Abstract
The asexual maturation of Plasmodium falciparum is accompanied by the transport of parasite-encoded proteins to the erythrocyte plasma membrane. Activation of G proteins by treatment with aluminum fluoride produced an accumulation within the erythrocyte cytosol of vesicles coated with Plasmodium homologues of COPII and N-ethylmaleimide-sensitive factor, proteins involved in intracellular transport between the Golgi apparatus and the endoplasmic reticulum. These vesicles contain malarial proteins that appear on the erythrocyte plasma membrane, as well as actin and myosin. It is proposed that the parasite adapted a process well established for intracellular transport to mediate the extracellular movement of its proteins through the erythrocyte cytosol to the surface membrane.
Introduction
During the development of the asexual stage of the human malaria parasite Plasmodium falciparum (Pf), the host cell erythrocyte is radically changed and acquires the ability to adhere to vascular endothelium. These alterations are believed to be largely because of the export of parasite proteins, which associate with the red blood cell (RBC) membrane. Protein targeting beyond the parasite plasma membrane (PPM) requires unusual pathways, given the parasite's intracellular location within a parasitophorous vacuolar membrane (PVM) and the lack of organelles, proteins, and biosynthetic machinery in the RBC. Pf erythrocyte membrane protein 1 (Pf EMP1) is a membrane-spanning protein that incorporates into the RBC membrane.1 The adhesive changes in parasite-infected red blood cells (IRBCs) are due to the expression of antigenically variant Pf EMP1, which appears to be concentrated on the exterior surface of RBCs in membrane protrusions termed knobs.1,2 The transport of this protein to the RBC membrane is of considerable interest, given its prominent role in cytoadherence and the pathology of cerebral malaria.3-5
The transport of transmembrane proteins like Pf EMP1 from the parasite to the RBC cytosol and surface membrane likely involves a vesicle-mediated process. An important question in proposing a vesicle-mediated trafficking pathway of parasite proteins from the PVM to the erythrocyte compartment is how vesicles form when mature RBCs do not contain proteins necessary to drive vesicle budding. For a transport vesicle to form from the PVM, a coating system would be required on its cytoplasmic side (ie, the RBC cytosol).
Homologues of proteins involved in intracellular vesicle transport were found in Pf.6,7 Albano et al8 identified a homologue of the guanosine triphosphate (GTP)-binding protein, Sar1p. Sar1p, in addition to Sec13/31p and Sec23/24p, comprises the COPII protein coat in higher eukaryotes.9,10 A Pf homologue of Sec31p was reported to be exported to structures in the RBC cytoplasm, suggested to be the Maurer clefts.11,12 A Pf homologue of N-ethylmaleimide-sensitive factor (NSF), which plays a central role in vesicular trafficking in eukaryotic cells, was localized to the parasite and associated with structures in the RBC cytosol.13 The major advance in uncovering a parasite-generated vesicle transport pathway was made by incubating IRBCs with aluminium tetrafluoride (AlF), an activator of G proteins, which revealed small (70- to 100-nm diameter), possibly coated vesicles in the RBC cytosol bearing the parasite proteins Pf EMP1 and Pf EMP3. These vesicles were organized in chains, which we suggested was evidence of cytoskeletal association.14 Taken together, these results led us to propose that malaria parasites export proteins into the host cell cytosol to spawn a vesicle-mediated protein trafficking pathway.14,15 We provide evidence that Pf adapted a well-established pathway for intracellular vesicle transport to mediate the extracellular movement of parasite proteins through the RBC cytosol to the surface membrane.
Materials and methods
Parasites
Antibodies
The production and characterization of the affinity-purified anti-Pf Sec31p,11 -Pf Sar1p,8 -Pf NSF,13 and -Pf EMP114 rabbit polyclonal antibodies was described previously. Preimmune rabbit serum was used as a control for nonspecific binding. A mouse monoclonal antibody SP1A6G5, specific for a 130-kDa Maurer cleft protein,18 was provided by Tobili Sam Yellowe (Cleveland, OH). Mouse monoclonal immunoglobulin G (IgG) against human β-actin (ab6276) and a beta terminal peptide of nonmuscle myosin heavy chain (ab684) were from Abcam Limited (Cambridge, United Kingdom). Aurion GP-UltraSmall gold (0.8-nm diameter) antimouse, antirabbit, and antirat IgG and IgM antibodies were obtained from Aurion (Fort Washington, PA).
Treatment of IRBCs with AlF
IRBCs at different stages of erythrocytic development were incubated with 100 or 250 μM AlF in RPMIc (RPMI 1640 containing 24 mM NaHCO3, 25 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), 21.1 mM glucose, 2 mM glutathione, 0.44 mM hypoxanthine, 66 mg mL-1 gentamycin, and 10% heat-inactivated human serum) at 2.5% hematocrit for 1 hour at 37°C. The cells were collected, washed twice with serum-free RPMI containing AlF, and processed for biochemical characterization or morphologic examination. Similar results were obtained with 100 or 250 μM AlF.
Preparation of RBC membranes and parasites from IRBCs treated with AlF
IRBCs (500 μL) were lysed with an equal volume of 0.15% saponin containing protease inhibitors (10 μg/mL leupeptin, pepstatin A, chymostatin, antipain, 0.5 mM PMSF (phenylmethylsulfonyl fluoride), and 2.5 mM diisopropylfluorophosphate). Parasites surrounded by the PVM were isolated by centrifugation at 3000 rpm, and RBC membranes were isolated from the parasite-free lysate by centrifugation at 14 100 rpm. Hemozoin was removed by ultrasonically lysing the parasites followed by centrifugation at 10 000 rpm. The protein concentration of the RBC membranes and hemozoin-free parasites was determined using the bicinchoninic acid (BCA) protein kit (Pierce, Rockford, IL).
Electrophoresis and immunoblotting
Uninfected RBCs were suspended in sample buffer (38 mM Tris (tris(hydroxymethyl)aminomethane)-HCl, 1 mM EDTA (ethylenediaminetetraacetic acid), 0.1 mM DTT (dithiothreitol), 4% SDS (sodium dodecyl sulfate), 0.3 M sucrose, 0.01% bromphenol blue, 0.6% L-methionine), boiled for 5 minutes, and analyzed by SDS-polyacrylamide gel electrophoresis (PAGE).19 A volume of 100 μL IRBCs (∼ 10% parasitemia) was resuspended in 500 μL Triton X-100 in phosphate-buffered saline (PBS; 1.9 mM NaH2PO4, 6.1 mM Na2HPO4, 140 mM NaCl, pH 7.4) with protease inhibitors (10 μg/mL leupeptin, pepstatin A, chymostatin, antipain, 0.5 mM PMSF, and 2.5 mM diisopropylfluorophosphate). The proteins were mixed and separated into Triton X-100 soluble and insoluble fractions by centrifugation for 20 minutes at 18 000g. The pellet was resuspended in 50 μL of 2% SDS in PBS and centrifuged at 18 000g for 20 minutes. The Triton X-100 and SDS soluble fractions were analyzed by SDS-PAGE,19 and Western blotting was performed by using enhanced chemiluminescence (ECL) detection. Equal numbers (∼ 1.5 × 108) of uninfected and infected erythrocytes (at ∼ 10% parasitemia) were solubilized, and equal aliquots were added to each lane in the gel. The primary antibody dilutions were Pf NSF (1:500), Pf Sar1p (1:500), and Pf Sec31p (1:1000). The secondary antibody was a donkey antirabbit antibody (Amersham, Arlington Heights, IL) used at a 1:3000 dilution.
To analyze the distribution of myosin in IRBCs, electrophoresis was performed on a 5% to 15% acrylamide linear gradient SDS gel containing 4 M urea, with a 5% stacking gel containing 2 M urea. The sample buffer contained 100 mM Tris-HCl, 10% β-mercaptoethanol, 4% SDS, 10% sucrose, 0.01% bromphenol blue, and 8 M urea. Equal amounts of protein were loaded for RBC membranes (82.5 μg) and PVM/parasite samples (39 μg) from untreated and AlF-treated IRBCs. Proteins were transferred onto Immobilon membranes and incubated with the antimyosin antibody (1: 1000) followed by a horseradish peroxidase-labeled goat antimouse antibody (1:10 000). Contamination of the parasite fraction by RBC membranes was assayed using an antihuman glycophorin A and B mouse monoclonal antibody (ab6396; Abcam). Parasite fractions were determined to be glycophorin (RBC membrane) free.
Immunofluorescence
IRBCs were fixed onto glass slides overnight at 4°C by using periodatelysine-paraforamaldehyde.17 Primary antibody dilutions were Pf NSF (1: 200), Pf Sar1p (1:25), Pf Sec31p (1:500), and myosin (1:100, 1:500). Binding was visualized by using a fluorescein isothiocyanate (FITC)-conjugated goat antirabbit IgG or FITC-conjugated goat antimouse IgG as appropriate. Immunofluorescence analysis (IFA) was performed on a Biorad Radiance 2000 laser scanning imaging system interfaced to an Olympus IX70 inverted epifluorescence microscope.
Electron microscopy
Morphology. IRBCs were fixed with 2% glutaraldehyde containing 1% tannic acid in 0.1 M sodium cacodylate buffer, followed by 2% osmium tetroxide and 1% uranyl acetate.14 The fixed IRBCs were centrifuged in warm agarose, dehydrated in graded steps of acetone, infiltrated, and embedded in Spurrs; the blocks were thin-sectioned (∼ 70 nm) with a Diatome diamond knife on a Reichert Jung Ultra Cut E ultramicrotome; the thin sections were poststained with 2.5% uranyl acetate and 2.5% bismuth and viewed with an Hitachi H-7000 electron microscope.
Immunoelectron microscopy. IRBCs were fixed with 2% paraformaldehyde containing 0.1% glutaraldehyde in 0.1 M sodium cacodylate buffer,14 centrifuged in warm agarose, partially dehydrated in ethanol, and infiltrated and embedded in L. R. White. Immunolabeling was performed on thin sections by incubation with antihuman β-actin (1:500), Pf EMP1 (1:30), Pf Sar1p (1:30), Pf NSF (1:30), Pf Sec31p (1:100), or Pf Maurer cleft antibodies (1:30), and labeling was visualized with the appropriate UltraSmall gold-conjugated secondary antibody. The gold particle size was enhanced by using an Aurion R-gent SE-EM (silver enhancement reagent for electron microscopy) initiator, activator, and enhancer. To insure specificity of antibody labeling, sections were incubated with the secondary antibodies only, which revealed a low level of nonspecific reactivity with the parasite nuclei. Sections were poststained for 5 minutes with 2.5% uranyl acetate and 2.5% sodium bismuth.
Results
Incubation of IRBCs with AlF produces vesicle chains in the RBC cytosol
Long, slender membranes with a translucent lumen termed Maurer clefts, circular unit membranes (0.2- to 1.0-μm diameter) with electron-dense contents and large, multiple membrane whorls are typically observed in the erythrocyte cytosol of IRBCs. The latter 2 structures were suggested to be PVM extensions.20 Typical Maurer clefts in IRBCs are shown in Figure 1A,D. Immunoelectron microscopy using monoclonal antibody (MAb) SP1A6G5, which is specific for a 130-kDa Maurer cleft protein, confirmed that these structures were clefts (Figure 1D). None of these membranes resembled classical coated vesicles.
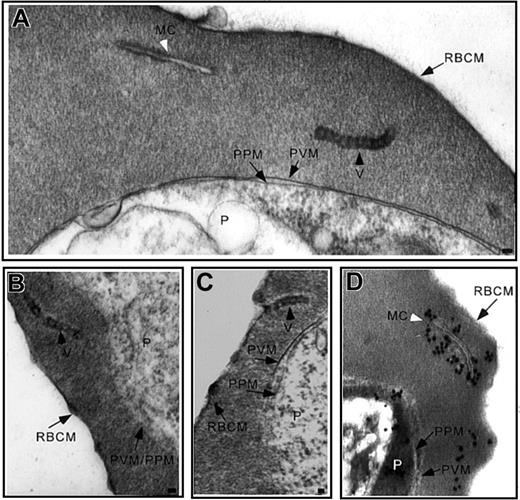
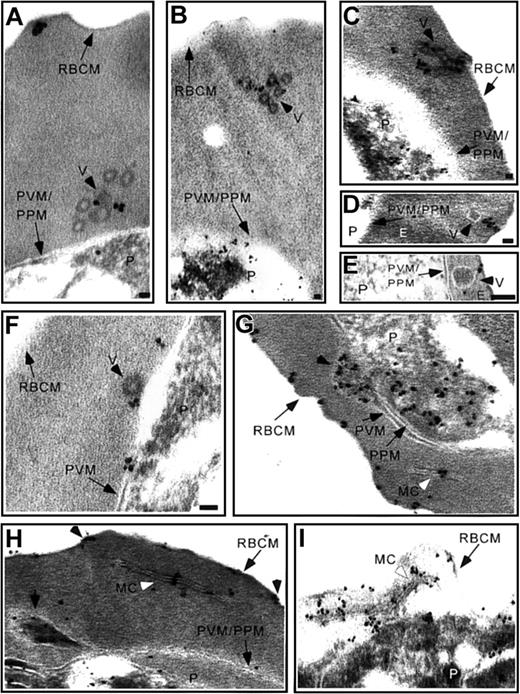
Incubation of IRBCs with AlF reveals vesicle chains. (A-B) Ultrastructural characterization of glutaraldehyde-fixed trophozoite stage IRBCs revealed vesicles with an electron-dense limiting membrane arranged in chains in the RBC cytosol (black arrowhead) or (C) closely apposed to the erythrocyte plasma membrane. A typical Maurer cleft is observed (A; white arrowhead). (D) Immunoelectron microscopy confirmed the long, slender unit membranes in the RBC cytosol are Maurer clefts. MC indicates Maurer cleft; P, parasite; PVM, parasitophorous vacuolar membrane; PPM, parasite plasma membrane, RBCM, red blood cell membrane; and V, vesicles. Scale bar = 70 nm.
Incubation of IRBCs with AlF reveals vesicle chains. (A-B) Ultrastructural characterization of glutaraldehyde-fixed trophozoite stage IRBCs revealed vesicles with an electron-dense limiting membrane arranged in chains in the RBC cytosol (black arrowhead) or (C) closely apposed to the erythrocyte plasma membrane. A typical Maurer cleft is observed (A; white arrowhead). (D) Immunoelectron microscopy confirmed the long, slender unit membranes in the RBC cytosol are Maurer clefts. MC indicates Maurer cleft; P, parasite; PVM, parasitophorous vacuolar membrane; PPM, parasite plasma membrane, RBCM, red blood cell membrane; and V, vesicles. Scale bar = 70 nm.
Examination of serial sections from more than 500 IRBCs by electron microscopy revealed in a few of those thousands of sections 1 or 2 of the 70- to 100-nm vesicles in the RBC cytosol, which appeared to be coated (data not shown). AlF is a valuable reagent for unraveling G protein-mediated vesicle transport between cellular compartments.21-25 The incubation of mature IRBCs with AlF caused the appearance of 70- to 100-nm vesicles with an electron dense periphery arranged in clusters (4-6 vesicles in a 70-nm section) or chains of up to 10 vesicles (Figure 1A-C) in the RBC cytosol, in agreement with previous results.14,15 Vesicles in clusters or chains were observed in a single thin section with a frequency of approximately 1 in every 25 IRBCs. These structures were not observed in serial sections from more than 500 AlF-treated uninfected RBCs. AlF had no effect on cell viability, as parasites progressed normally through the erythrocytic cycle when AlF was removed from the culture medium. The vesicle complexes often abutted the RBC membrane (Figure 1C). The vesicles in the chains were 1 section thick (∼ 70 nm). Inclusion of ruthenium red in the incubation medium ruled out the possibility that these were coated pits or tubuloendocytic vesicles of RBC origin, because these structures were not labeled (data not shown).
Pf Sar1p and Pf Sec31p are associated with transport vesicles and Maurer clefts in the erythrocyte cytosol
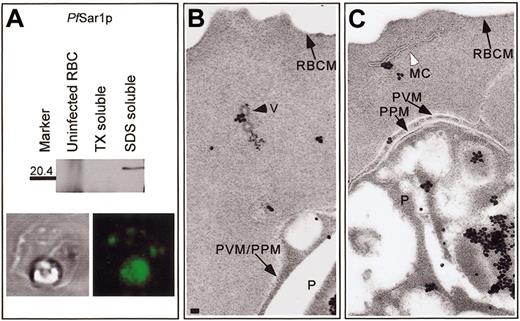
Pf homologues of Sar1p (Figure 2A) and Sec31p (data not shown) were detected by Western blotting in IRBCs solubilized with SDS, but not Triton X-100, in agreement with previous studies.8,11 These proteins were absent in Western blots of uninfected RBCs, although there was some ECL reactivity from higher and lower molecular weight species because of the peroxidase-like activity of hemoglobin monomers and tetramers as noted previously.8 IFA showed these proteins were localized to the parasite compartment and structures present in the RBC cytosol (Pf Sar1p, Figure 2A; Pf Sec31p, not shown). The distribution of Pf Sar1p and Pf Sec31p in the RBC cytosol of IRBCs incubated with AlF was similar to untreated IRBCs (data not shown). Pf Sar1p was localized to the parasite compartment, with 70- to 100-nm coated vesicles in the RBC cytosol (Figure 2B), similar to those observed in Figure 1A-C, and with Maurer clefts (Figure 2C). A similar labeling pattern was obtained for IRBCs probed with an anti-Pf Sec31p antibody (data not shown). Chains and clusters of coated vesicles bearing Pf Sar1p or Pf Sec31p were observed only in IRBCs treated with AlF. Thus, 2 components of a putative PfCOPII complex were localized to the 70- to 100-nm transport vesicles and Maurer clefts in the RBC cytosol.
Pf Sar1p is associated with coated vesicles and Maurer clefts. (A) Western blot analysis of uninfected and IRBCs incubated with AlF demonstrated that Pf Sar1p was an approximate 23-kDa Triton X-100-insoluble, SDS-soluble protein that was absent in uninfected RBCs. IFA showed Pf Sar1p localized to the parasite compartment and structures in the RBC cytosol. Immunoelectron microscopy revealed Pf Sar1p associated with (B) coated vesicles (V), black arrowhead, and (C) Maurer clefts (MC), white arrowhead, within the RBC cytosol. Scale bar = 70 nm.
Pf Sar1p is associated with coated vesicles and Maurer clefts. (A) Western blot analysis of uninfected and IRBCs incubated with AlF demonstrated that Pf Sar1p was an approximate 23-kDa Triton X-100-insoluble, SDS-soluble protein that was absent in uninfected RBCs. IFA showed Pf Sar1p localized to the parasite compartment and structures in the RBC cytosol. Immunoelectron microscopy revealed Pf Sar1p associated with (B) coated vesicles (V), black arrowhead, and (C) Maurer clefts (MC), white arrowhead, within the RBC cytosol. Scale bar = 70 nm.
Parasite protein Pf EMP1 is transported by coated vesicles to the Maurer clefts and the erythrocyte plasma membrane
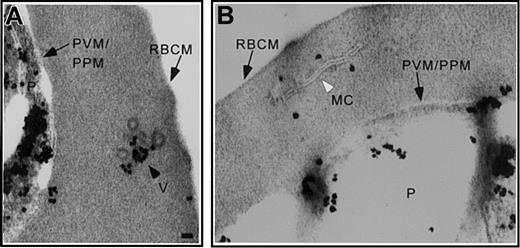
We suggested that the parasite proteins Pf EMP1 and Pf EMP3 were exported to the RBCs by a vesicle-mediated process.14 In IRBCs incubated with AlF, Pf EMP1 was detected in the parasite and associated with coated vesicles (Figure 3A) and Maurer clefts (Figure 3B) in the RBC cytosol. The vesicles transporting Pf EMP1 were similar in appearance to vesicles bearing Pf Sar1p and Pf Sec31p.
Pf EMP1 is associated with coated vesicles and Maurer clefts. Trophozoite stage IRBCs were treated with AlF, processed for immunoelectron microscopy and probed with an α-Pf EMP1 antibody. Pf EMP1 associated with (A) the parasite compartment and coated vesicles in the RBC cytosol and (B) Maurer clefts. Scale bar = 70 nm.
Pf EMP1 is associated with coated vesicles and Maurer clefts. Trophozoite stage IRBCs were treated with AlF, processed for immunoelectron microscopy and probed with an α-Pf EMP1 antibody. Pf EMP1 associated with (A) the parasite compartment and coated vesicles in the RBC cytosol and (B) Maurer clefts. Scale bar = 70 nm.
Pf homologue of NSF is associated with transport vesicles and Maurer clefts in the erythrocyte cytosol
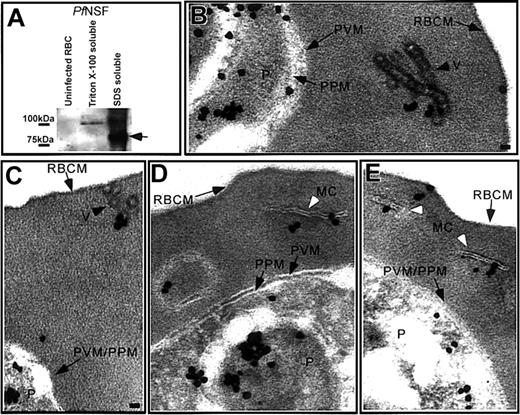
A Pf homologue of NSF, which is involved in vesicle docking and fusion processes,26 was exported to the erythrocyte cytosol of IRBCs.13 In agreement with previous results, Pf NSF is absent in uninfected RBCs, insoluble in Triton X-100, and SDS soluble (Figure 4A), suggesting it is associated with cytoskeleton and/or membranes. IFA confirmed that Pf NSF was exported into the RBC cytosol (data not shown). Immunoelectron microscopy showed that Pf NSF was associated with vesicle chains in the RBC cytosol (Figure 4B), coated vesicles abutting the RBC plasma membrane (Figure 4C), Maurer clefts (Figure 4D-E), a large, double membrane structure in the RBC cytosol (Figure 4D), and at transitional regions between the Maurer clefts and the erythrocyte plasma membrane (Figure 4E). Pf NSF-coated vesicles were not observed in untreated IRBCs.
Pf NSF is associated with coated vesicles and Maurer clefts. (A) Western blot analysis of uninfected RBCs and IRBCs showed that Pf NSF was an approximate 89-kDa Triton X-100-insoluble, SDS-soluble protein that was absent in uninfected RBCs. Immunoelectron microscopy showed Pf NSF associated with (B) chains of coated vesicles in the RBC cytosol, black arrowhead, (C) coated vesicles closely apposed to the RBC membrane, black arrowhead, (D-E) the Maurer clefts, white arrowhead, (D) a large, double membrane structure in the RBC cytosol, and (E) transitional areas between a Maurer cleft and the RBC membrane. Scale bar = 70 nm.
Pf NSF is associated with coated vesicles and Maurer clefts. (A) Western blot analysis of uninfected RBCs and IRBCs showed that Pf NSF was an approximate 89-kDa Triton X-100-insoluble, SDS-soluble protein that was absent in uninfected RBCs. Immunoelectron microscopy showed Pf NSF associated with (B) chains of coated vesicles in the RBC cytosol, black arrowhead, (C) coated vesicles closely apposed to the RBC membrane, black arrowhead, (D-E) the Maurer clefts, white arrowhead, (D) a large, double membrane structure in the RBC cytosol, and (E) transitional areas between a Maurer cleft and the RBC membrane. Scale bar = 70 nm.
Actin is associated with transport vesicles in the erythrocyte cytosol
The organization of coated transport vesicles in chains in AlF-treated IRBCs suggested they might be transported along a cytoskeletal network. Immunoelectron microscopy showed that actin was associated with the RBC membrane, clusters of coated vesicles in the RBC cytosol (Figure 5A-B), and vesicle chains extending to the RBC plasma membrane (Figure 5C). Other larger vesicles with electron dense contents, which were morphologically distinct from the coated 70- to 100-nm vesicles, appeared to interact with the RBC membrane through an association with actin (Figure 5D-E). Actin was localized in the vicinity of a budding, coated vesicle (Figure 5F) and other PVM protrusions (Figure 5G).
Actin is associated with intraerythrocytic transport vesicles. Trophozoite stage IRBCs were treated with AlF and processed for immunoelectron microscopy using an antiactin antibody. Actin associated with the RBC membrane and (A-B) coated vesicles in the RBC cytosol, (C) vesicle chains in the RBC cytosol closely apposed to the RBC membrane, and (D-E) vesicles (arrowhead) with a translucent limiting membrane and electron dense contents closely apposed to the RBC membrane. (F) Actin in close proximity to a coated vesicle budding from the PVM, black arrowhead, (G) concentrated in protruding areas of the PVM, black arrowhead, (H) associated with the Maurer clefts, which appeared to extend to actin-rich areas (black arrowheads) and a protruding, electron-dense area of the PVM (short arrow) that was similar in appearance (translucent limiting membrane and electron-dense contents) to the vesicles in panels D and E. (I) Maurer clefts in IRBCs permeabilized with streptolysin O maintained their parallel orientation and appeared to be tethered to the RBCs and parasite membranes via actin. Scale bar = 70 nm.
Actin is associated with intraerythrocytic transport vesicles. Trophozoite stage IRBCs were treated with AlF and processed for immunoelectron microscopy using an antiactin antibody. Actin associated with the RBC membrane and (A-B) coated vesicles in the RBC cytosol, (C) vesicle chains in the RBC cytosol closely apposed to the RBC membrane, and (D-E) vesicles (arrowhead) with a translucent limiting membrane and electron dense contents closely apposed to the RBC membrane. (F) Actin in close proximity to a coated vesicle budding from the PVM, black arrowhead, (G) concentrated in protruding areas of the PVM, black arrowhead, (H) associated with the Maurer clefts, which appeared to extend to actin-rich areas (black arrowheads) and a protruding, electron-dense area of the PVM (short arrow) that was similar in appearance (translucent limiting membrane and electron-dense contents) to the vesicles in panels D and E. (I) Maurer clefts in IRBCs permeabilized with streptolysin O maintained their parallel orientation and appeared to be tethered to the RBCs and parasite membranes via actin. Scale bar = 70 nm.
Intraerythrocytic transport vesicles do not contain erythrocyte, PVM, or Maurer cleft proteins
To gain further insight into the origin and composition of the intraerythrocytic transport vesicles in IRBCs, we used immunoelectron microscopy to investigate whether proteins from the erythrocyte membrane (glycophorin), PVM (exp-1), or the Maurer clefts (the 130-kDa protein recognized by MAb SP1A6G5) were associated with the vesicles. None of these proteins were localized to the vesicles (data not shown).
Maurer clefts in the RBC cytosol are tethered to the RBC plasma membrane via actin
We showed here that Pf EMP1, Pf Sec31p, Pf Sar1p, and Pf NSF associate with the Maurer clefts. Wickham et al12 observed that Pf EMP1, Pf EMP3, Pf HRP1, and Pf Sar1p colocalized with the Maurer clefts and suggested the clefts were an elaboration of a canonical secretory pathway that is transposed outside the parasite into the host cell. Maurer clefts were proposed to be associated with the host cell cytoskeleton,27,28 and this interaction may regulate the release of proteins from the clefts.29 The first direct evidence that Maurer clefts are connected to the RBCs is shown in Figure 5H-I. Actin was associated with the body of a cleft, and each end of the cleft appeared to extend out to actin-enriched regions of the RBC membrane (Figure 5H). In IRBCs treated with streptolysin O to remove RBC cytosol, the clefts maintained their parallel orientation to the RBC membrane. The cleft appeared to be connected to the parasite compartment and RBC membrane by actin-rich areas (Figure 5I). We suggest that the Maurer clefts are tethered to the host cell membrane by association with actin and possibly other RBC cytoskeletal proteins and play a direct role in the trafficking of parasite proteins to the host cell membrane.
Erythrocyte myosin is stabilized at the PVM in AlF-treated IRBCs
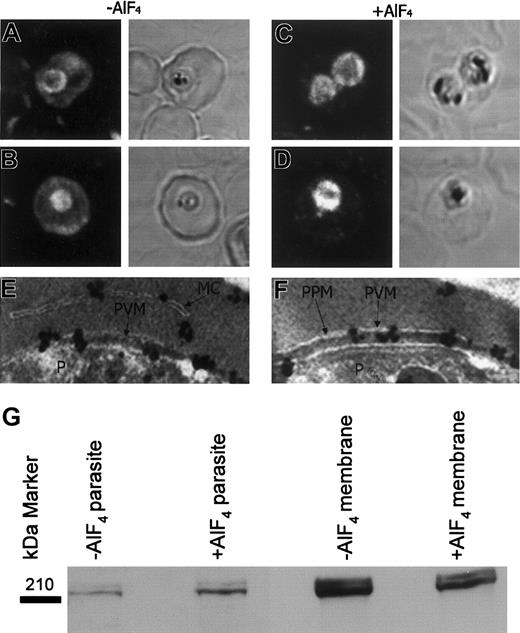
Myosins are a conserved class of actin-based motor proteins found in virtually all eukaryotes that play a role in many diverse cellular tasks, including endocytosis and exocytosis and vesicle transport. RBCs contain about 6000 copies per cell of a nonmuscle myosin II of approximately 200 kDa30 ; parasites contain an unusual approximately 105 kDa (Myosin XIV), which is present only at the end of the erythrocytic cycle and is part of an actin-myosin motor required for parasite invasion.31 By immunofluorescence, myosin was associated with the RBC membrane and was diffusively distributed in the RBC cytosol of uninfected RBCs (data not shown) in agreement with previous results.30 In IRBCs, myosin was also peripherally associated with the parasite, presumably with the PVM (Figure 6A-B). AlF treatment perturbed the myosin distribution, causing a decrease in the erythrocyte compartment and an increased accumulation at the outside edge of the parasite (Figure 6C-D). Immunoelectron microscopy of AlF-treated IRBCs showed myosin was associated with the PVM and the Maurer clefts (Figure 6E-F) and on rare occasion with coated vesicles in the RBC cytosol (data not shown). Myosin was also associated with Maurer clefts in untreated IRBCs. Western blotting of RBC membranes and parasites surrounded by the PVM prepared by saponin lysis of IRBCs, normalized for protein as described in “Materials and methods,” confirmed that AlF treatment stabilized myosin binding to the PVM (Figure 6G).
RBC myosin association with the PVM appears to be G protein-mediated. (A-B) In the absence of AlF, myosin was associated with the RBC membrane and PVM and diffusely distributed in the RBC cytosol. (C-D) AlF perturbs the myosin distribution to an almost exclusive association with the PVM and some structures in the RBC cytosol. (E-F) Immunogold EM revealed myosin to be associated with the PVM and Maurer clefts in AlF-treated IRBC. (G) Western blots showing that AlF causes a loss of myosin from the RBC membrane and stabilization at the PVM. In A-D, 1 mm equals 700 nm. Original magnification E-F, × 20 000.
RBC myosin association with the PVM appears to be G protein-mediated. (A-B) In the absence of AlF, myosin was associated with the RBC membrane and PVM and diffusely distributed in the RBC cytosol. (C-D) AlF perturbs the myosin distribution to an almost exclusive association with the PVM and some structures in the RBC cytosol. (E-F) Immunogold EM revealed myosin to be associated with the PVM and Maurer clefts in AlF-treated IRBC. (G) Western blots showing that AlF causes a loss of myosin from the RBC membrane and stabilization at the PVM. In A-D, 1 mm equals 700 nm. Original magnification E-F, × 20 000.
Vesicle formation and transport in the erythrocyte cytosol
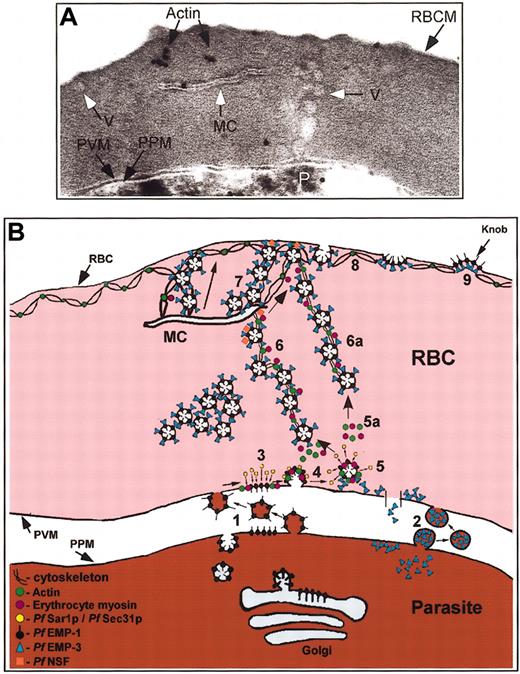
Many of the steps of the proposed erythrocytic vesicle-mediated protein transport pathway in IRBCs were visualized in a single thin section by electron microscopy (Figure 7A). Vesicles that were morphologically similar to those observed in Figures 1 to 6 budded from the PVM and appeared to be transported on a network to a Maurer cleft that was tethered to the RBC membrane by actin. The synthesis of the individual results obtained in Figures 1 to 6 and 7A into a model for a parasite-generated, vesicle-mediated intraerythrocytic protein transport pathway in IRBC is presented in Figure 7B.
Proposed scheme for the transport of parasite proteins to the erythrocyte plasma membrane. (A) Many of the individual events depicted in Figures 1 to 5 are captured in a single (∼ 70 nm) section from an IRBC treated with AlF. Coated vesicles budding from the PVM form a vesicle chain that spans the RBC cytosol and associates with one end of a Maurer cleft. A vesicle in line with the (left-hand side) of a Maurer cleft is observed at the RBC membrane (white arrow). The Maurer cleft is anchored to the RBC plasma membrane via actin. Original magnification, × 20 000. (B) Schematic representation of the transport of Pf EMP1 and Pf EMP3 (and other parasite proteins) to the RBC cytosol and plasma membrane. Steps 1 and 2 are hypothetical pathways. Pf Sec31, Pf Sar1p, and possibly Pf Sec23p (which is in the Pf genome but is uncharacterized) are exported to the RBC cytosol in which they form Pf COPII, facilitating vesicle formation at the PVM (steps 3-5). Vesicle budding and transport may be actin-myosin-mediated processes (step 5, 5a). Vesicles containing Pf EMP1 and Pf EMP3 uncoat (step 5) and are transported across the RBC cytosol to the Maurer clefts (step 6) or directly to the erythrocyte plasma membrane (step 6a) by an actin-myosin-mediated process. Pf NSF associates with the vesicles prior to their interaction with the clefts or the erythrocyte plasma membrane. Vesicles could be transported along actin-tethered Maurer clefts to the RBC membrane (step 7), or vesicles could bud from the ends of the Maurer cleft and diffuse to the RBC membrane. The vesicles associate with the RBC cytoskeleton (step 8) leading to knob formation.9 The appearance of extended chains of vesicles in the RBC cytosol and at the RBC membrane in IRBCs treated with AlF suggests an AlF-sensitive factor (eg, small GTPases such as Pfsar1p and/or Pf Rabs) may prevent vesicle uncoating (step 5) and block vesicle fusion at the clefts and/or erythrocyte plasma membrane, respectively, causing the vesicles to backup into chains.
Proposed scheme for the transport of parasite proteins to the erythrocyte plasma membrane. (A) Many of the individual events depicted in Figures 1 to 5 are captured in a single (∼ 70 nm) section from an IRBC treated with AlF. Coated vesicles budding from the PVM form a vesicle chain that spans the RBC cytosol and associates with one end of a Maurer cleft. A vesicle in line with the (left-hand side) of a Maurer cleft is observed at the RBC membrane (white arrow). The Maurer cleft is anchored to the RBC plasma membrane via actin. Original magnification, × 20 000. (B) Schematic representation of the transport of Pf EMP1 and Pf EMP3 (and other parasite proteins) to the RBC cytosol and plasma membrane. Steps 1 and 2 are hypothetical pathways. Pf Sec31, Pf Sar1p, and possibly Pf Sec23p (which is in the Pf genome but is uncharacterized) are exported to the RBC cytosol in which they form Pf COPII, facilitating vesicle formation at the PVM (steps 3-5). Vesicle budding and transport may be actin-myosin-mediated processes (step 5, 5a). Vesicles containing Pf EMP1 and Pf EMP3 uncoat (step 5) and are transported across the RBC cytosol to the Maurer clefts (step 6) or directly to the erythrocyte plasma membrane (step 6a) by an actin-myosin-mediated process. Pf NSF associates with the vesicles prior to their interaction with the clefts or the erythrocyte plasma membrane. Vesicles could be transported along actin-tethered Maurer clefts to the RBC membrane (step 7), or vesicles could bud from the ends of the Maurer cleft and diffuse to the RBC membrane. The vesicles associate with the RBC cytoskeleton (step 8) leading to knob formation.9 The appearance of extended chains of vesicles in the RBC cytosol and at the RBC membrane in IRBCs treated with AlF suggests an AlF-sensitive factor (eg, small GTPases such as Pfsar1p and/or Pf Rabs) may prevent vesicle uncoating (step 5) and block vesicle fusion at the clefts and/or erythrocyte plasma membrane, respectively, causing the vesicles to backup into chains.
Discussion
Treatment of IRBCs with AlF provided the key evidence for an intraerythrocytic vesicle-mediated secretory pathway and suggested that it was G-protein mediated. G proteins are divided into large, trimeric GTP-binding proteins and small, monomeric GTPases belonging to the Ras superfamily. The Ras/rab gene family are regulators of vesicle budding, motility, and fusion.32 Control of vectorial transport is mediated by these small GTPases that cycle between a membrane-associated (GTP-bound) and a soluble (guanosine diphosphate [GDP]-bound) form, which requires hydrolysis of GTP. Six Rab proteins were identified to date in the Pf genome (www.plasmodb.org); the genome is devoid of heterotrimeric G proteins. Therefore, the latter are not the target of AlF action. Although human RBCs contain both classes of G proteins, the lack of effect of AlF on uninfected RBCs and the association of PfCOPII homologues with the vesicles in the RBC cytosol of IRBCs incubated with AlF suggests the involvement of exported parasite G proteins in the vesicle-mediated transport process.
We do not know what steps in the vesicle transport process were affected by AlF. Fluoride complexes bind next to the bound GDP on G proteins and mimic the γ-phosphate of GTP, promoting the switch to a GTP-like conformation.22,33 Small G proteins bind fluoride complexes in the presence of their corresponding GTPase activating proteins (GAPs).22-24 AlF inhibited GTP hydrolysis on RasGTP and RabGTP because of the formation of a quaternary G protein · GDP · AlF · GAP complex,23,24 thereby inhibiting the uncoating of coated vesicles and preventing their fusion with the target membrane. AlF may interact with small Pf G proteins (eg, Pf Rabs) and an as of yet undescribed Pf Rab-GAP to inhibit the GTP hydrolysis of Pf RabGTP, causing the coated vesicles bearing parasite proteins to back up into vesicle chains at the Maurer clefts or RBC membrane because of their inability to fuse. There is evidence for this interpretation, as uncoating vesicles appeared to be in a state of arrested fusion at the erythrocyte membrane of IRBCs treated with AlF.14,15 It is also possible that a Pf Sec23p-AlF-Pf Sar1p complex formed, preventing GDP hydrolysis and disassembly of the coat, thereby inhibiting fusion with target membranes.
The transport of vesicles between intracellular organelles and the cell surface is accomplished either by energy-dependent (long-range) movement on microtubules or local movement by an actin-myosin motor system. The distances between the PVM and the Maurer clefts and erythrocyte plasma membrane are small and could be traversed in seconds by random diffusion of the vesicles; we do not favor this model because transport is directional (from the PVM outward), specific parasite proteins are transported to specific destinations in the RBC cytosol, and the vesicles are organized into small clusters or extended chains, suggesting they are associated with a platform. Vesicular transport along microtubules in IRBCs seems unlikely because RBCs do not contain microtubules. On the basis of the localization of actin and myosin to the PVM, chains of transport vesicles containing parasite proteins, the Maurer clefts, and erythrocyte plasma membrane, we propose that vesicle formation and transport may involve an actin-myosin-mediated process. Most of the RBC actin under normal conditions is present in stable 14-subunit oligomers, with only a small fraction in the cytosol that is below the critical concentration for assembly. Asexual parasites contain proteases that degrade the RBC cytoskeletal proteins α and β-spectrin, 4.1, actin, and ankyrin.34,35 It is possible that the action of these proteases disrupts this balance, raising the free concentration of cytosolic actin or produces free actin filaments extending from the RBCs that could interact with other proteins. It cannot be ruled out that the actin involved in vesicle transport could be supplied by the parasite, because the antibody we used was raised against the conserved N-terminal of β-actin and cross-reacts with Pf actin.
There is some evidence for myosin II as a vesicle transporter. In mammalian cells, myosin II is Golgi associated during vesicle budding.36-38 Myosin II binding to isolated Golgi was accompanied by the recruitment of actin. Myosin II binding to newly budded Golgi vesicles was stabilized by GTP-γS or AlF, suggesting it binds transiently to budding vesicle membranes under the regulation of G proteins. AlF stabilized myosin binding to the PVM in Figure 6. It is tempting to speculate that RBC myosin might move along actin filaments to either pull a vesicle bud or to move away vesicles from the PVM and across the RBC cytosol.
We were surprised that Pf NSF was membrane bound, associating with Maurer clefts, transport vesicles, and the RBC membrane. The soluble proteins α-SNAP (soluble N-ethylmaleimide-sensitive factor attachment protein) and NSF exist predominantly in a membrane-bound form in adrenal chromaffin cells, being found predominantly at the membrane of chromaffin secretory vesicles.39 The observation of Pf NSF-coated vesicles in the RBC cytosol suggests this interaction could be important for the acquisition of competence for docking. We suggest that Pf NSF may play a role in the targeting and fusion of the PfCOPII-coated vesicles bearing parasite proteins (eg, Pf EMP1 and Pf EMP3) to the Maurer clefts or the RBC membrane.
Our results suggest that intraerythrocytic parasites export proteins into the RBC cytosol to establish a vesicle-mediated system for transporting parasite proteins to the erythrocyte membrane. These proteins include Pf homologues of the COPII proteins Sar1p and Sec31p, well known for their involvement in intracellular transport between the Golgi and endoplasmic reticulum. This is the first report of COPII proteins being secreted to a distant location (eg, the cytosol of a host cell) to establish a vesicle-mediated protein transport system. Treatment of IRBCs with AlF resulted in the appearance of 70- to 100-nm coated vesicles arranged in extended chains or clusters in the erythrocyte cytosol of IRBCs. The localization of Pf Sar1p, Pf Sec31p, Pf NSF, Pf EMP1, Pf EMP3,14 actin, and erythrocyte myosin to the chains/clusters of morphologically similar vesicles strongly suggests that these proteins are associated with the same vesicles. Because of the small size of the vesicles and the moderate reactivity of some of the antibodies, it was necessary to use UltraSmall gold-conjugated antibodies and silver enhancement to better visualize the antibody deposition. This use results in gold particles of varying size, making it impossible to colocalize multiple proteins to the same vesicles by immunoelectron microscopy. Further biochemical characterization of the transport vesicle proteins will require a proteomic analysis of the purified transport vesicles.
Prepublished online as Blood First Edition Paper, July 17, 2003; DOI 10.1182/blood-2003-05-1448.
Supported by National Institutes of Health grant AI41761 (T.F.T.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked ”advertisement” in accordance with 18 U.S.C. section 1734.
We thank Akin Adisa and Frank Albano for providing the Pf Sec31p and Pf Sar1p antibodies and Tobili Sam-Yellowe for the anti-Maurer cleft antibody. We thank Louis Casta for figure preparation and helpful discussions.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal