Abstract
Signals provided by the erythropoieitin receptor (EpoR) are required for erythroid development beyond the erythroid colony-forming unit (CFU-e) stage and are propagated via the EpoR-tethered Janus kinase, JAK2. JAK2 functions, in part, to phosphorylate 8 conserved EpoR phosphotyrosine (PY) sites for the binding of a diverse set of signaling factors. However, recent studies in transgenic and knock-in mice have demonstrated substantial bioactivity for PY-null EpoR forms. Presently, the activities of a PY-null EpoR-HM form in primary progenitor cells from knock-in mice were further assessed using optimized Epo dose-dependent proliferation, survival, and differentiation assays. As compared with the wild-type (wt)–EpoR, EpoR-HM activity was compromised several-fold in each context when Epo was limited to physiologic concentrations. Possible compensatory increases in serum growth factor levels also were investigated, and as assayed using embryonic stem (ES) cell–derived erythroid G1E2 cells, activities in serum from EpoR-HM mice were substantially elevated. In addition, when challenged with phenylhydrazine-induced anemia, EpoR-HM mice failed to respond with efficient splenic stress erythropoiesis. Thus, the function of this JAK2-coupled but minimal PY-null EpoR-HM form appears to be attenuated in several contexts and to be assisted in vivo by compensatory mechanisms. Roles normally played by EpoR PY sites and distal domains therefore should receive continued attention.
Introduction
The cell surface receptors for several clinically important colony-stimulating factors and interleukins form a type 1 receptor superfamily.1,2 Common features include (1) extracellular 2-fold 7 β-strand FNIII subdomains that function in ligand binding3,4 ; (2) an approximately 50 amino acid juxamembrane cytoplasmic box 1 domain5,6 in β- and γ-chains that tethers these receptors to Janus kinase (JAK) protein tyrosine kinases;7,8 and (3) the occurrence (especially in receptor β-chains) of cytoplasmic tyrosine sites that are phosphorylated by JAKs and function as docking sites for diverse sets of SH2 domain–encoding signal transduction factors.9,10 Investigations using box 1 region mutants,11,12 dominant-negative JAKs,13,14 and/or mice with JAK kinase gene disruptions15,16 have revealed the essential nature (and upstream positioning) of JAKs in several type 1 receptor systems. For at least certain cytokine receptors, studies of phosphotyrosine (PY) site function also have pointed to important contributing roles for at least select PY sites (and coupled transduction pathways). Examples include key roles for PY440 of the interferon-γ receptor-1 chain in engaging signal transducer and activator of transcription-1 (Stat1) and interferon (IFN) response pathways17,18 and for a PY704 Stat3 (and/or Stat1) binding site in the granulocyte colony-stimulating factor (G-CSF) receptor, which recently has been shown to be critical for neutrophil function in a targeted transgenic mouse model.19,20 For other receptors, however, controversy exists regarding the relative functional importance of PY sites and linked events, and meaningful bioactivity has been shown to be exerted by receptor forms that couple to JAK kinases but lack cytoplasmic PY sites.21,22 Effects of stimulating JAK2-only pathways also have been probed in cell line models23,24 and recently in primary cells via the ectopic expression of JAK2 catalytic domain constructs.25 For example, EpoR-JAK2 and epidermal growth factor (EGF) receptor–JAK2 chimeras have been shown to promote proliferation in FDCP1 and 32D cells23,24 as has an AP20187 binding site–JAK2 catalytic domain fusion construct in BaF3 cells.25 In primary hematopoietic cells, this latter construct interestingly cofunctions selectively with either stem cell factor (SCF) or Flt3 ligand to promote early hematopoietic progenitor cell expansion with Stat5 dependency.25
In the case of the erythropoietin receptor (EpoR), biofunction of PY-null receptor forms recently has been demonstrated in transgenic26,27 as well as knock-in mouse models.28 Specifically, our laboratory has expressed PY-containing and PY-null EpoR forms in transgenic mice from a Gata1 gene vector (as hEGF receptor–EpoR EE chimeras) and has reported on the high activity of a Stat5-coupled PY343-containing form and compromised activity in growth and differentiation assays of a derived Y343F PY-null EpoR form.26,27 By comparison, Zang et al28 have studied essentially equivalent EpoR forms via their introduction into the endogenous EpoR locus. Based on in vivo performance and colony-forming assays, a Y343-retaining EpoR-H form possessed similarly elevated activities, while a PY-null form (EpoR-HM) was somewhat less active than wild-type (wt)–EpoR controls but overall conferred only mild in vivo biosignaling defects. In the present studies, bioactivities of this knocked-in PY-null EpoR-HM form have been analyzed further through the optimization of Epo dose-dependent in vitro assays for EpoR-supported primary adult erythroid progenitor cell proliferation, differentiation, and survival. Studies reveal Epo dose-dependent deficiencies in the activities of this JAK2-coupled but PY-null EpoR form in each context. In addition, in vivo analyses also reveal not only elevated serum growth factor activity in EpoR-HM mice but, also, compromised stress-induced erythropoiesis. Together, these findings indicate that at least certain EpoR PY–coupled events can contribute in important ways to a core EpoR-JAK2 signaling axis.
Materials and methods
Mice
EpoR-HM mice expressing a targeted transgenic PY-null EpoR form from the endogenous EpoR gene were those developed by Zang et al.28 These and control wild-type EpoR C57BL/6 mice (Charles River Laboratories, Wilmington, MA) used in experiments were 6 to 8 weeks old. Genomic polymerase chain reaction (PCR) with primers 5′-GAGTTTGAGGGTCTCTTCACC-3′ (exon 7) and 5′-TAGGCTGGAGTCCTAGGAGC-3′ (exon 8) was used to confirm genotypes. In stress erythropoiesis experiments, phenylhydrazine (Sigma, St Louis, MO) was injected subcutaneously (60 mg/kg) at 0 and 24 hours.
Marrow and spleen cell preparations
Progenitor cells were prepared from bone marrow as described.29 Specifically, mice were humanely killed with CO2, femurs and tibiae were isolated, and marrow cells were gently flushed from cavities using 21ga (femur) or 23ga (tibia) needles with a total volume of 10 mL (per mouse) Iscove modified Dulbecco medium (IMDM) plus 2% fetal bovine serum (FBS). Cells then were passed thrice slowly through a 21ga needle and a 70-μm strainer; were collected and resuspended initially in 1 mL of 138 mM NaCl, 2.7 mM KCl, 1.2 mM KH2PO4, 8.1 mM Na2HPO4, pH 7.4 (phosphate-buffered saline [PBS]); and were exposed for 2 minutes to 9 mL potassium bicarbonate–buffered 0.8% ammonium chloride, 0.1 mM Na2 EDTA (ethylenediaminetetraacetic acid) solution, pH 7.5. 10 × PBS (1.1 mL) was added, and cells were collected through 15 mL of 50% FBS in PBS, washed in IMDM plus 2% FBS, and resuspended initially in 1.5 mL hematopoietic cell expansion medium (HCEM) as IMDM supplemented with 0.75% bovine serum albumin (BSA), 7.5 μg/mL insulin, 150 μg/mL iron-saturated human transferrin (Stem Cell Technologies, Vancouver, BC), 0.1 mM β-mercaptoethanol, 12% FBS (catalog no. SH30070; HyClone, Logan, UT), plus penicillin (100 U/mL), streptomycin (100 μg/mL), and amphotericin B (0.25 μg/mL) (PSF). In cell counts, cells (0.1 mL) were exposed for 2 minutes to 3% acetic acid (3.9 mL) and 0.44 mL of 10-fold concentrated PBS was added. These cells (0.5 mL) then were combined with precisely 105 fluorospheres (catalog no. 752410F; Coulter, Miami, FL) in 0.5 mL PBS. Cells numbers (per milliliter) then were determined via flow analysis of 10 000 microsphere events (and were confirmed via direct manual counting). Splenocytes were prepared at 120 hours after phenylhydrazine injection as detailed by Zhang et al.30
3HdT incorporation assays
Marrow preparations were adjusted in HCEM to 1 × 106 cells per milliliter and plated (50 μL per well). Cytokines (Epo as epoetin alfa; Amgen, Thousand Oaks, CA) (murine interleukin-3 [mIL-3]; Peprotech, Rocky Hill, NJ) at 2 × concentrations in HCEM then were added (50 μL). At the indicated intervals, 3H-thymidine (3HdT) incorporation over a 2-hour period was determined (2.0 mCi/mmol [74 MBq/mmol], catalog no. 2407005; ICN Pharmaceuticals, Santa Ana, CA) (1 μCi [0.037 MBq] per well in 10 μL PBS, 0.1% BSA). Alternatively, for use in proliferation assays, cells were expanded for 65 hours in the dexamethasone-based media developed by Panzenbock et al,31 and at 24 and 48 hours, equal volumes of new medium were added. In Epo-dependent proliferation assays, dexamethasone, β-estradiol, SCF, and Epo were omitted, and washed cells were plated at 5 × 105 per milliliter. Rates of Epo-dependent 3H-thymidine (3HdT) incorporation then were assayed at 24 hours of culture.
Assays of Ter119, CD71, and phosphatidylserine (PS) expression
Marrow preparations at 1 × 106 cells per milliliter were cultured in HCEM supplemented with Epo at indexed concentrations. Epo-dependent Ter119-positive cell formation was assayed using a phycoerythrin (PE)–Ter119 antibody (BD-Pharmingen, San Diego, CA), and transferrin receptor expression was assayed using a fluorescein isothiocyanate (FITC) anti-CD71 antibody (BD-Pharmingen). Assays involved stepwise 4°C incubations of washed cells in 0.3 mL PBS, 0.1% BSA with murine immunoglobulin G (IgG) Fc fragment (5 μg; Pierce Biotechnology, Rockford, IL) for 30 minutes and with PE-Ter119 (1 μg) and/or FITC–anti-CD71 antibody (2 μg) for 45 minutes. In annexin V binding assays, Ter119-stained cells were incubated for 45 minutes in 0.3 mL of 140 mM NaCl, 2.5 mM CaCl2 10 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid)/NaOH, pH 7.4, with 5 μL FITC–annexin V (catalog no. 65874X; BD-Pharmingen) and were washed in this buffer prior to flow cytometry (Coulter XL system).
TUNEL assays
Marrow preparations were cultured in HCEM with Epo at 1.6, 0.1, or 0 U/mL, and at 42 hours, levels of apoptotic Ter119-positive cells were assayed using a DeadEnd fluorometric terminal deoxyuridine triphosphate nick-end labeling (TUNEL) system (Promega, Madison, WI). In brief, 107 cells were washed in PBS, 0.5% BSA; were resuspended in 0.5 mL PBS; and were incubated at 4°C for 20 minutes with 5 mL of 1% MeOH-free formaldehyde in PBS (Polyscience, Niles, IL). Cells then were washed in PBS, 0.5% BSA; incubated for 5 minutes at 4°C in 5 mL of 0.2% Triton X-100 in PBS; and washed in PBS, 0.5% BSA. In TUNEL reactions, cells were incubated at 37°C for 60 minutes in 45 μL equilibration buffer, 5 μL nucleotide mix, and 1 μL (10 units) terminal deoxynucleotidyl-transferase. Reactions were terminated (by the addition of 1 mL of 20 mM Na2EDTA, pH 8.0), and cells then were washed; resuspended in 0.3 mL PBS, 0.5% BSA; and stained with PE-Ter119.
Assays of serum growth factors activity
Whole blood (0.3 mL) was obtained by cardiac puncture and was used to prepare sterile plasma. Growth factor activity in samples from 8-week-old EpoR-HM and C57BL/6 mice then was assayed using embryonic stem (ES) cell–derived G1E2 cells.32 G1E2 cells were maintained in IMDM, 12% FBS, 0.1 mM monothioglycerol, PSF, 50 ng/mL mSCF, and 2 U/mL Epo. In proliferation assays, mSCF was decreased to 10 ng/mL, Epo was omitted, and cells (at 5 × 105/mL) were challenged with plasma at increasing concentrations. 3HdT incorporation rates were determined at 24 hours of culture.
Results
Epo dose-dependent deficiencies in EpoR-HM signaling of erythroid progenitor cell proliferation, survival, and differentiation
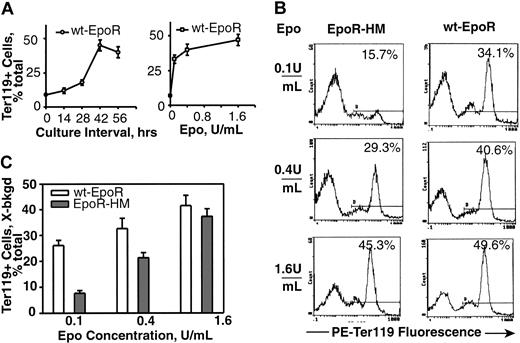
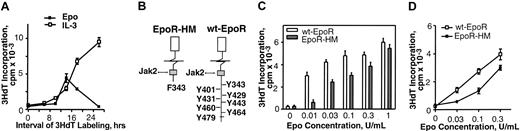
Pilot experiments first sought to develop a useful Epo dose-dependent proliferative response assay using marrow cell preparations from adult mice. In assays of Epo-dependent erythroid progenitor cell (EPC) differentiation (Figure 2), the development of Ter119-positive cells from these primary preparations occurred at meaningful frequencies within 28 hours of culture. From this it was predicted that normal marrow might contain well-represented cohorts of Ter119– yet Epo-responsive EPCs. This was tested by 3HdT pulse-labeling of Epo-exposed primary marrow cell preparations at early culture intervals (Figure 1A). At approximately 14 hours of culture, Epo-induced 3HdT incorporation rates were relatively high but subsequently decreased during EPC maturation. By comparison, rates of IL-3–stimulated 3HdT incorporation were low at early time points but then markedly increased. Useful Epo dose-response profiles also were obtained at 14 hours of culture. When this assay was used to compare EpoR-HM versus wt-EpoR activities in primary EPCs, several-fold decreases in 3HdT incorporation rates were observed for EPCs from EpoR-HM mice (Figure 1B-C). To extend this finding, EPCs also were cultured in the presence of dexamethasone to inhibit differentiation and to expand SCF- and Epo-dependent EPCs.31 Expanded cells then were plated at equivalent densities (in the absence of dexamethasone, β-estradiol, and SCF) and were challenged with Epo. EPCs from EpoR-HM mice again were significantly attenuated in their ability to support 3HdT incorporation rates in response to physiological concentrations of Epo (Figure 1D).
EpoR-HM is attenuated in its ability to support the development of Ter119-positive erythroid progenitor cells. (A) Marrow preparations from wt-EpoR mice were cultured in the presence of Epo at 1.6 U/mL, and Ter119-positive cell formation was assayed at 0, 14, 28, 42, and 56 hours (left). At 42 hours of culture, Epo concentration-dependent profiles for Ter119-positive cell development also were defined (right). (B) Bone marrow preparations from EpoR-HM or wt-EpoR mice were cultured in the presence of Epo at 0.1, 0.4, and 1.6 U/mL, and at 42 hours the Ter119-positive cell formation was assayed. Representative flow cytometric profiles are shown, and frequencies of Ter119-positive cells are indexed (as the percent of total events). The gate for positive events was set using unstained EPCs. (C) Differences in the abilities of EpoR-HM versus the wt-EpoR to support Ter119-positive EPC formation are summarized. Values are normalized means (less background levels for positive events in the absence of Epo stimulation) (n = 3 EpoR-HM and n = 3 wt-EpoR mice). Percents refer to positive events among totals.
EpoR-HM is attenuated in its ability to support the development of Ter119-positive erythroid progenitor cells. (A) Marrow preparations from wt-EpoR mice were cultured in the presence of Epo at 1.6 U/mL, and Ter119-positive cell formation was assayed at 0, 14, 28, 42, and 56 hours (left). At 42 hours of culture, Epo concentration-dependent profiles for Ter119-positive cell development also were defined (right). (B) Bone marrow preparations from EpoR-HM or wt-EpoR mice were cultured in the presence of Epo at 0.1, 0.4, and 1.6 U/mL, and at 42 hours the Ter119-positive cell formation was assayed. Representative flow cytometric profiles are shown, and frequencies of Ter119-positive cells are indexed (as the percent of total events). The gate for positive events was set using unstained EPCs. (C) Differences in the abilities of EpoR-HM versus the wt-EpoR to support Ter119-positive EPC formation are summarized. Values are normalized means (less background levels for positive events in the absence of Epo stimulation) (n = 3 EpoR-HM and n = 3 wt-EpoR mice). Percents refer to positive events among totals.
EpoR-HM is attenuated in its ability to support Epo concentration-dependent 3HdT incorporation in bone marrow–derived EPCs. (A) Marrow cell preparations were cultured in HCEM in the presence (or absence) of either Epo (0.2 U/mL) or IL-3 (10 ng/mL). At the indicated intervals, rates of cytokine-dependent proliferation were assayed based on stimulated rates of 3HdT incorporation. (mean incorporation rates ± SD, n = 3). (B) Diagrammed are EpoR-HM and wt-EpoR forms together with box1-associated JAK2 kinase and cytoplasmic phosphotyrosine sites within the wt-EpoR. (C) Marrow preparations from EpoR-HM or wt-EpoR control mice were cultured in the presence of Epo at the indexed concentrations. At 14 hours of culture, rates of Epo-dependent 3HdT incorporation were determined. Mean incorporation values (± SD) for 3 wt-EpoR and 3 EpoR-HM mice are shown. (D) To confirm apparent differences in Epo-dependent 3HdT incorporation as supported by EpoR-HM versus wt-EpoR, EPCs from EpoR-HM and control mice also were expended in the presence of dexamethasone, SCF, and Epo for 65 hours and were then tested for Epo responsiveness. Values are means ± SD of triplicate samples for 2 wt-EpoR and EpoR-HM mice (and outcomes are representative of 2 independent repeated experiments).
EpoR-HM is attenuated in its ability to support Epo concentration-dependent 3HdT incorporation in bone marrow–derived EPCs. (A) Marrow cell preparations were cultured in HCEM in the presence (or absence) of either Epo (0.2 U/mL) or IL-3 (10 ng/mL). At the indicated intervals, rates of cytokine-dependent proliferation were assayed based on stimulated rates of 3HdT incorporation. (mean incorporation rates ± SD, n = 3). (B) Diagrammed are EpoR-HM and wt-EpoR forms together with box1-associated JAK2 kinase and cytoplasmic phosphotyrosine sites within the wt-EpoR. (C) Marrow preparations from EpoR-HM or wt-EpoR control mice were cultured in the presence of Epo at the indexed concentrations. At 14 hours of culture, rates of Epo-dependent 3HdT incorporation were determined. Mean incorporation values (± SD) for 3 wt-EpoR and 3 EpoR-HM mice are shown. (D) To confirm apparent differences in Epo-dependent 3HdT incorporation as supported by EpoR-HM versus wt-EpoR, EPCs from EpoR-HM and control mice also were expended in the presence of dexamethasone, SCF, and Epo for 65 hours and were then tested for Epo responsiveness. Values are means ± SD of triplicate samples for 2 wt-EpoR and EpoR-HM mice (and outcomes are representative of 2 independent repeated experiments).
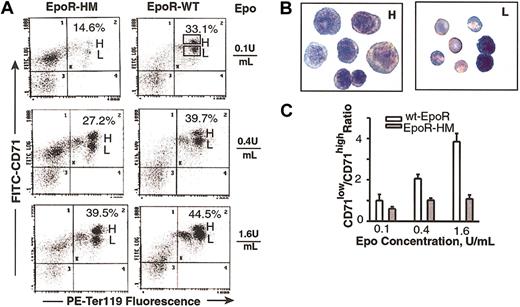
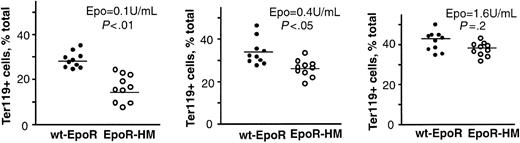
As EPCs develop beyond the erythroid colony-forming unit (CFU-e) stage, a lineage- and stage-specific cell surface antigen, Ter119, is expressed that is thought to occur in association with glycophorin A.33 This Epo-dependent developmental event was used to establish a second quantitative Epo bioassay. First, a time course for Ter119-positive cell formation from normal marrow-derived EPCs was defined (Figure 2A, left panel), and maximum frequencies of Ter119-positive cells were observed to develop within 42 hours of culture. The Epo concentration-dependent nature of this response also was defined (Figure 2A, right panel) and at 0.4 U/mL and 0.1 U/mL was limited to submaximal levels. This optimized assay next was used to study the Epo dose-dependent EPCs from EpoR-HM and wt-EpoR mice. As Epo was limited from 1.6 to 0.4 to 0.1 U/mL, an attenuated ability of PY-null EpoR-HM receptors to support Ter119-positive EPC formation was discovered (Figure 2B-C). At an Epo concentration of 0.1 U/mL (and as corrected for background levels of Ter119-positive cells) the efficiency of EpoR-HM signaling of this response was 3.4-fold below that of the wt-EpoR. In Epo at 0.4 U/mL and 1.6 U/mL, this defect was reversed with near wild-type activity observed for EpoR-HM at 1.6 U/mL. To possibly more narrowly define the stage(s) of EPC development that might be most affected by deficient EpoR-HM signaling, cells cultured in Epo at 0.1, 0.4, and 1.6 U/mL were costained with PE-Ter119 and FITC–anti-CD71 antibodies (at a penultimate stage of erythropoiesis, CD71 levels sharply increase). Costaining resolved a subpopulation of early CD71high Ter119-positive cells from a derived and more mature subpopulation of CD71low Ter119-positive cells (Figure 3A). Analyses of the frequencies of these CD71high to CD71low subpopulations as they formed from EPCs isolated from EpoR-HM mice (versus wt-EpoR mice) revealed an attenuated ability of EpoR-HM to support CD71low, Ter119-positive EPC formation. This finding also was analyzed as the ratios of these cell subtypes at varied Epo doses (Figure 3C). In addition, these differently staged Ter119-positive EPCs were isolated by fluorescence-activated cell sorting (FACS), and CD71high EPCs were observed to correspond to proerythroblasts and basophilic erythroblasts, while derived CD71low EPCs correspond to chromatophilic erythroblasts (Figure 3B). In Figure 4, direct values for Epo dose-dependent Ter119-positive cell formation from EpoR-HM versus wt-EpoR EPCs are summarized for assays using 10 mice.
Development of EPCs from EpoR-HM mice is attenuated at a Ter119-positive, CD71high stage. (A) EPCs in marrow preparations from EpoR-HM and wt-EpoR mice were expanded in the presence of Epo at 0.1, 0.4, or 1.6 U/mL. At 42 hours of culture, Ter119 and CD71 expression levels were assayed. Indicated in representative flow profiles (as percents of total events) are frequencies of Ter119-positive CD71+ populations (H gate, Ter119-positive CD71high; L gate, Ter119-positive CD71low EPCs). (B) Ter119-positive CD71high and CD71low EPCs were isolated by FACS and stained as cytospin preparations. For each, representative sets of cells are shown (predominantly proerythroblasts and basophilic erythroblasts as CD71high EPCs and chromatophilic erythroblasts as CD71low EPCs) (original magnification, × 400). (C) Graphed are mean ratios (plus or minus standard deviation) of CD71low/CD71high Ter119-positive cells formed in the presence of Epo at 0.1, 0.4, or 1.6 U/mL for EpoR-HM versus wt-EpoR mice (n = 3 + n = 3 mice).
Development of EPCs from EpoR-HM mice is attenuated at a Ter119-positive, CD71high stage. (A) EPCs in marrow preparations from EpoR-HM and wt-EpoR mice were expanded in the presence of Epo at 0.1, 0.4, or 1.6 U/mL. At 42 hours of culture, Ter119 and CD71 expression levels were assayed. Indicated in representative flow profiles (as percents of total events) are frequencies of Ter119-positive CD71+ populations (H gate, Ter119-positive CD71high; L gate, Ter119-positive CD71low EPCs). (B) Ter119-positive CD71high and CD71low EPCs were isolated by FACS and stained as cytospin preparations. For each, representative sets of cells are shown (predominantly proerythroblasts and basophilic erythroblasts as CD71high EPCs and chromatophilic erythroblasts as CD71low EPCs) (original magnification, × 400). (C) Graphed are mean ratios (plus or minus standard deviation) of CD71low/CD71high Ter119-positive cells formed in the presence of Epo at 0.1, 0.4, or 1.6 U/mL for EpoR-HM versus wt-EpoR mice (n = 3 + n = 3 mice).
Overall outcomes for Epo-dependent Ter119-positive cell formation from EpoR-HM versus wt-EpoR EPCs. Graphed are the frequencies of Ter119-positive cells formed in the presence of Epo at 0.1, 0.4, and 1.6 U/mL for marrow preparations from 10 EpoR-HM and 10 wt-EpoR mice. Differences between means (horizontal bars) were tested for significance (P values) using Student t distribution.
Overall outcomes for Epo-dependent Ter119-positive cell formation from EpoR-HM versus wt-EpoR EPCs. Graphed are the frequencies of Ter119-positive cells formed in the presence of Epo at 0.1, 0.4, and 1.6 U/mL for marrow preparations from 10 EpoR-HM and 10 wt-EpoR mice. Differences between means (horizontal bars) were tested for significance (P values) using Student t distribution.
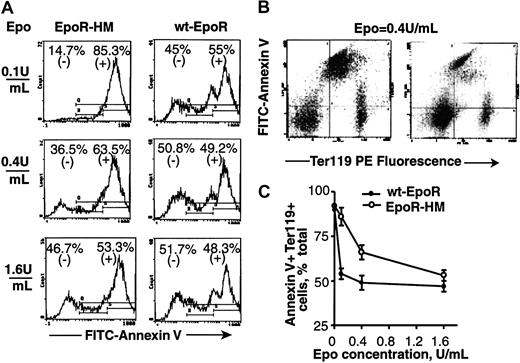
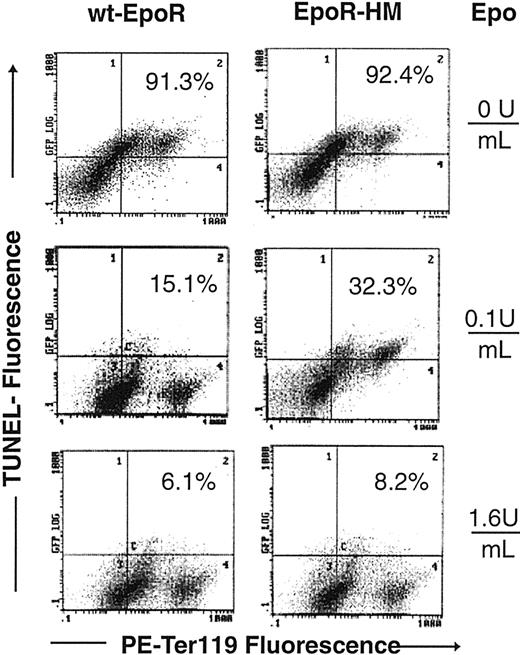
The EpoR is also known to promote red cell production by inhibiting EPC apoptosis.34 For Ter119-positive cells as they develop from EPCs in the above expansion system, frequencies of potentially apoptotic cells therefore also were assayed based initially on the staining of cell surface PS moieties with FITC-annexin V (Figure 5A-C). Clear Epo concentration-dependent differences were observed in annexin V staining of EpoR-HM Ter119-positive EPCs, and at an Epo dose of 0.1 U/mL levels of annexin V–negative cells were 3-fold greater for wt-EpoR EPCs (14.7%) than for EpoR-HM EPCs (45%). In Figure 6, overall outcomes for 2 independent Epo dosing and annexin V staining experiments are summarized. Annexin V previously has been used to assay apoptotic cells in Ter119-positive splenocytes from phenylhydrazine-treated mice.35 However, plasma membrane phospholipid compositions are modulated during red cell development,36 and differences in annexin V staining of EPCs from EpoR-HM versus wt-EpoR mice might, in part, reflect altered development. Apoptosis among Ter119-positive cells from EpoR-HM and control wt-EpoR mice therefore was also assayed by TUNEL (Figure 7). At an Epo dose of 0.1 U/mL (and at 42 hours culture), frequencies of TUNEL-positive Ter119-positive EPC cells from EpoR-HM mice were increased approximately 2-fold over wt-EpoR EPCs. At a high Epo dose (1.6 U/mL) this apparent defect in EPC survival was reversed, and this is in keeping with physiologic dose-response profiles observed in analyses of Epo-dependent EPC proliferation and development.
Cell surface phosphatidylserine levels are increased among EpoR-HM EPCs. (A) Bone marrow preparations from EpoR-HM and wt-EpoR mice were cultured in the presence of Epo at 0.1, 0.4, or 1.6 U/mL. At 42 hours of culture, frequencies of FITC–annexin V-positive (and -negative) cells were determined and are indicated as percents of total events. The gate for positive cells was set using unstained cells. Primary data are shown for representative EpoR-HM and wt-EpoR EPCs. (B) Also shown is a representative annexin V versus Ter119 flow cytometry profile for EpoR-HM EPCs (left) versus wt-EpoR EPCs (right) cultured in Epo at 0.4 U/mL (mid-range dose). (C) Graphed are direct frequencies of annexin V–positive and Ter119-positive cells formed from EpoR-HM (n = 3) and wt-EpoR (n = 3) mice at increasing doses of Epo (means ± SD).
Cell surface phosphatidylserine levels are increased among EpoR-HM EPCs. (A) Bone marrow preparations from EpoR-HM and wt-EpoR mice were cultured in the presence of Epo at 0.1, 0.4, or 1.6 U/mL. At 42 hours of culture, frequencies of FITC–annexin V-positive (and -negative) cells were determined and are indicated as percents of total events. The gate for positive cells was set using unstained cells. Primary data are shown for representative EpoR-HM and wt-EpoR EPCs. (B) Also shown is a representative annexin V versus Ter119 flow cytometry profile for EpoR-HM EPCs (left) versus wt-EpoR EPCs (right) cultured in Epo at 0.4 U/mL (mid-range dose). (C) Graphed are direct frequencies of annexin V–positive and Ter119-positive cells formed from EpoR-HM (n = 3) and wt-EpoR (n = 3) mice at increasing doses of Epo (means ± SD).
Overall outcomes for annexin V analyses of Ter119-positive EPCs from EpoR-HM versus wt-EpoR mice. Summarized are direct frequencies of annexin V–stained Ter119-positive EPCs formed in the presence of Epo at the indicated doses (0.1, 0.4, 1.6 U/mL) (events scored are percent annexin V–positive cells among Ter119-positive cells). Data points are frequencies for individual mice (and are derived from 2 representative but independent experiments). Horizontal lines represent mean values.
Overall outcomes for annexin V analyses of Ter119-positive EPCs from EpoR-HM versus wt-EpoR mice. Summarized are direct frequencies of annexin V–stained Ter119-positive EPCs formed in the presence of Epo at the indicated doses (0.1, 0.4, 1.6 U/mL) (events scored are percent annexin V–positive cells among Ter119-positive cells). Data points are frequencies for individual mice (and are derived from 2 representative but independent experiments). Horizontal lines represent mean values.
Epo dose-dependent increases in TUNEL-positive EPCs from EpoR-HM mice. Marrow preparations from EpoR-HM and wt-EpoR mice were cultured in the presence of Epo at 0, 0.1, or 1.6 U/mL. At 42 hours of culture, cells were processed through TUNEL reactions and stained for Ter119 expression. As compared with wt-EpoR controls, Ter119-positive EPCs from EpoR-HM mice had increased frequencies of TUNEL-positive cells (eg, 32.3% TUNEL-positive EpoR-HM EPCs versus 15.1% TUNEL-positive EPCs for wt-EpoR at 0.1 U/mL Epo). Percents are frequencies of TUNEL-positive cells among Ter119-positive EPCs.
Epo dose-dependent increases in TUNEL-positive EPCs from EpoR-HM mice. Marrow preparations from EpoR-HM and wt-EpoR mice were cultured in the presence of Epo at 0, 0.1, or 1.6 U/mL. At 42 hours of culture, cells were processed through TUNEL reactions and stained for Ter119 expression. As compared with wt-EpoR controls, Ter119-positive EPCs from EpoR-HM mice had increased frequencies of TUNEL-positive cells (eg, 32.3% TUNEL-positive EpoR-HM EPCs versus 15.1% TUNEL-positive EPCs for wt-EpoR at 0.1 U/mL Epo). Percents are frequencies of TUNEL-positive cells among Ter119-positive EPCs.
In EpoR-HM mice, serum growth factors are elevated and stress erythropoiesis is compromised
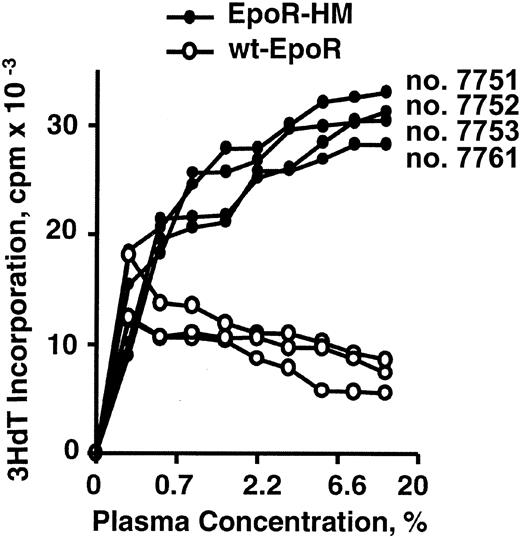
The limited ability of the PY-null EpoR-HM receptor form to support EPC development in the presence of Epo at limiting concentrations suggests that the in vivo function of this receptor form might be assisted by compensatory mechanisms. To test this basic hypothesis, serum was isolated from adult age- and sex-matched EpoR-HM and wt-EpoR mice and was assayed for activity in promoting the proliferation of an ES cell–derived erythroid cell line, G1E2. For serum samples from all EpoR-HM mice analyzed, significant increases in activity (stimulated 3HdT incorporation) were discovered (Figure 8). Available systems for the immunoassay of murine Epo37 proved to be quite variable, and the extent to which elevated Epo levels might account for increases in EpoR-HM serum growth factor activity presently is uncertain.
Serum growth factor activity is elevated in EpoR-HM mice. Sterile plasma samples were prepared from 8-week-old EpoR-HM and wt-EpoR mice and were used to challenge G1E2 cells in a 3HdT incorporation assay format. Data shown are for samples from individual EpoR-HM and wt-EpoR mice, and outcomes are representative of 2 independent experiments.
Serum growth factor activity is elevated in EpoR-HM mice. Sterile plasma samples were prepared from 8-week-old EpoR-HM and wt-EpoR mice and were used to challenge G1E2 cells in a 3HdT incorporation assay format. Data shown are for samples from individual EpoR-HM and wt-EpoR mice, and outcomes are representative of 2 independent experiments.
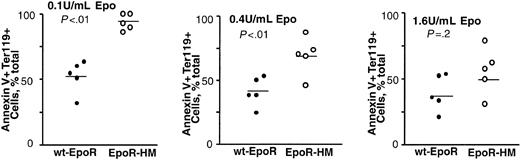
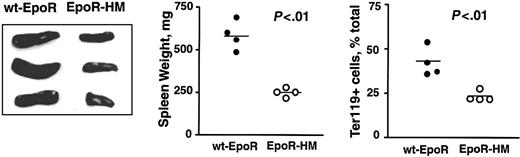
Again based on the above-observed attenuated Epo dose-dependent in vitro development of EpoR-HM EPCs, we also investigated whether in vivo erythropoiesis also might be perturbed due to stress. Here, EpoR-HM and control wt-EpoR mice were treated with phenylhydrazine (60 mg/kg at 0 and 24 hours), and at 120 hours increases in splenic erythropoiesis due to induced hemolytic anemia were analyzed. As expected, spleens from wt-EpoR mice were increased markedly in size (and weight) and contained relatively high frequencies of Ter119-positive EPCs (Figure 9). By direct comparison, spleens from EpoR-HM mice failed to support efficient erythroid hyperplasia and also contained lower frequencies of Ter119-positive cells. These outcomes suggest that despite the apparent existence of in vivo compensatory mechanisms, EpoR-HM mice falter in significant ways to support stress erythropoiesis in this hemolytic anemia model.
Attenuated splenic stress erythropoiesis in EpoR-HM mice in response to phenylhydrazine-induced anemia. EpoR-HM and wt-EpoR mice were treated at 0 and 24 hours with phenylhydrazine. At 120 hours, spleens were isolated, photographed, weighed, disrupted, and analyzed for Ter119 expression. Plotted values are spleen weights (mg) and direct frequencies of Ter119-positive splenic EPCs from 4 EpoR-HM and 4 wt-EpoR mice. The significance (P values) of differences between overall mean values (horizontal bars) was tested using Student t distribution.
Attenuated splenic stress erythropoiesis in EpoR-HM mice in response to phenylhydrazine-induced anemia. EpoR-HM and wt-EpoR mice were treated at 0 and 24 hours with phenylhydrazine. At 120 hours, spleens were isolated, photographed, weighed, disrupted, and analyzed for Ter119 expression. Plotted values are spleen weights (mg) and direct frequencies of Ter119-positive splenic EPCs from 4 EpoR-HM and 4 wt-EpoR mice. The significance (P values) of differences between overall mean values (horizontal bars) was tested using Student t distribution.
Discussion
In type 1 and 2 cytokine receptor systems, JAKs are well established as essential upstream signaling components.15,16 Less clear is whether JAK-targeted receptor PY sites also contribute in basic ways to biofunction or perhaps play only modulatory roles. The EpoR system provides an interesting case, and although specific signaling events have been at least tentatively assigned to 8 evolutionarily conserved PY residues,38 PY-null EpoR forms nonetheless exert substantial erythropoietic activity via JAK2-coupled routes per se when expressed at physiological levels in knock-in28 and transgenic mouse models.26,27 If EpoR PY sites are important, these prior studies predict first that PY-null EpoR-HM function in vivo might be assisted by compensatory mechanisms and, second, that primary erythroid progenitor cells from EpoR-HM mice also might display Epo dose-dependent biosignaling defects in vitro. The present investigations were performed to test these hypotheses. In vitro analyses of Epo-stimulated 3HdT incorporation, Ter119-positive cell formation, cell surface phosphatidylserine levels, and TUNEL-positive EPCs each revealed Epo dose-dependent deficiencies for erythroid progenitor cells from EpoR-HM mice. In addition, serum growth factor activities were elevated in EpoR-HM mice, and capacities to respond to phenylhydrazine-induced anemia were attenuated. Aspects of these findings selected for discussion include (1) insight gained into the functional capacities of this minimal JAK2-coupled PY-null EpoR form through in vitro Epo dosing experiments; (2) major wt-EpoR–directed events that may be attenuated during PY-null EpoR-HM signaling and possibly compensated for during JAK2-only signaling; (3) the possible nature of elevated serum growth factors in EpoR-HM mice; and (4) factors that may limit EpoR-HM–mediated stress erythropoiesis.
One primary action of Epo is to inhibit progenitor cell death; this involves, at least in part, Epo-dependent increases in Bcl-xl expression39 (and roles for Bcl-xl also appear to extend to late stages including reticulocytes).40 It therefore is possible that the presently observed deficiencies in EpoR-HM–supported EPC proliferation and differentiation may reflect survival defects. For proliferation outcomes, until direct evidence for Epo effects on EPC mitogenesis or cell cycle parameters is generated, this clearly should be considered. Differentiation outcomes, however, can be considered in more specific contexts. With regard first to the stage at which EpoR-HM–dependent EPC formation appears to falter, Ter119 and annexin V binding assays indicate that this is most prominent for a Ter119-positive CD71low EPC pool and less prominent for a less mature Ter119-positive CD71high population. Therefore, these EPCs at defined narrow stages are proposed to depend most upon wt-EpoR PY sites and linked pathways. Annexin V binding assays may well reflect differences in survival potential, and this is supported by the outcomes of TUNEL assays for Ter119-positive EpoR-HM versus wt-EpoR EPCs. However, erythroid cell membrane compositions may change during late differentiation,36 and EpoR-HM–altered differentiation events therefore might also be detected by annexin V binding assays. This does not discount the significance of observed differences between EpoR-HM and wt-EpoR EPCs but does raise possibly interesting questions regarding survival-versus differentiation-based explanations. Questions concerning underlying mechanisms ultimately must be advanced through direct analyses of transduction events (and modulated genes) in purified stage-specific EPCs from EpoR-HM and wt-EpoR mice.
The possible nature of EpoR PY–dependent events that provide an advantage for wt-EpoR EPC development (versus those pathways that support PY-independent EpoR-HM function) is also of interest to consider. EpoR-HM retains juxtamembrane sites for JAK2 binding and PY-null yet JAK2-coupled EpoR forms previously have been shown to efficiently stimulate Myc gene transcription.41 In 32D cell sublines, a transfected EpoR-HM construct has also been shown to efficiently activate Bcl-x gene transcription,42 but in FDCW2 cells this was assisted by an EpoR PY343 Stat5 binding site,43 and Epo-dependent Bcl-x gene expression also has been reported to be attenuated in splenic erythroblasts from Stat5a–/–5b–/– mice.35 Thus, the issue of possible PY343 and Stat5 contributions to EpoR and JAK2-dependent Bcl-x gene expression is presently unresolved (and in EpoR-HM mice, this issue may be complicated further by apparent compensatory mechanisms). Within the EpoR system, JAK2 also has been reported to interact with Vav44 and to bind and perhaps cofunction with a Src kinase (especially Src or Lyn).45,46 Given the substantial bioactivity retained by EpoR-HM, the nature of presently poorly understood EpoR PY–independent JAK2-linked pathways should be interesting to discover. Beyond PY343 and Stat5, an EpoR PY479 site for p85/phosphatidylinositol-3 kinase (PI3K) coupling previously has been shown to likewise efficiently restore function in transduced fetal liver cells47 and to act through proposed Akt kinase and forkhead transcription factor routes to sustain erythroid progenitor cell survival.48 Together these studies underline PY343-plus Stat5 and PY479-plus p85/PI3K as prime candidates for supporting the PY-dependent enhanced activity of the wt-EpoR. When considering activities of EpoR-HM (as well as EpoR PY343 or EpoR PY479 forms), it also is important to consider effects of the absence of EpoR PY sites that recruit negatively acting factors (including Cis, SOCS3, and SHP-1)49,50 as well as the possible effects of EpoR truncations.51
In EpoR-HM mice, 2 in vivo perturbations also were discovered: G1E2 cell–based bioassays revealed an increase in serum growth factor activities, and EpoR-HM mice displayed a restricted capacity to mount splenic stress erythropoiesis in response to phenylhydrazine-induced anemia. In EpoR-HM serum, one elevated component may be Epo, and this is an essential factor for the sustained proliferation of the G1E2 cells used in bioassays. However, when FDCW2-EpoR cells were used (versus control parental FDCW2 cells), this EpoR-HM elevated serum activity was not so obviously detected, and this outcome raises questions as to whether levels of additional hematopoietic growth factors might also be increased in EpoR-HM mice (although their nature presently is unknown). Elevated levels of Epo (and/or other growth factors) might also account in part for a previously observed limited capacity of EpoR-HM mice to increase erythropoietic output in response to administered erythropoietin.28 Finally, the limited ability of EpoR-HM mice to respond to stress-induced anemia with robust splenic erythropoiesis merits consideration. In EpoR-HM mice, this defect exists despite sharp increases in Epo levels that are induced by phenylhydrazine52 and appears to perhaps be stronger than perturbations in steady-state EPC formation. Stress erythropoiesis is known to have several unique features. This includes roles in early EPCs for glucocorticoid receptor action,53 emerging roles for bone morphogenetic proteins (BMPs),54 and heightened roles for SCF action.55 Whether these, or perhaps other collateral signaling factors, integrate with EpoR PY–coupled events likewise should be interesting to assess in future studies, as should the extent to which other clinically important erythropoietic stress agents might differentially stimulate splenic and marrow erythropoiesis in EpoR-HM (and EpoR-H) knock-in mice.
Prepublished online as Blood First Edition Paper, July 17, 2003; DOI 10.1182/blood-2003-01-0078.
Supported by NIH R01 HL44491 (D.M.W.).
M.P.M. and V.G.K. contributed equally to this study.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank Dr James Ihle (St Jude Children's Research Hospital) for the generous provision of EpoR-HM mice and Elain Kunze and Susan Margargee (The Pennsylvania State University) for expert assistance with flow cytometric analyses.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal