each TCD was interpreted at the Medical College of Georgia (Augusta, GA) without knowledge of its source and categorized by TAMMvel values as normal (all recordings lower than 170 cm/s); conditional (at least 170 but lower than 200 cm/s); abnormal (TAMMvel of at least 200 cm/s in either the ICA or MCA); or inadequate. The highest TAMMvel on either side in an allowable segment was used to categorize the study. If velocities in the MCA and ICA were lower than 170 cm/s but velocities in the ACA, PCA or basilar artery were at least 170 cm/s, the study was also classified as conditional. Unless one side was clearly abnormal, a study that did not provide readings from right and left MCA/ICA areas was classified as inadequate. Conditional studies were further subdivided as follows: Conditional 2A studies were those in which MCA/ICA velocities on one or both arteries were between 170 and 199 cm/s; conditional 2B studies were those in which MCA/ICA velocities on both sides were lower than 170 cm/s but the posterior or basilar artery on one or both sides was at least 170 cm/s; and conditional 2C were studies in which the MCA/ICA velocities on both sides were no more than 170 cm/s, the PCA and basilar arteries were lower than 170 cm/s, but the ACA on one or both sides was at least 170 cm/s.
Determination of stroke. The STOP protocol attempted to capture all neurological events, whether reported acutely or discovered on quarterly evaluation. Primary endpoints were cerebral infarction and intracranial hemorrhage (ICH). Focal symptoms consistent with cerebral infarction or ICH were required unless the presentation suggested subarachnoid hemorrhage. The MRI after an event was compared with the baseline MRI for changes consistent with stroke. In the absence of new MRI findings, persistent neurological abnormalities were classified as a stroke, as were transient symptoms in the face of a new MRI lesion appropriate to the patient's clinical presentation. Two neurologists blinded to randomization status independently determined whether an event was a stroke, with a third neurologist available in cases of disagreement. One neuroradiologist, blinded to the anatomic area of clinical suspicion, examined the baseline and event MRI studies for new lesions.
When a possible stroke was reported in a TCD-screened patient who was not randomized, a single neurologist (E.S.R.) examined the medical records related to the event, including hospital admission or clinic visit reports, neurological consultations, and radiology reports, and categorized the episode as a stroke or other neurological event.
Data analyses
Stroke. Stroke-free survival was calculated as a function of either the baseline TCD TAMMvel alone or all TCD TAMMvel data prior to the stroke by classifying the patient's stroke risk on the basis of the TCD with the highest category recorded at any time prior to the stroke. In all cases, the TAMMvel used to categorize the study result was from either the MCA or ICA, and not the anterior, posterior cerebral, or basilar arteries. The highest TCD velocity was not always the last study prior to stroke, and time was analyzed from the first abnormal TCD rather than when the highest velocity was recorded. In addition, the baseline and highest TAMMvel were used as continuous predictors in a separate analysis. For that analysis, time was started from the TCD with the highest TAMMvel, not necessarily the first abnormal TCD. Patients who died were censored at the time of death and those who were lost to follow-up were censored at the point at which their stroke status was last known. For randomized patients, strokes were categorized as ischemic or intracranial hemorrhagic on the basis of the STOP reports of the MRI/MRA Reading Panel. For nonrandomized patients, computed tomography (CT) and/or MRI written reports from the centers were used.
A 2-sample rank test (Wilcoxon rank sum) was used to compare age in children with hemorrhage and those with ischemic stroke and for comparing the timing between first and second TCD in children who initially had abnormal TCDs with those from other result groups. The comparisons of stroke risk were made by means of the log-rank statistic.8
Conversion to abnormal TCD. In addition to stroke, the second outcome of interest was conversion of TCD from low- or intermediate-risk categories (or inadequate, in which risk is unknown) to the high-risk (abnormal) category. The occurrence and timing of TCD rescreening were not mandated, but the protocol suggested early rescreening of children with abnormal (2 to 4 weeks) or conditional/inadequate studies (2 to 12 weeks) and rescreening at longer intervals (6 to 9 months) for children with normal TCD. Whether and when a patient had a repeated TCD was also affected by compliance, prior TCD results, and whether a patient had the first TCD early or late in the study screening period. In the ancillary TCD study, the timing of the follow-up TCD was not specified.
Repeated TCD studies were used to estimate the probability of later development of a first abnormal TCD (conversion) after an initial study that was normal or borderline. The investigators evaluated predictor variables for conversion, including age at time of first TCD, absolute TCD velocity, and interval from initial to subsequent TCD testing. Patient data were censored if a stroke or decision to give a patient a transfusion for any reason took place. Because only those patients who (1) had more than one TCD study, (2) were not found to have abnormal TCD at baseline, and (3) did not have a stroke prior to subsequent TCD could contribute to this analysis, this group was a subset of the larger sample. A Weibull regression model was used to evaluate the time to an abnormal TCD examination. Follow-up time was defined as the time from the initial normal or conditional TCD examination to either the time of an abnormal TCD or the end of follow-up. Patients who developed abnormal TCDs were considered interval-censored since the exact time of conversion to abnormal was unknown. Patients who did not have a subsequent abnormal examination were considered right-censored at the last examination. For this analysis, TCD was categorized as normal (lower than 170 cm/s), low conditional (170 to 184 cm/s in the MCA or ICA) and high conditional (185 to 199 cm/s in the MCA or ICA) in an effort to better define transition from low to high risk.
Results
TCD testing
A total of 2324 children (2018 during the first phase of the study and 306 additional patients during the ancillary study) underwent 5613 TCD examinations. Baseline results were as follows: normal, 1558 (67%), mean velocity of 133 ± 19.32 cm/s; conditional, 409 (17.6%), mean velocity of 177 ± 13.33 cm/s; abnormal, 217 (9.3%), mean velocity of 224 ± 26.76 cm/s; inadequate, 140 (6%). Overall follow-up time was 48 ± 18 months (range, 0.2-67 months). The timing of repeated TCDs was, as expected, influenced by prior results. Mean separations in months from first to second TCD by initial result category were as follows: normal, 12 ± 9 months (median, 8.5); conditional, 5 ± 7 months (median, 2.1); abnormal, 2.5 ± 5 months (median, 0.95); and inadequate, 8.7 ± 12 months (median, 3.4; P < .001, abnormal different from other result groups). Children entered by the ancillary study were slightly younger (7.3 years mean versus 8.2 years) than those screened for the trial, but the distribution of TCD findings was approximately the same as in the original screened group: 11% versus 10% with abnormal; 20% versus 19% with conditional; 70% versus 72% with normal, indicating that, with respect to stroke risk, these patients were not different from the larger group.
A change from normal, conditional, or inadequate TCD to abnormal TCD was infrequent: 1% converted to abnormal at the second examination and 1.5% changed to high-risk status from the second to the third examination. No patient whose third TCD was normal was abnormal at the fourth examination. Abnormal examinations were in some instances followed by normal TCD: 2 (4%) of 50 patients whose first examination was abnormal had a normal second examination; 3 (8%) of 38 whose second examination was abnormal had a normal third examination, and 3 (15%) of 20 patients whose third examination was abnormal had a normal fourth TCD. Changes in TCD were not due to transfusion, as a child's data were censored at the start of chronic transfusion. We do not have data on hydroxyurea use, and it is possible that the TCD results in some children may have been influenced by this treatment.
Seventy-six children had 2 abnormal TCDs, but were not enrolled in the randomized trial. Of these, 9 had stroke; 23 at some point went on transfusion before stroke; 8 were lost to follow-up after the second TCD; and the remainder had further follow-up of varying lengths of time.
Stroke
There were 64 children who had stroke: 10 (16%) with intracranial hemorrhage and 54 (84%) with ischemic infarction. Eighteen of the 64 strokes were from the randomized trial (16 with cerebral infarction and 2 with intracranial hemorrhage). One of the hemorrhages occurred before randomization, and this case does not contribute to further analyses (which refer to 9 hemorrhages). The other hemorrhage in the randomized group was a subarachnoid hemorrhage. Forty-six others who were TCD-screened but not randomized had stroke. Diagnosis was based on history and examination and confirmatory tests (both MRI and CT, n = 23; MR only, n = 15; CT only, n = 7; and autopsy n = 1). Eight of these were intracranial hemorrhages (4 subarachnoid hemorrhages, 2 intraparenchymal hemorrhages, 1 mixed intracranial-subarachnoid hemorrhage, 1 epidural hematoma with venous infarction); 1 intraparenchymal cerebellar hemorrhage was fatal. The remaining 38 children had nonfatal ischemic strokes.
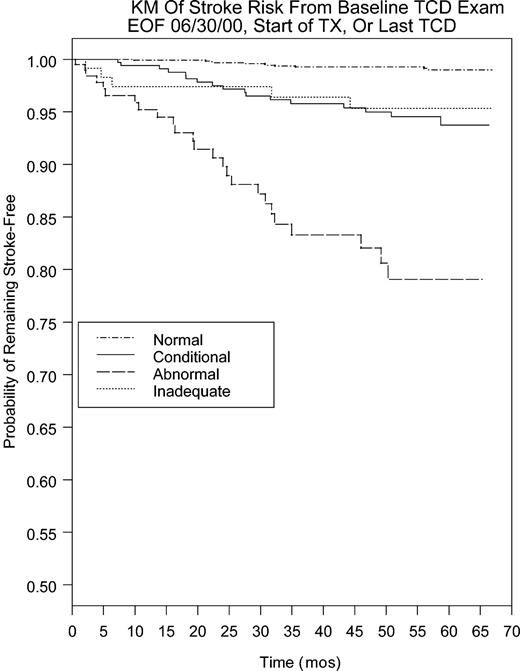
Stroke was highly correlated with TCD. The relationship of stroke to TCD was assessed by the stroke-free survival on the basis of either the baseline (or first) TCD (Figure 1), or in cases of multiple studies, the TCD that showed the highest result any time before stroke. Six of the 64 stroke cases did not contribute to survival analysis as their data were censored because they started transfusion prior to stroke (5) or were enrolled after stroke (1). The 5 patients with stroke after censoring for transfusion were as follows: the single case of stroke in the transfusion arm of STOP (last TCD abnormal 26 months prior to stroke); a patient randomized to transfusion but who discontinued treatment 14 months prior to stroke (last TCD abnormal 8 months prior to stroke); 2 patients randomized to standard care who adopted clinical transfusion after trial closure yet had stroke 31 and 28 months after starting transfusion (last adequate TCD was abnormal in both cases, and the last performed TCD inadequate 9 and 11 months prior to stroke); and 1 nonrandomized case who went on transfusion 9 months prior to stroke for unclear reasons (last TCD abnormal 26 months prior to stroke).
Kaplan-Meier plot of stroke risk after baseline TCD to either end of follow-up, start of chronic transfusion. or June 2000.
Kaplan-Meier plot of stroke risk after baseline TCD to either end of follow-up, start of chronic transfusion. or June 2000.
Risk of stroke with abnormal TCD is much higher than with normal (P < .001), conditional (P < .001), or inadequate TCD (P = .002) examination results. Risk with conditional but not inadequate TCD is higher than with normal TCD (P < .001), regardless of whether the baseline or highest TCD is used. If the baseline TCD is used, 11 (19%) of 58 stroke cases had a normal TCD, 17 (29%) had conditional, 5 (9%) had inadequate TCD, and 25 (43%) had an abnormal TCD. Of the 5 cases that had stroke after starting transfusion, 4 had persistent high velocities despite treatment. Case 1 was the single case of stroke in the transfusion arm of STOP (maximum velocity [Vmax], 225 cm/s at 9 months prior to stroke). Case 2 was a child randomized to transfusion but who discontinued transfusion after trial closure and 14 months before stroke. This patient's TCD had dropped to normal with transfusion but reverted to abnormal (Vmax was 221 cm/s at 8 months prior to stroke) after cessation of therapy. The data are censored because stroke took place after the patient had been started on transfusion even though the child was not on transfusion at the time of stroke. Two children randomized to the standard-care arm switched to transfusion after trial closure 28 and 31 months before stroke. The last Vmax was 222 cm/s recorded 14 months prior to stroke in the first case and 254 cm/s 29 months prior to stroke in the second case. A never-randomized patient started transfusion 9 months prior to stroke; the last Vmax was 223 cm/s recorded 26 months prior to stroke.
If the highest TCD is considered, 9 (19%) of 53 had a normal TCD (5 cases did not contribute because they had only inadequate TCD, and no velocity could be determined); 12 (23%) had a conditional, while 31 (58%) had an abnormal TCD. Using the highest velocity shows primarily that some of the children with initial TCD in the conditional range later have abnormal TCD. The highest maximum TAMMvel (excluding those with inadequate examinations) was also a significant stroke predictor (P < .0001) when used as a continuous variable. For each 10 cm/s increase in TCD TAMMvel, the risk of stroke increased by 29.3%.
Children with hemorrhage (n = 9) were older than those with ischemic stroke (n = 49) at the time of stroke, although the difference was not statistically significant (11.4 ± 3.4 versus 8.7 ± 3.8 years; P = .055). Hemorrhages were less clearly predicted by TCD than ischemic stroke. Although there were only 9 hemorrhages, which limits the strength of the analysis, if one considers hemorrhages alone the baseline TCD result category did not predict hemorrhage. However, baseline velocity used as a continuous variable demonstrated that higher velocity was associated with greater hemorrhage risk (P = .0027) but the association was less robust than for ischemic stroke (P < .001).
Stroke in a child with a recent normal TCD was relatively uncommon. Of the 46 children with stroke who were not in the randomized portion of the study, 13 had a TCD within 12 months before the stroke. One patient had a normal TCD, while the others had abnormal (n = 8), conditional (n = 3), or inadequate (n = 2) TCD results. A total of 21 other children with stroke had TCD between 12 and 24 months prior to the stroke; 7 of these (2 with hemorrhage) had a normal TCD, 5 were conditional, 5 were abnormal, and 1 study was inadequate. Only 8 of these 35 children had a normal TCD in the 24 months prior to stroke (3 of these had velocities greater than 150 cm/s), while 15 children had abnormal studies. Fourteen of these 15 children with recent abnormal TCD had ischemic stroke. Of the 18 randomized patients who had a stroke at any time in the trial or follow-up study, the last TCD prior to stroke was abnormal in all cases, regardless of the treatment at the time of the event.
Time to stroke in relation to velocity
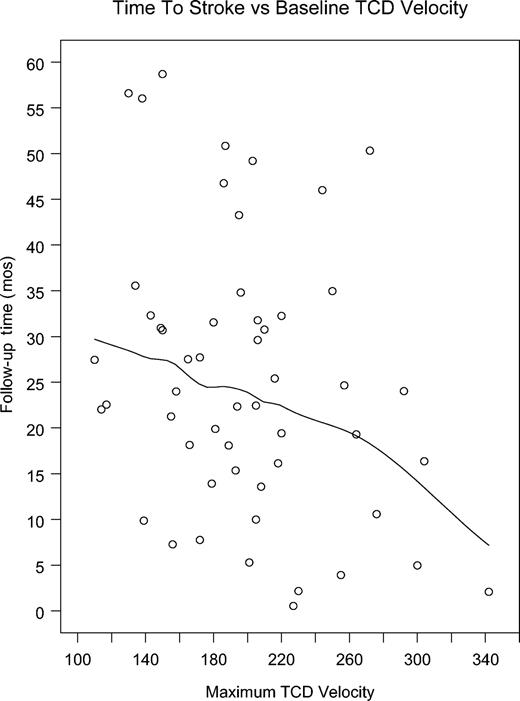
We compared the absolute TCD velocity in the 34 patients whose last TCD was abnormal prior to stroke with the latency in months from the TCD to the stroke. There was a wide range of velocities, from 201 cm/s to 343 cm/s, and stroke occurred from 3 days to 34.5 months after TCD. Four patients had a stroke less than a month after TCD, with velocities of 202, 210, 218, and 343 cm/s. The patient who was observed for 34.5 months before stroke had TCD velocity of 244 cm/s, and the second longest latency of 32 months was seen in a child with TCD velocity of 220 cm/s on the last TCD before stroke. Although an individual's risk is hard to predict, our data suggest that higher velocities indicate both higher risk and, at some level, more proximate risk. We also analyzed the 317 patients who had at least one abnormal TCD. In this group there were 31 strokes. When the group is divided into quartiles on the basis of absolute TCD velocity, velocity ranged from 200 cm/s to 366 cm/s. Survival analysis comparing the 4 groups demonstrated a significant difference between the highest quartile and the lower 3 (P = .004, 75th percentile, 227 cm/s), indicating that, with the highest velocities, the time-to-stroke interval is lower than with lower (but still abnormal) TCDs. A plot of time to stroke in relation to baseline velocity illustrates the trend to shorter latencies with high velocities but considerable variance is also demonstrated (Figure 2). The figure includes a Loess smoothed curve to demonstrate trend (Spearman rank correlation, –0.275; P = .038).9
Plot of time to stroke versus baseline highest TCD velocity. The fitted line is a Loess smoothed curve to demonstrate trend.
Plot of time to stroke versus baseline highest TCD velocity. The fitted line is a Loess smoothed curve to demonstrate trend.
Risk of conversion to abnormal TCD
Of 2324 children who had at least one TCD, those with an initial abnormal examination, only one examination, or only inadequate TCD examinationss were removed because these patients could not contribute to the risk of conversion analyses. Patients with initial inadequate but later adequate examinations were classified on the basis of their first adequate examination. Data from 3629 examinations in 1215 children were used in this analysis.
Of the 1215 subjects, 559 had 2, 364 had 3, and 292 had 4 or more TCD studies prior to development of either abnormal TCD or stroke. The mean age of these patients at entry was 7.8 years, and they were followed for 22 ± 15 months (0.5-58 months). We observed 9% (n = 114) with one or more abnormal TCDs on follow-up. Prediction based on the Weibull model suggests that a child 2 years of age at the first examination, with a normal first examination, had a 4% chance of having an abnormal examination in the next 12 months. In contrast, a child of the same age with a high-conditional first examination had a 50% chance. Table 1 shows the age distribution of the converters and demonstrates that conversion is much less common in children older than 10 years of age than in younger children.
Age distribution, length of follow-up, and probability of conversion from lower risk to abnormal TCD
Age, y . | No. children . | Median LOF (inner quartile range) . | Converters*(% within each group) . |
|---|---|---|---|
| 2-4 | 243 | 17.0 (29.5) | 31 (12.8)† |
| 4-6 | 233 | 14.7 (28.6) | 36 (15.5) |
| 6-8 | 196 | 23.3 (28.8) | 17 (8.7) |
| 8-10 | 183 | 15.4 (30.1) | 15 (8.2) |
| 10-12 | 156 | 25.6 (26.0) | 6 (3.9) |
| 12-14 | 112 | 13.3 (18.9) | 6 (5.4) |
| 14-16 | 85 | 11.0 (10.3) | 3 (3.5) |
| 16+ | 5 | 6.9 (1.5) | 0 (0.0) |
| Total | 1213 | 15.6 (27.4) | 114 (9.4) |
Age, y . | No. children . | Median LOF (inner quartile range) . | Converters*(% within each group) . |
|---|---|---|---|
| 2-4 | 243 | 17.0 (29.5) | 31 (12.8)† |
| 4-6 | 233 | 14.7 (28.6) | 36 (15.5) |
| 6-8 | 196 | 23.3 (28.8) | 17 (8.7) |
| 8-10 | 183 | 15.4 (30.1) | 15 (8.2) |
| 10-12 | 156 | 25.6 (26.0) | 6 (3.9) |
| 12-14 | 112 | 13.3 (18.9) | 6 (5.4) |
| 14-16 | 85 | 11.0 (10.3) | 3 (3.5) |
| 16+ | 5 | 6.9 (1.5) | 0 (0.0) |
| Total | 1213 | 15.6 (27.4) | 114 (9.4) |
LOF indicates length of follow-up.
Converters are defined as children with 2 or more TCD examinations, with normal or conditional first TCD and with subsequent test with abnormal result.
Significant difference in median follow-up time across age (P < .0001).
If the first 2 examinations are used as predictors for conversion to abnormal, the effect of age and prior TCD is more pronounced. This study sample is a further subset of the overall cohort, composed of patients who had normal or conditional results on the first 2 examinations. Follow-up time started at the second examination and ended at an abnormal TCD result. If no abnormal examination occurred, follow-up time was censored at the last examination. A total of 2513 examinations from 657 children were used. Of the 657, a total of 339 had 3, 151 had 4, and 167 had 5 or more examinations during the follow-up period. The mean age at entry was 7.5 ± 3.6 years, and these children were followed for an average of 30 ± 14 months. Sixty-one patients developed an abnormal examination during the follow-up period. The converters are listed in Table 2 on the basis of prior examination results. Conversion risk with 2 normal studies (1.4%) contrasts markedly with the likelihood of a subsequent abnormal result after 2 high-conditional studies (55%). Table 3 further shows the interaction of age and TCD velocity on subsequent conversion risk at 1, 2, and 3 years, derived from the Weibull model, and demonstrates that the conversion takes place primarily, but not exclusively, in the young patients. From the shaded areas of Table 3, it can be seen that even after 2 normal TCDs, a child entering screening at age 2 years still has a 4.4% chance of conversion in 4 years, contrasted with a less than 1% chance in those screened at age 14 years. On the other hand, 2 high conditionals in the youngest children were almost always followed by conversion (97%), but only about 13% of the time in children first screened at age 14 years.
Conversion risk based on first 2 TCD examinations
TCD history . | No. children (% of total) . | Converters (% of each group) . |
|---|---|---|
| Normal | ||
| To normal | 370 (56.3) | 5 (1.4) |
| To low conditional | 54 (8.2) | 5 (9.2) |
| To high conditional | 10 (1.5) | 4 (40) |
| Low conditional | ||
| To normal | 80 (12.2) | 5 (6.2) |
| To low conditional | 63 (9.6) | 12 (19) |
| To high conditional | 29 (4.4) | 14 (48) |
| High conditional | ||
| To normal | 19 (2.9) | 4 (21) |
| To low conditional | 21 (3.2) | 6 (29) |
| To high conditional | 11 (1.7) | 6 (55) |
TCD history . | No. children (% of total) . | Converters (% of each group) . |
|---|---|---|
| Normal | ||
| To normal | 370 (56.3) | 5 (1.4) |
| To low conditional | 54 (8.2) | 5 (9.2) |
| To high conditional | 10 (1.5) | 4 (40) |
| Low conditional | ||
| To normal | 80 (12.2) | 5 (6.2) |
| To low conditional | 63 (9.6) | 12 (19) |
| To high conditional | 29 (4.4) | 14 (48) |
| High conditional | ||
| To normal | 19 (2.9) | 4 (21) |
| To low conditional | 21 (3.2) | 6 (29) |
| To high conditional | 11 (1.7) | 6 (55) |
See Table 1 for definition of converters.
* The proportion of converters is not equal across all groups (P < .0001).
Predicted probability of converting to abnormal TCD status within 1, 2, or 3 years, on the basis of age and TCD history
Age at first screening, TCD history . | Probability of conversion . | . | . | ||
|---|---|---|---|---|---|
| . | 1y . | 2y . | 3y . | ||
| 2y | |||||
| Normal | |||||
| To normal | .017 | .031 | .044 | ||
| To low conditional | .093 | .165 | .228 | ||
| High conditional | |||||
| To low conditional | .334 | .530 | .661 | ||
| To high conditional | .728 | .910 | .968 | ||
| 6y | |||||
| Normal | |||||
| To normal | .006 | .011 | .016 | ||
| To low conditional | .033 | .061 | .086 | ||
| High conditional | |||||
| To low conditional | .132 | .231 | .314 | ||
| To high conditional | .364 | .568 | .700 | ||
| 10y | |||||
| Normal | |||||
| To normal | .002 | .004 | .006 | ||
| To low conditional | .012 | .022 | .031 | ||
| High conditional | |||||
| To low conditional | .048 | .088 | .123 | ||
| To high conditional | .146 | .254 | .343 | ||
| 14y | |||||
| Normal | |||||
| To normal | .001 | .001 | .002 | ||
| To low conditional | .004 | .008 | .011 | ||
| High conditional | |||||
| To low conditional | .017 | .031 | .045 | ||
| To high conditional | .054 | .097 | .136 | ||
| 16y | |||||
| Normal | |||||
| To normal | < .001 | .001 | .001 | ||
| To low conditional | .002 | .004 | .007 | ||
| High conditional | |||||
| To low conditional | .010 | .019 | .027 | ||
| To high conditional | .032 | .058 | .083 | ||
Age at first screening, TCD history . | Probability of conversion . | . | . | ||
|---|---|---|---|---|---|
| . | 1y . | 2y . | 3y . | ||
| 2y | |||||
| Normal | |||||
| To normal | .017 | .031 | .044 | ||
| To low conditional | .093 | .165 | .228 | ||
| High conditional | |||||
| To low conditional | .334 | .530 | .661 | ||
| To high conditional | .728 | .910 | .968 | ||
| 6y | |||||
| Normal | |||||
| To normal | .006 | .011 | .016 | ||
| To low conditional | .033 | .061 | .086 | ||
| High conditional | |||||
| To low conditional | .132 | .231 | .314 | ||
| To high conditional | .364 | .568 | .700 | ||
| 10y | |||||
| Normal | |||||
| To normal | .002 | .004 | .006 | ||
| To low conditional | .012 | .022 | .031 | ||
| High conditional | |||||
| To low conditional | .048 | .088 | .123 | ||
| To high conditional | .146 | .254 | .343 | ||
| 14y | |||||
| Normal | |||||
| To normal | .001 | .001 | .002 | ||
| To low conditional | .004 | .008 | .011 | ||
| High conditional | |||||
| To low conditional | .017 | .031 | .045 | ||
| To high conditional | .054 | .097 | .136 | ||
| 16y | |||||
| Normal | |||||
| To normal | < .001 | .001 | .001 | ||
| To low conditional | .002 | .004 | .007 | ||
| High conditional | |||||
| To low conditional | .010 | .019 | .027 | ||
| To high conditional | .032 | .058 | .083 | ||
We confirmed only 2 cases of Sβ0 thalassemia among 238 children tested for genotype during eligibility evaluation. This suggests that about 1% of the children in this study had this genotype although the genotype of the remaining patients was not known. Owing to the small number of cases, we did not test for difference in TCD or stroke risk with Sβ0 thalassemia compared with Hb SS.
Discussion
TCD has been used as a surrogate marker for stroke risk, and high-risk TCD has been correlated with absence of alpha thalassemia mutation10 and abnormal neurophsychological testing.11 TCD results indicating risk,12 such as stroke,13 have been reported to cluster among siblings. The STOP study is the largest prospective application of TCD (or any testing modality) for stroke prediction yet reported, and the screening and follow-up studies of STOP provide a unique opportunity to further the knowledge of TCD as a predictor for stroke in this setting. The study confirms prior reports that TCD velocity is a powerful predictor of stroke due to SCD, and adds new information regarding change in TCD over time. This study design underestimated prediction because many at-risk patients began transfusions before having a stroke. Further limitations of this study are that repeated TCD in nonrandomized individuals was not mandated and the timing of repeated TCD was often influenced by prior TCD results.
The Kaplan-Meier curve shows that baseline TCD is a good predictor for risk, extending about 4 years from the baseline study. If the results of subsequent TCD are considered, and a patient is labeled at high risk on the basis of any abnormal study, more strokes are predicted. Repeated screening can identify more patients at risk for stroke, but more screening has to be done. The results could be expected to be higher in a natural history study, because in STOP, transfusion therapy prevented many strokes in children with abnormal TCD, and these data are for this reason not directly comparable to studies performed before transfusion for primary prevention was initiated. The additional children who were identified to be at risk for stroke on follow-up studies had first TCDs that were conditional or, rarely, normal. Thus, repeated screening can prevent more strokes. However, since more children will be identified as being at risk, the additional stroke prevention will come at the cost of treating more children with transfusions.
Intracranial hemorrhage does not appear to be reliably predicted by TCD. There were 9 patients with intracranial hemorrhage (6 with subarachnoid hemorrhage [SAH], 1 with ICH, and 2 with both SAH and ICH), but only 1 had an abnormal TCD. Only one patient with SAH had an aneurysm, and the cause of SAH in these individuals is not clear. Our results suggest that, while SAH is not unusual in children with SCD, it is not predicted by TCD. Not all ischemic strokes were predicted by TCD, although fewer than 1% of the patients whose last TCD was normal had stroke. Whether these individuals represent cases of flawed examinations, absence of timely rescreening, or pathophysiological mechanisms not detected by TCD (eg, small vessel disease) is unclear.
An important contribution of this work is to provide numerical estimates of the probability of conversion from low- to high-risk TCD. In STOP, follow-up TCD examination often altered the assigned risk category. Younger patients and those with velocities near a category boundary were the most likely to change categories with repeated TCD and require the most intense surveillance on that basis.
How these data should be applied will be a matter of clinical judgment. Some consider the untreated stroke risk of 10% per year defined by TCD too high to tolerate, while others may not consider this risk sufficient to justify chronic transfusion. The stroke rate of 2% to 5% per year in an individual with a conditional TCD result may also be unacceptable to some, as it is considerably higher than the 0.5% to 1% risk of unselected individuals in some age categories predicted by the Cooperative Study of Sickle Cell Disease.1 Regardless of the inclination of the patient or the clinician, the information from TCD screening will give credible estimates of stroke risk and should help direct TCD screening resources. Application of these data is likely to be influenced by the results of STOP II, a randomized withdrawal of transfusion after at least 30 months of transfusion during which no stroke occurred and the TCD reverted to normal. If short-term transfusion can reduce the stroke risk and if treatment can then be safely withdrawn under TCD surveillance, then shorter treatment intervals could limit both the complications and the cost of long-term transfusion for primary stroke prevention.
Whether adding other measures to TCD would better select children for treatment needs to be studied. Recently, nocturnal oxygen desaturation has been studied prospectively in a cohort of 95 children with SCD. Children with desaturation (hypoxemia) were found to be a high risk, and the combination of this measure with clinical and laboratory features and TCD velocities added to risk assessment. Larger studies with multiple modes of assessment, although difficult to carry out, might lead to a more predictive model for stroke prediction and improve patient selection for treatment.14
Appendix
In addition to the authors, the STOP Investigative Team included the following:
Investigators
Brian Berman, Charles Daeschner, Catherine Driscoll, Beatrice Files, Lewis Hsu, Ann Jensen-Hurlet, Abdullah Kutlar, Virgil McKie, Scott Miller, Nancy Olivieri, Charles Scher, and Winfred Wang.
New England Research Institutes
Sarah Estow, Robert Lagos, Sonja McKinlay, Steve Weiner, and Maria Yelle.
Clinical Coordinators
Kim Brock, Eldrida Carter, Kathy Chiarucci, Mary Debarr, Pansy Feron, Sylvia Harris, Laura Hoey, Kathy Jacques, Lisa Kuisel, Norma Lewis, Ramona Lindsey, Brenda Martin, Claire McMeechan, Maria Muracca, Kathy Rey, Greta Roath, Ekua Hackney-Stephens, Linda Sumter, Aimee Talbot, Gayle Taplin, Carol Whittle, and Patrice Woods.
Medical College of Georgia
Jeannette Harbin, Leslie Holley, Brenda Jackson, Ferdane Kutlar, Bonnie Miller, Nadine Odo, Judi S. Schweitzer, Mary Sahm, and Amy Winstead.
TCD Examiners
Frank Allen, Katie Allen, Michael Beasley, Archana Ejantkar, Eric Houston, David Hunter, Jeff Ottenlips, and Sam Trocio.
Prepublished online as Blood First Edition Paper, January 29, 2004; DOI 10.1182/blood-2003-08-2733.
Supported by Cooperative Agreements (U10 HL 52193 and U10 HL 52016) with the National Heart, Lung, and Blood Institute.
Charles H. Pegelow died on November 18, 2002.
A complete list of the members of the STOP Study Investigative Team appears in the “Appendix.”
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors wish to thank Rayna Wright, Judi Schweitzer, and Angela Pelletier for assistance with preparation of the manuscript and to acknowledge the contribution of the entire STOP Investigative Team and the patients and families for the successful execution of the STOP study from which these data were derived.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal