Abstract
Macrophage receptor with collagenous structure (MARCO) is a scavenger receptor expressed in peritoneal macrophages and in a subpopulation of macrophages in the marginal zone of the spleen and in the medullary cord of lymph nodes. By global gene expression analysis, it has been found that the MARCO mRNA was one of the most up-regulated in splenic dendritic cells (DCs) following lipopolysaccharide or bacterial activation and in granulocyte-macrophage colony-stimulating factor (GM-CSF)–treated microglial cells. Here we show that MARCO is expressed on splenic DCs at late time points after activation and that its expression correlates with profound changes in actin cytoskeleton organization in DCs and microglia. During maturation, DCs undergo profound rearrangements of actin cytoskeleton. Immature DCs are adherent with visible actin cables, while fully mature, MARCO-expressing, splenic DCs are nonadherent, round in shape, and have an actin cytoskeleton with a punctate distribution. The simple expression of MARCO was sufficient to induce these cytoskeleton modifications in DCs. MARCO-transfected immature DCs acquired a typical morphology of mature DCs and did not rearrange the actin cytoskeleton following activation. Moreover, DCs in which MARCO was knocked down did not reach the mature phenotype and maintained the typical morphology of transitional DCs. MARCO expression in DCs and microglial cells was also associated with a decrease of antigen internalization capacity. Thus, the MARCO receptor is important for actin cytoskeleton rearrangements and the down-regulation of antigen uptake function during DC and microglial cell maturation.
Introduction
Scavenger receptors (SRs) are membrane glycoproteins able to bind chemically modified low-density lipoprotein (LDL).1 There are 3 classes of SRs: A, B, and C.1 SRs-A are homotrimeric glycoproteins, with collagenous structure, composed of 77-kDa monomers distinct in 3 types. Type I SRs-A differ from type II in that they have a 110–amino acid SR cysteine-reach (SRCR) domain at the C terminus of the protein.2 Type III SRs-A have an altered C-terminal domain trapped within the endoplasmic reticulum and can act as dominant-negative isoforms.3 SRs-A are widely expressed in macrophages in various tissues (liver, lung, gut, spleen, thymus, and lymph nodes) and function as pattern recognition molecules.1 They can directly bind both Gram-positive and Gram-negative bacteria by interacting with their cell wall components, such as lipoteichoic acid (LTA) and lipopolysaccharide (LPS).2,4
Macrophage receptor with collagenous structure (MARCO) is an SR-A, identified in mouse and human, with a structure similar to type I molecules. It is a trimer containing a collagenous domain and an SRCR portion at the C-terminal domain.5,6 It can bind both Gram-positive and Gram-negative bacteria but not yeast.6
By ectopic expression of MARCO in fibroblasts, a role in actin cytoskeleton rearrangement has been attributed to the cysteine-reach domain V of this protein. MARCO-expressing fibroblasts show, indeed, complete disassembly of stress fibers and focal adhesions and the formation of structures similar to lamellipodia and long dendritic processes.7 In a different manner from the other type I SR-As, MARCO is constitutively expressed only in peritoneal macrophages and in a subpopulation of macrophages in the marginal zone of the spleen and in the medullary cord of lymph nodes.6 Nevertheless, its expression can be induced, following bacterial infection or intravenous injection of LPS, also in different types of macrophages, such as Kupffer cells in the liver and alveolar macrophages.8-10 Recently, by performing global gene expression analysis for the identification of genes involved in the process of dendritic cell (DC) maturation, the mRNA encoding for MARCO, absent in immature DCs, was identified as one of the most up-regulated genes 6 hours following LPS or bacterial activation.11,12 Analogously, MARCO mRNA was found as one of the most up-regulated by global gene expression analysis of microglial cells following exposure to granulocyte-macrophage colony-stimulating factor (GM-CSF).11
DCs are professional antigen-presenting cells able to prime an antigen-specific immune response.13 Their principal functions are antigen uptake, processing, and initiation of acquired immune responses. Immature, antigen-capturing DCs are located in nonlymphoid organs, such as the skin and mucosae where they continuously monitor the environment for incoming pathogens, or in the marginal zone of the spleen. Once activated by inflammatory stimuli such as bacteria, bacterial cell products and inflammatory cytokines lose the uptake function and migrate to the draining lymph node or from the marginal zone to the T-cell area of the spleen to activate T-cell responses.14 Thus, following the encounter of antigen/pathogen they undergo phenotypic and functional changes that transform them from antigen-capturing cells to antigen-presenting cells. During this process, DCs also undergo profound cytoskeleton rearrangements. Immature DCs are slightly adherent, low motile cells with organized actin-based cytoskeleton, while matured DCs are nonadherent cells with disassembled actin cytoskeleton.15
Microglial cells originate from bone marrow and migrate in the brain where they finish their differentiation under the influence of growth factors and cytokines released by resident cells.16-18 Macrophage CSF (M-CSF) and GM-CSF are 2 cytokines believed to be particularly important in the process of microglial cell differentiation. Both stimulate proliferation of neonatal microglia but differently affect their phenotype and functions.19 In particular, the capacity to produce cytokines and present antigens to T cells are differentially regulated by microglia exposure to the CSF cytokines. GM-CSF–treated microglial cells show enhanced ability to process and present antigens and enhanced antigen-presentation capacity.20,21 Given the described role of MARCO in the induction of actin cytoskeleton rearrangements in fibroblast and given the strong up-regulation of MARCO mRNA in maturing DCs and microglial cells, we have hypothesized that MARCO could be involved in regulating the profound actin cytoskeleton rearrangements occurring in maturing DCs and microglial cells. Since the actin cytoskeleton is strongly involved in the phagocytic processes we have also hypothesized that MARCO expression could influence the ability of antigen internalization.
In the present study, we show that MARCO is transiently expressed at the surface of mouse splenic DCs at late time points after LPS stimulation. We also show that it is one of the molecules responsible for actin cytoskeleton rearrangements and, surprisingly, for the decrease of the phagocytic function of DCs. Analogously, we provide evidence that MARCO-expressing microglial cells appear round, slightly adherent, and have a reduced phagocytic activity if compared with MARCO-negative microglial cells.
Materials and methods
DCs and microglial cells
D1 cells were cultured in Iscove modified Dulbecco medium (IMDM; Sigma, St Louis, MO) containing 10% heat-inactivated fetal bovine serum (Gibco-BRL, Gaithersburg, MD), 100 IU penicillin, 100 μg/mL streptomycin, 2 mM l-glutamine (all from Sigma), and 50 μM β-mercaptoethanol (in complete IMDM) with 30% supernatant from R1 medium (supernatant from NIH3T3 fibroblasts transfected with GM-CSF).22
To obtain fresh splenic DCs, unfractionated spleen cells were cultured at a density of 3 × 105 to 5 × 105 cells/mL in R1-conditioned medium. Cultures were fed with fresh R1-conditioned medium every 3 to 4 days. DC-enriched cultures were collected at days 12 to 15.
For preparation of microglial cells, neonatal microglia were derived from newborn (< 24 hours after birth) SJL/J strain mice as described.19 After removal of the meninges under a dissecting microscope, brains were mechanically disrupted and filtered through 100-μm cell strainers. Cells were seeded in modified essential medium (Gibco-BRL) and supplemented with 10% fetal calf serum (FCS), 5 μg/mL insulin (Gibco-BRL), and 2.0 mg/mL l-glucose (Sigma) for 12 to 14 days. Confluent mixed glial cultures were shaken overnight on an orbital shaker (first shake). Adherent glial cells were trypsinized, split, and reseeded for an additional 10 to 12 days of culture. The procedure was repeated twice (second and third shakes). The purity of each preparation was assessed by CD11b staining and was always more than 93%. GM-CSF (10 ng/mL) and M-CSF (5 ng/mL) were added at the beginning of the cultures and again every 3 days.
Sample preparation and array hybridization
Antisense cRNA was prepared following Affymetrix (Santa Clara, CA) recommendations. Briefly, total RNA was extracted from frozen pellets using the Trizol procedure. The Oligotex kit from Qiagen (Chatsworth, CA) was used to purify mRNA. Double-stranded cDNA was retrotranscribed using a modified oligo dT primer with a 5′ T7 RNA polymerase promoter sequence and the Superscript Choice System for cDNA synthesis (Life Technologies, Gaithersburg, MD). Double-stranded cDNA (1 μg) was transcribed to cRNA with the ENZO kit (Affymetrix). cRNA was purified on an affinity column (RNeasy; Qiagen) and then fragmented to an average size of 50 to 200 bases, by incubation for 35 minutes at 94°Cin40mMTris (tris(hydroxymethyl)aminomethane)–acetate (pH 8.1), 100 mM potassium acetate, and 30 mM magnesium acetate. Samples were diluted in the hybridization solution (1 M NaCl; 10 mM Tris [pH 7.6]; 0.005% Triton X-100; 0.1 mg/mL herring sperm DNA; and BioB-, BioC-, BioD-, and cre-control cRNAs at a concentration of 1.5, 5, 25, and 100 pM, respectively) at a final concentration of 0.05 μg/mL and heated at 94°C for 5 minutes. Analysis of the samples was performed by hybridizing the fragmented cRNAs to the Affymetrix Mu6500 GeneChip array, representing approximately 6500 murine genes and expressed sequence tags (ESTs). Probe array hybridizations were carried out by placing the samples in the hybridization cartridge at a final volume of 200 μL/chip. Hybridizations were performed under rotation at 45°C for 16 hours. Following hybridization, the chips were rinsed with 6 × SSPE-T (0.9 M NaCl, 60 mM NaH2PO4, 6 mM EDTA [ethylenediaminetetraacetic acid], and 0.005% Triton X-100 adjusted to pH 7.6) and 0.5 × SSPE-T and stained by incubation with 2 μg/mL streptavidin-phycoerythrin (PE) (Molecular Probes, Eugene, OR) and 1 mg/mL acetylated bovine serum albumin (BSA; Sigma). The arrays were read at a resolution of 7.5 μm using a confocal scanner (Affymetrix) and analyzed with the MicroArray Suite 4.0 Gene Expression analysis program (Affymetrix).
Real-time reverse transcriptase–polymerase chain reaction (RT-PCR)
Total RNA was extracted from 106 cells using the TRIZOL reagent according to the recommended procedure (Gibco-BRL). Single-strand cDNA was synthesized using Superscript Reverse Transcriptase kit (Gibco-BRL). mRNA quantitation was performed using the ABI Prism 7700 Sequence Detection System as described,23 and amplification was performed using the Gene-Amp Gold PCR Reagent Kit (Perkin-Elmer, Applied Biosystem Division, Foster City, CA). For normalization, βactin mRNA was used.
Immunofluorescence staining
Microglial cells or DCs were plated on untreated coverslips and incubated overnight at 37°C. Cells were then fixed in 1% paraformaldehyde (PFA) for 15 minutes at room temperature and permeabilized in phosphate-buffered saline (PBS) containing 3% BSA and 0.01% Triton X-100 for 5 minutes. After washing twice with PBS, cells were incubated with 20 μg/mL ED318 anti-MARCO antibody (Serotec, Raleigh, NC) for 1 to 2 hours. After an extensive wash with PBS, cells were incubated with 20 μg/mL cytochrome 3 (CY3)–conjugated goat anti–rat immunoglobulin G (IgG; Jackson, West Grove, PA). For filamentous actin staining, 1 ng/mL fluorescein isothiocyanate (FITC)–conjugated phalloidin (Sigma) was added to the secondary antibody. All the antibodies were diluted in PBS containing 0.5% BSA.
For filamentous actin staining of green fluorescent protein (GFP)–positive cells, GFP-positive cells were incubated after the permeabilization step with 0.1 μg/mL TRITC (tetramethylrhodamine-5(and 6)-isothiocyanate)–conjugated phalloidin (Sigma). In some experiments, D1 cells were pretreated with 10 μg/mL LPS (Escherichia coli serotype 026:B6; Sigma) for varying times between 2 to 24 hours and then stained with phalloidin.
Immunohistochemistry
Sections (7 μm) from spleen of C57BL/6 mice were fixed in ice-cold acetone for 10 minutes. The sections were air-dried for 5 minutes, washed in PBS containing 0.1% Triton X-100 for 10 minutes, and extensively rinsed with PBS at room temperature. Sections were then incubated with FITC-conjugated anti-CD11c (50 μg/mL) or FITC-conjugated isotype control antibodies (Becton and Dickinson, Mountain View, CA) for 30 minutes at 4°C, and, after extensive wash with PBS, were further incubated with undiluted (200 μg/mL) anti-MARCO or isotype control antibodies for 15 minutes at 4°C. Finally, the secondary antibody, CY3-conjugated goat anti–rat IgG (Jackson), was added at a concentration of 20 μg/mL for 30 minutes at 4°C. All the antibodies were diluted in PBS containing 0.5% bovine serum albumin.
Expression construct
MARCO cDNA was obtained by polymerase chain reaction (PCR) amplification from LPS-activated D1 cells. The following primers were used for the PCR: sense, 5′GGC CGT CGA CTT TGG CCA CCT ATA AAG CTT3′; antisense, 5′GGC CGG ATC CGA CAC ACT GAT GAC CTC TCG3′. MARCO cDNA has been inserted in the PINCO retroviral vector24 using XhoI and BamHI restriction sites. CDNA amplification and cloning were performed using established molecular biology methods. All the reagents used were from Invitrogen (Carlsbad, CA).
Retroviral infection of DCs
The PINCO retroviral vector used in this study24 encodes the enhanced green fluorescent protein (EGFP) under the transcriptional control of an SV40 promoter and MARCO cDNA under the control of the retroviral long terminal repeat (LTR). The production of high-titer vectors has been previously described.25 For the infection, D1 cells were cultured in the presence of viral supernatant (filtered with 0.45-μm filters) supplemented with 4 μg/mL polybrene (Sigma) for 3 hours. There were 3 infection cycles performed. After infection, D1 cells were plated in complete IMDM supplemented with 30% R1 medium. The efficiency of transduction was then evaluated by fluorescence-activated cell sorter (FACS) analysis; the efficiency was usually around 30%.
Microbeads and bacterial uptake
To evaluate the ability to phagocytose bacteria, DH5α bacteria expressing the fluorescent molecule DS-red (Clontech, Palo Alto, CA) were used. DCs were incubated at 37°C in antibiotic-free medium with DS-red bacteria for 1.5 hours, with a cell-bacteria ratio of 1:10. Cells were then observed under a confocal microscope (Biorad, Hercules, CA) to evaluate their uptake.
To evaluate the ability of DCs and microglial cells to phagocytose microbeads, cells were incubated 20′ at 4°C as control or at 37°C with a cell-bead ratio of 1:100. Cells were then washed with PBS and incubated 5′ with 0.05% Trypsin 0.53 mM EDTA (Gibco-BRL), to eliminate any residual nonphagocytosed bead. PE-conjugated microbeads (1 μm) were from Molecular Probes. Cells were eventually fixed in PFA 1% PBS. The uptake was evaluated by FACScan (Becton and Dickinson) analysis.
GeneBlock treatment
Both control and anti-MARCO ribozymes and the liposomes (GeneBlock) were gently given by Atugen (Berlin, Germany). Liposomes were loaded with the ribozymes by 30′ incubation at 37°C with a liposome-ribozyme ratio of 1:1. Cells were then incubated with loaded liposomes for one hour. Following the treatment with liposomes, some of the cells were activated using 10 μg/mL LPS. Afterward, cells were fixed in PFA 1% PBS and stained with phalloidin.
Results
MARCO is expressed in mouse DCs following LPS activation
We performed a differential transcription analysis of 6-hour and 18-hour LPS-activated versus immature DCs,11 using Affymetrix high-density oligonucleotide arrays displaying probes for 6500 genes and ESTs.26 For this analysis, we took advantage of the well-characterized DC line, D1.11,12 D1 cells are splenic myeloid DCs that can be maintained indefinitely in culture in the immature state in the presence of a conditional medium (fibroblast supernatant containing GM-CSF) and can be driven to full maturation upon stimulation with inflammatory products, mimicking the in vivo DC maturation process. MARCO-coding mRNA resulted as one of the most up-regulated molecules following LPS treatment. MARCO mRNA was absent in immature DCs, it was strongly up-regulated 6 hours after LPS treatment, and it was down-regulated again in fully mature DCs 18 hours after LPS treatment. The kinetic of MARCO mRNA up-regulation was confirmed by TaqMan analysis (Figure 1A-B). Diversely to the kinetic of mRNA expression, the protein was maximally expressed at the surface of D1 cells 18 to 24 hours after LPS treatment (Figure 1C), and it was down-regulated at 48 hours (data not shown). Figure 2 shows the immunofluorescence analysis of MARCO expression on D1 cells.
MARCO expression on DCs. (A) Fold increase of MARCO mRNA levels in LPS-activated D1 cells versus nonactivated cells revealed by GeneChip analysis.12 (B) Real-time PCR showing MARCO mRNA increase in D1 cells after LPS stimulation at the indicated time points with respect to nonactivated cells. Error bars indicate standard deviation of 3 different samples. (C) D1 cell surface expression of MARCO (monoclonal antibody [mAb] ED31) after LPS activation at the indicated time points analyzed by flow cytometry.
MARCO expression on DCs. (A) Fold increase of MARCO mRNA levels in LPS-activated D1 cells versus nonactivated cells revealed by GeneChip analysis.12 (B) Real-time PCR showing MARCO mRNA increase in D1 cells after LPS stimulation at the indicated time points with respect to nonactivated cells. Error bars indicate standard deviation of 3 different samples. (C) D1 cell surface expression of MARCO (monoclonal antibody [mAb] ED31) after LPS activation at the indicated time points analyzed by flow cytometry.
Immunofluorescence analysis of MARCO expression on LPS-activated D1 cells. D1 cells were grown on a glass coverslip. Immature (A,C) and 24-hour LPS-activated (B,D) D1 cells were stained with DAPI (4,6 diamidino-2-phenylindole), to identify the nuclei, and with the anti-MARCO mAb (A-B) or an irrelevant control (C-D). The MARCO expression was analyzed by fluorescence microscopy. Original magnification, × 400.
Immunofluorescence analysis of MARCO expression on LPS-activated D1 cells. D1 cells were grown on a glass coverslip. Immature (A,C) and 24-hour LPS-activated (B,D) D1 cells were stained with DAPI (4,6 diamidino-2-phenylindole), to identify the nuclei, and with the anti-MARCO mAb (A-B) or an irrelevant control (C-D). The MARCO expression was analyzed by fluorescence microscopy. Original magnification, × 400.
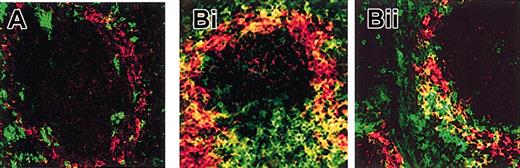
In vivo, the expression of MARCO was limited to the marginal zone of the spleen, and it was not associated with DCs in mice kept in pathogen-free conditions (Figure 3A). Conversely, MARCO-positive DCs were clearly present in the spleen marginal zone of mice that were maintained in a conventional non–pathogen-free animal house facility (Figure 3B).
Immunohistochemical analysis of MARCO expression in vivo. Frozen sections (7 μm) of spleens from mice kept in pathogen-free (A) or in conventional (B) animal house conditions were double stained with anti-CD11c (green) for DC identification and anti-MARCO ED31 (red) mAbs. Stained sections were analyzed by confocal microscopy. MARCO and CD11c double-positive cells (yellow) were present in spleens only of animals maintained in conventional animal house conditions (B). Original magnification, × 400.
Immunohistochemical analysis of MARCO expression in vivo. Frozen sections (7 μm) of spleens from mice kept in pathogen-free (A) or in conventional (B) animal house conditions were double stained with anti-CD11c (green) for DC identification and anti-MARCO ED31 (red) mAbs. Stained sections were analyzed by confocal microscopy. MARCO and CD11c double-positive cells (yellow) were present in spleens only of animals maintained in conventional animal house conditions (B). Original magnification, × 400.
Constitutive expression of MARCO induces actin cytoskeleton rearrangements in DCs
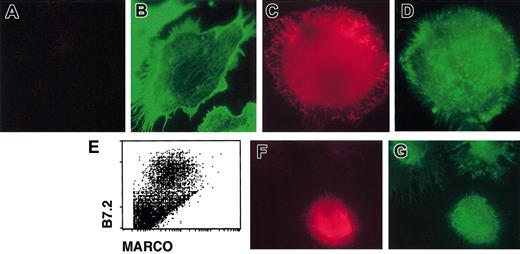
Immature DCs are able to internalize bacteria, but they lose this function once activated by external stimuli. MARCO expression at the surface of DCs appears at late time points after the encounter of the activation stimulus, when DCs have lost the uptake capacity.27 Thus, the kinetic of MARCO expression on DCs is not compatible with the putative MARCO function of bacteria phagocytosis. Since it has been observed that MARCO was responsible for the actin cytoskeleton rearrangement in MARCO-expressing fibroblasts,7 we first analyzed the actin cytoskeleton in immature D1 cells and in mature MARCO-expressing cells by staining with phalloidin. Immature D1 cells exhibited some heterogeneity in morphology. They were MARCO negative and mostly adherent with visible actin cables (Figure 4A-B). Mature, MARCO-expressing, D1 cells were nonadherent, round in shape, and had an actin cytoskeleton showing a punctate distribution (Figure 4C-D). When fresh DCs were derived from spleen, cells expressing a high level of B7.2 (mature DCs) were clearly MARCO positive (Figure 4E). Similar to D1 cells, all of the MARCO-expressing fresh splenic DCs were nonadherent, round in shape, and had a punctate actin (Figure 4F-G).
Actin cytoskeleton organization in immature and mature DCs. Immature (A-B) and 24-hour, LPS-activated (C-D) D1 cells were double stained for (A,C) MARCO (red) and (B,D) filamentous actin (green) and analyzed using a fluorescence microscope. Only nonactivated cells showed organized actin cytoskeleton with visible actin cables, while mature cells showed actin with a punctate distribution. (E) MARCO expression on LPS-activated, fresh spleen-derived DCs. Fresh spleen DCs were activated with LPS and double stained with anti-B7.2 and anti-MARCO antibodies; only activated B7.2-positive DCs were also MARCO positive. (F-G) Double staining of fresh LPS-activated splenic DCs for MARCO (red) and filamentous actin (green). MARCO-positive cells showed an actin cytoskeleton with a punctate distribution. Original magnification, × 600.
Actin cytoskeleton organization in immature and mature DCs. Immature (A-B) and 24-hour, LPS-activated (C-D) D1 cells were double stained for (A,C) MARCO (red) and (B,D) filamentous actin (green) and analyzed using a fluorescence microscope. Only nonactivated cells showed organized actin cytoskeleton with visible actin cables, while mature cells showed actin with a punctate distribution. (E) MARCO expression on LPS-activated, fresh spleen-derived DCs. Fresh spleen DCs were activated with LPS and double stained with anti-B7.2 and anti-MARCO antibodies; only activated B7.2-positive DCs were also MARCO positive. (F-G) Double staining of fresh LPS-activated splenic DCs for MARCO (red) and filamentous actin (green). MARCO-positive cells showed an actin cytoskeleton with a punctate distribution. Original magnification, × 600.
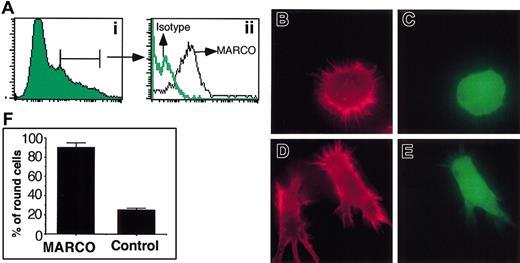
To investigate the role of MARCO in maturing DCs we analyzed the phenotype of DCs that expressed MARCO in a constitutive way. D1 cells were transduced with the cDNA encoding the full-length protein using the retroviral vector PINCO.24 This construct carries the enhanced green fluorescent protein (EGFP)–encoding cDNA under the control of SV40 promoter and MARCO cDNA under the control of the retroviral LTR. As shown in Figure 5A, GFP-expressing cells were also MARCO positive. Actin cytoskeleton was analyzed in MARCO-expressing cells. Strikingly, more than 90% of them showed the same phenotype: they were nonadherent, round in shape, and had a punctate actin (Figure 5). In contrast, control cells, infected with the empty PINCO vector, showed the typical phenotype of immature DCs, indicating that the simple expression of MARCO was sufficient to induce these morphologic modifications.
Effect of MARCO expression in D1 cell morphology. D1 cells were infected with the retroviral expression vector PINCO, encoding the EGFP and MARCO proteins, or the empty PINCO vector, as control, encoding only the EGFP protein. (A) MARCO-transduced cells were analyzed for MARCO expression by flow cytometry after staining with the anti-MARCO mAb or an irrelevant isotype-matched mAb. (Ai) EGFP expression on infected cells. (Aii) MARCO expression on EGFP-positive gated cells. (B) Virally infected cells were stained for filamentous actin (red) 48 hours after infection. Filamentous actin organization of MARCO-transduced D1 cells (C) identified as EGFP positive. (D) Filamentous actin organization of D1 cells infected with the empty retroviral vector (E) encoding only EGFP. Original magnification, × 600. (F) Percentage of MARCO-expressing or control cells showing a round morphology. A total of 400 cells were counted in randomly selected fields. The experiment was repeated 3 times with similar results. Error bars indicate SD.
Effect of MARCO expression in D1 cell morphology. D1 cells were infected with the retroviral expression vector PINCO, encoding the EGFP and MARCO proteins, or the empty PINCO vector, as control, encoding only the EGFP protein. (A) MARCO-transduced cells were analyzed for MARCO expression by flow cytometry after staining with the anti-MARCO mAb or an irrelevant isotype-matched mAb. (Ai) EGFP expression on infected cells. (Aii) MARCO expression on EGFP-positive gated cells. (B) Virally infected cells were stained for filamentous actin (red) 48 hours after infection. Filamentous actin organization of MARCO-transduced D1 cells (C) identified as EGFP positive. (D) Filamentous actin organization of D1 cells infected with the empty retroviral vector (E) encoding only EGFP. Original magnification, × 600. (F) Percentage of MARCO-expressing or control cells showing a round morphology. A total of 400 cells were counted in randomly selected fields. The experiment was repeated 3 times with similar results. Error bars indicate SD.
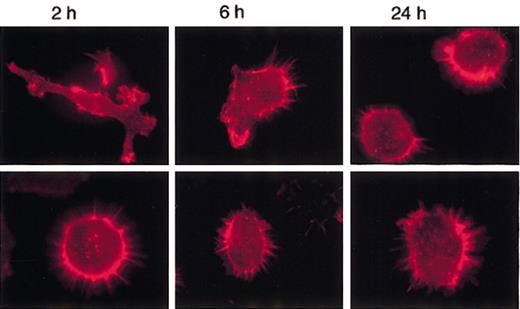
To verify if MARCO-expressing D1 cells could undergo actin cytoskeleton rearrangements following LPS treatment, cells were stimulated with LPS at different time points and the morphologic changes analyzed. Nontransfected and control cells showed similar behavior (data not shown). Following activation, control cells became very homogeneous: 2 hours after LPS treatment, all of them were strongly adherent with some veils and visible actin cables; after 6 hours they were polarized with lamellipodia and retraction fibers; and at 24 hours they were nonadherent, round in shape, and had a punctate actin (Figure 6). In contrast, MARCO-expressing cells never became adherent; more than 90% remained round in shape at 2 and 6 hours after LPS treatment (Figure 6). The actin cytoskeleton was completely depolymerized in some MARCO-expressing cells at 24 hours (data not shown). Thus, the expression of MARCO made the cells unresponsive to the morphologic changes induced by LPS, and it had a drastic effect in some cells at late time points, when, probably, the effect of the transduced MARCO could synergize with the effect of the endogenously produced MARCO.
MARCO-transduced D1 cells do not undergo actin cytoskeleton rearrangements following LPS activation. D1 cells infected with the MARCO-expressing (MARCO-positive cells; bottom row) or empty (control cells; top row) PINCO vector were activated with LPS and stained for filamentous actin (red) at the indicated times following LPS activation. Original magnification, × 600.
MARCO-transduced D1 cells do not undergo actin cytoskeleton rearrangements following LPS activation. D1 cells infected with the MARCO-expressing (MARCO-positive cells; bottom row) or empty (control cells; top row) PINCO vector were activated with LPS and stained for filamentous actin (red) at the indicated times following LPS activation. Original magnification, × 600.
Actin cytoskeleton modification in MARCO-expressing microglial cells
It has been shown that neonatal microglial cells grown in the presence of GM-CSF but not M-CSF express MARCO at the cell surface.28 We thus investigated if MARCO-expressing microglial cells showed differences in actin cytoskeleton organization with respect to untreated or M-CSF–treated cells. As shown in Figure 7, the 3 types of cells (untreated, M-CSF treated, or GM-CSF treated) showed different phenotypes. Untreated cells were MARCO negative and showed a high dendrite number (Figure 7A-B). Once activated with LPS for 24 hours they exhibited a very long cell body and remained MARCO negative (Figure 7G-H). As well as untreated cells, M-CSF–cultured cells were MARCO negative, remained negative after LPS treatment (Figure 7C-D,I-J), and were extremely vacuolated. In contrast, nonactivated GM-CSF–grown cells were in many cases nonadherent and round in shape (Figure 7E-F), a phenotype that correlated with MARCO expression and that was more evident after LPS treatment (Figure 7K-L).
MARCO expression and actin cytoskeleton organization on untreated, M-CSF–, and GM-CSF–treated microglial cells. (A-F) Nonactivated and (G-L) LPS-activated microglial cells were double stained for filamentous actin (green) and MARCO (red). MARCO-positive, nonactivated, and activated GM-CSF–treated microglial cells are mostly round and slightly adherent; some do not show any phalloidin staining (arrows). Original magnifications: nonactivated microglial cells (A, B, G, H), × 600; all other panels, × 400.
MARCO expression and actin cytoskeleton organization on untreated, M-CSF–, and GM-CSF–treated microglial cells. (A-F) Nonactivated and (G-L) LPS-activated microglial cells were double stained for filamentous actin (green) and MARCO (red). MARCO-positive, nonactivated, and activated GM-CSF–treated microglial cells are mostly round and slightly adherent; some do not show any phalloidin staining (arrows). Original magnifications: nonactivated microglial cells (A, B, G, H), × 600; all other panels, × 400.
Knocking down of MARCO leads to inhibition of morphologic, LPS-induced effects at late time points
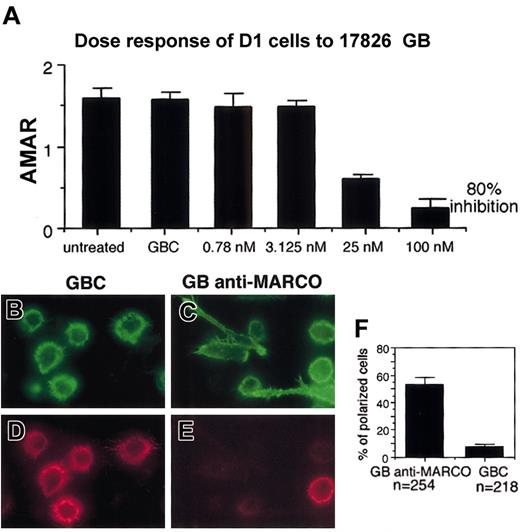
MARCO expression was knocked down in D1 cells using GeneBlocks, a new generation of ribozymes, from Atugen. The ability of different GeneBlocks to inhibit MARCO mRNA induction in D1 cells was tested using real-time PCR (Table 1). The GeneBlock giving 80% inhibition of MARCO expression was used in the following experiment together with a control GeneBlock, a ribozyme identical to the first but with a substitution in the sequence that made it nonfunctional. A dose inhibition test was performed to define the concentration of GeneBlock giving the maximal inhibition (Figure 8A). MARCO and control GeneBlocks were delivered inside the cells for 24 hours using liposomes; then the cells were stimulated with LPS in the presence of ribozymes. Morphologic modifications were tested 24 hours later. Cells treated with control GeneBlock expressed MARCO and were able to undergo cytoskeleton rearrangements; they showed typical morphology of mature DCs (Figure 8B,D,F). In contrast, cells treated with MARCO GeneBlock were much more heterogeneous; about 50% were adherent and lengthened or polarized, typical morphologies of transitional DCs (Figure 8C,E,F). Nevertheless, some nonadherent, round in shape, MARCO-negative DCs could also be detected, indicating that MARCO is sufficient to induce the morphologic modifications observed in mature DCs but is not the only molecule able to cause them.
MARCO knock-down by different GeneBlocks
GB identification no. . | % knock-down of the MARCO mRNA relative to GBC . |
|---|---|
| 17822 | 30.6 |
| 17823 | 72 |
| 17824 | 45.3 |
| 17825 | 72.8 |
| 17826 | 70.7 |
| 17827 | 47.8 |
| 17828 | 22.8 |
| 17829 | 26.8 |
GB identification no. . | % knock-down of the MARCO mRNA relative to GBC . |
|---|---|
| 17822 | 30.6 |
| 17823 | 72 |
| 17824 | 45.3 |
| 17825 | 72.8 |
| 17826 | 70.7 |
| 17827 | 47.8 |
| 17828 | 22.8 |
| 17829 | 26.8 |
MARCO mRNA levels have been analyzed by real-time PCR. The percent knock-down is obtained by setting the value of average MARCO-actin ratio (Figure 8) for control GeneBlock (GBC) as 100% and calculating the remaining value of average MARCO-actin ratio of GeneBlock (GB)—treated samples in relation to that of GBC. GBs were used at a concentration of 100 nM.
Effect of MARCO knock-down on DC morphology following LPS activation. (A) MARCO mRNA quantification by real-time PCR in LPS-activated D1 cells, treated with the indicated amounts of MARCO GeneBlock (GB). The average MARCO-actin ratio is the quotient of the fluorescent signal obtained from the MARCO mRNA amplification with a specific TaqMan ampliconset for MARCO divided by the value of the fluorescent signal obtained from the actin mRNA amplification with the TaqMan ampliconset for actin. These values are taken from a point where the amplification is in a linear range. The average is taken from triplicates. GBC indicates control GeneBlock. The percent knock-down is obtained by setting the value of average MARCO-actin ratio (AMAR) obtained with the GBC as 100% and calculating the remaining value of the GB-treated sample in relation to that of GBC. Double staining for filamentous actin (B-C) and MARCO (D-E) of D1 cells after treatment with anti-MARCO (GB anti-MARCO) and control (GBC) GeneBlocks cells were treated with GeneBlocks for 48 hours and activated with LPS during the last 24 hours. Knock-down of MARCO in D1 cells inhibits the progression toward the terminal maturation stage, and many cells show a morphology typical of transitional cells. Original magnifications, × 400. (F) Percentage of polarized or lengthened cells counted in randomly selected fields. The experiment was repeated twice with similar results. Error bars in panels A and F represent standard deviations from the mean calculated on the randomly selected fields.
Effect of MARCO knock-down on DC morphology following LPS activation. (A) MARCO mRNA quantification by real-time PCR in LPS-activated D1 cells, treated with the indicated amounts of MARCO GeneBlock (GB). The average MARCO-actin ratio is the quotient of the fluorescent signal obtained from the MARCO mRNA amplification with a specific TaqMan ampliconset for MARCO divided by the value of the fluorescent signal obtained from the actin mRNA amplification with the TaqMan ampliconset for actin. These values are taken from a point where the amplification is in a linear range. The average is taken from triplicates. GBC indicates control GeneBlock. The percent knock-down is obtained by setting the value of average MARCO-actin ratio (AMAR) obtained with the GBC as 100% and calculating the remaining value of the GB-treated sample in relation to that of GBC. Double staining for filamentous actin (B-C) and MARCO (D-E) of D1 cells after treatment with anti-MARCO (GB anti-MARCO) and control (GBC) GeneBlocks cells were treated with GeneBlocks for 48 hours and activated with LPS during the last 24 hours. Knock-down of MARCO in D1 cells inhibits the progression toward the terminal maturation stage, and many cells show a morphology typical of transitional cells. Original magnifications, × 400. (F) Percentage of polarized or lengthened cells counted in randomly selected fields. The experiment was repeated twice with similar results. Error bars in panels A and F represent standard deviations from the mean calculated on the randomly selected fields.
MARCO-expressing DCs and microglia show reduced phagocytic activity
To investigate if MARCO expression could have any effect in bacterial internalization, D1 cells, constitutively expressing MARCO, were compared with control immature cells for their ability to internalize bacteria. Cells were incubated with DH5α E coli expressing the DS-red protein for 1.5 hours at a multiplicity of infection of 10. The number of bacteria bound to the cell membrane or internalized was counted by scanning the cells using confocal microscopy. More than 200 cells per group were tested, and for each cell horizontal and vertical sections were analyzed. Surprisingly, MARCO-expressing DCs were strongly impaired in the bacterial internalization function (Figure 9). In control cells, the bacteria were contained inside big vacuoles (data not shown) that were completely absent in MARCO-expressing cells and mature cells, which showed a very similar behavior (data not shown). In contrast, the number of bacteria bound at the cell surface was higher in MARCO-expressing cells. The expression of MARCO was sufficient to induce some morphologic and functional modifications typical of mature DCs, such as actin cytoskeleton modifications and loss of the ability to internalize bacteria. Nevertheless, MARCO-expressing cells showed an immature phenotype when analyzed for the expression of major histocompatibility complex (MHC) class II and costimulatory molecules that could be up-regulated following LPS stimulation (data not shown). The reduced phagocytic activity of MARCO-expressing cells was confirmed by measuring the ability of D1 cells constitutively expressing MARCO and the ability of MARCO-positive (GM-CSF–treated) microglial cells to internalize PE-conjugated latex beads compared with control D1 cells and MARCO-negative (M-CSF–treated) microglial cells, respectively. As shown in Figure 9, MARCO-negative cells could internalize latex particles with a very high efficiency, while MARCO-expressing cells showed a strongly reduced phagocytic activity.
Effect of MARCO expression in antigen internalization. (A) D1 cells infected with the MARCO-expressing (MARCO) or the empty PINCO vector (control) were incubated with Ds-red E coli for 1.5 hours. Random fields of cells were counted and scored for the number of bacteria internalized (top panel) or bound to the cell membrane (bottom panel) by confocal microscopy. For each cell, horizontal and vertical sections were analyzed to count only internalized bacteria. The experiment was repeated twice with similar results. Error bars represent the standard deviations from the mean calculated on the indicated number of cells. (B) D1 cells infected with the MARCO-expressing (MARCO) or empty PINCO vector (control) were incubated for 20 minutes with PE-conjugated microbeads with a cell-bead ratio of 1:100. Uptake of microbeads at 4°Cor37°C has been measured on gated green cells by flow cytometry. MARCO-expressing cells show a reduced phagocytic activity. (C) GM-CSF– or M-CSF–grown microglial cells were treated as in panel B, and the efficiency of the beads' uptake at 4°Corat37°C was investigated by flow cytometry. GM-CSF–treated microglial cells show a reduced phagocytic activity. The experiment was repeated 3 times with similar results. Percentages in panels B and C represent the percentage of cells that have internalized beads.
Effect of MARCO expression in antigen internalization. (A) D1 cells infected with the MARCO-expressing (MARCO) or the empty PINCO vector (control) were incubated with Ds-red E coli for 1.5 hours. Random fields of cells were counted and scored for the number of bacteria internalized (top panel) or bound to the cell membrane (bottom panel) by confocal microscopy. For each cell, horizontal and vertical sections were analyzed to count only internalized bacteria. The experiment was repeated twice with similar results. Error bars represent the standard deviations from the mean calculated on the indicated number of cells. (B) D1 cells infected with the MARCO-expressing (MARCO) or empty PINCO vector (control) were incubated for 20 minutes with PE-conjugated microbeads with a cell-bead ratio of 1:100. Uptake of microbeads at 4°Cor37°C has been measured on gated green cells by flow cytometry. MARCO-expressing cells show a reduced phagocytic activity. (C) GM-CSF– or M-CSF–grown microglial cells were treated as in panel B, and the efficiency of the beads' uptake at 4°Corat37°C was investigated by flow cytometry. GM-CSF–treated microglial cells show a reduced phagocytic activity. The experiment was repeated 3 times with similar results. Percentages in panels B and C represent the percentage of cells that have internalized beads.
Discussion
Cells of the innate immune system, such as DCs and macrophages, recognize micro-organisms through the expression of receptors with a broad specificity for microbial molecular patterns.29 A family of receptors capable of binding micro-organisms is represented by scavenger receptors. The MARCO receptor belongs to the family of the scavenger receptors and it has been described as constitutively expressed on macrophages residing in the spleen marginal zone and in the lymph node medullary cord.6 It has been observed by global gene expression analysis of LPS and bacterial activated DCs and GM-CSF–differentiated microglial cells that MARCO mRNA was one of the most up-regulated.11,12,28 In the present study, starting from this experimental observation we have defined a role for MARCO on actin cytoskeleton rearrangements and regulation of antigen internalization in DCs and microglia.
During the process of maturation, DCs undergo profound actin cytoskeleton rearrangements. In particular, immature DCs appear adherent with well-organized actin cables, while mature DCs are nonadherent with altered actin cytoskeleton.15 Surface MARCO expression is induced in DCs late following LPS activation. A clear correlation can be found between MARCO expression and the rearranged actin cytoskeleton of mature DCs showing a punctate distribution. The same type of correlation between MARCO expression and actin cytoskeleton modifications can be observed in GM-CSF–treated microglial cells. These MARCO-expressing cells are nonadherent, round in shape, and have rearranged actin when compared with MARCO-negative untreated or M-CSF–treated microglial cells. In this study, we provide evidence that DC actin cytoskeleton modifications can be directly induced by MARCO expression. It is sufficient to constitutively express MARCO in immature splenic DCs for the cells to become round, nonadherent, and have punctate actin. In agreement with this observation, blocking MARCO expression in activated DCs, using anti-MARCO ribozymes, impedes, in a significant number of the cells, actin cytoskeleton rearrangements typical of maturing DCs and freezes them in the state of transient cells. Moreover the constitutive MARCO expression on immature DCs make them unresponsive to further cytoskeleton modifications induced by LPS and causes, in some cells, a complete depolymerization of actin cytoskeleton (data not shown) late after LPS activation when DCs start to express endogenous MARCO, maybe because the effect of the transduced MARCO synergizes with the effect of the endogenously produced MARCO.
MARCO expression per se is sufficient to induce actin cytoskeleton rearrangements without the necessity to stimulate MARCO via the binding with its natural ligands, such as Gram-positive or Gram-negative bacteria. This observation has been made also using other cell types. In particular, the simple ectopic expression of MARCO in fibroblasts was sufficient to induce actin cytoskeleton rearrangements and, in some cases, the protrusion of long cellular processes.7 Nevertheless, it cannot be excluded that MARCO is able to bind glass or some serum proteins. As an alternative, we can hypothesize that MARCO exerts its effect by interacting with another cell surface protein, as has been observed for the integrin α6Aβ1 in embryonic cells.30 In fact, the expression of this integrin in embryonic stem cells induced the extension of numerous filopodia and lamellipodia and migration, phenomena that were not associated with a direct engagement by α6Aβ1 of an extracellular matrix ligand but that appeared to depend on the association of α6Aβ1 with CD81, a member of the tetraspanin superfamily.30
The signaling pathway activated by MARCO remains to be defined. In immature human monocyte-derived DCs cytoskeletal architecture is strongly dependent on small GTPases Rho, Rac, and Cdc4231 that are involved in the formation of filopodia and podosomes, highly specialized adhesion structures important for cell motility, typical of immature DCs and absent in mature DCs. Perpetuation of cell movements and cytoskeleton architecture in immature human DCs depend on continued signaling and cooperative activation of Cdc42, Rac, and Rho.31 It cannot be excluded that MARCO is one of the receptors that contributes to the down-regulation of activated Cdc42 levels, although the MARCO-mediated effect on cytoskeleton rearrangements observed in some fibroblasts was not dependent on Cdc42 but was only partially dependent on Rac1.7 In fact, in mouse bone marrow–derived DCs, activated Cdc42 is regulated during maturation and it is detectable only in immature DCs.32 Down-regulation of activated Cdc42 during the process of DC maturation has been associated with the decrease of endocytic activity,32 a phenomenon regulated by actin cytoskeleton. However, the dependence on the endocytic activity on activated Cdc42 has been observed only in bone marrow–derived DCs and not in splenic DCs,33 while the expression of MARCO can be induced only on splenic DCs and not on bone marrow–derived DCs (data not shown). In maturing splenic DCs the control of the endocytic process, associated with actin cytoskeleton rearrangements, seems not to be dependent on Cdc42 but on mechanisms downstream of this molecule.33 Our attempts to investigate the signaling pathway activated by MARCO have failed because of the impossibility of obtaining a population uniformly expressing MARCO. Transfectant D1 cells lose the ability to divide and do not propagate in culture.
Initially, both human and mouse MARCOs were detected exclusively in macrophages in the marginal zone of the spleen and in the medullary cord of lymph node. Subsequently, it has been observed that MARCO expression was inducible on lung and liver macrophages in mice infected with Klebsiella pneumoniae,34 and human MARCO has been found in normal lavages of diseased lung.9 Moreover, MARCO could also be detected in alveolar macrophages of untreated mice that were maintained in a conventional animal house facility.9 Analogously, we found MARCO on splenic DCs in normal mice not housed in germ-free conditions. Thus, it is possible that a basal level activation due to spontaneous exposure of a less clean environment is necessary for MARCO expression.
Microglial cells express MARCO after GM-CSF treatment. This cytokine induces microglial cell molecular modifications that enable them to become fully competent antigen-presenting cells. Infiltrating T cells during an infectious process represent the major source of GM-CSF in adult brain. The expression of MARCO on microglial cells and DCs induces not only actin cytoskeleton rearrangements but, accordingly, also a decreasing of the phagocytic activity. Why should the expression of a scavenger receptor down-regulate the ability of antigen internalization? One possibility could be that microglial cells and mature DCs, showing a reduced phagocytic activity, maintain the ability to bind bacteria for blocking their propagation. The simple binding of bacteria could hamper their divisions and slow down the invasion process. Moreover, the presence of DCs in the spleen marginal zone exposing bacteria on their surface could favor the interaction with antigen-specific marginal zone B cells35 and the subsequent activation of the early humoral immune response.35
Prepublished online as Blood First Edition Paper, July 3, 2003; DOI 10.1182/blood-2002-12-3651.
Supported by grants from AIRC (Italian Association Against Cancer), Biopolo, and 5th EC Programs (DC strategies 00470 and TAGAPO 00202).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Klaus Giese and Frauke Lenders from Atugen (Berlin) for the anti-MARCO GeneBlock, Maura Francolini (CNR, Milan) for the confocal microscopy analyses, and Luisa Lanfrancone (IEO, Milan) for the PINCO vector.

![Figure 1. MARCO expression on DCs. (A) Fold increase of MARCO mRNA levels in LPS-activated D1 cells versus nonactivated cells revealed by GeneChip analysis.12 (B) Real-time PCR showing MARCO mRNA increase in D1 cells after LPS stimulation at the indicated time points with respect to nonactivated cells. Error bars indicate standard deviation of 3 different samples. (C) D1 cell surface expression of MARCO (monoclonal antibody [mAb] ED31) after LPS activation at the indicated time points analyzed by flow cytometry.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/8/10.1182_blood-2002-12-3651/6/m_h82035081001.jpeg?Expires=1770038392&Signature=Da5ShIsiZK9SGGGAH0A~2sial7dWUnUww9Fsq55xw6rhjys7YYJn6YIhlFjva8jBHtAheG9ayYmL-XWN7t~mKun5mLcEl9tOezKx3U-nzTBGCumdMAG~C1fNSu08ayLqv71BG9Yb0Bli9kN344isEVCeiLfTs22FF3RGNc6v931PalnO5QB0dUIzbj7O7ky~oN5sS~H--YyOgufbMokKCcVADflvH845MTPZLQjt8QMrxpL-tWp0hT5CDZFdsCGsomdCcey5Ssc42ONzdROjHReWkdZJslbYsFWDiUEPcANovDRW3e8ZRrOfGYWApvXR9KgtMol~2K9vYQUb0DnVxg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal