Abstract
Using multivalent protein probes, an evolutionarily conserved endogenous ligand for EMR2, a human myeloid cell–restricted EGF-TM7 receptor, was identified on the surface of a number of adherent cell lines. In addition, in situ staining of the ligand has revealed specific in vivo patterns consistent with a connective tissue distribution. The interaction is conserved across species and mediated exclusively by the largest EMR2 isoform containing 5 epidermal growth factor (EGF)–like modules. Antibody-blocking studies subsequently revealed that the fourth EGF-like module constitutes the major ligand-binding site. The largest isoform of CD97, a related EGF-TM7 molecule containing an identical EGF-like module, also binds to the putative EMR2 ligand. Through the use of mutant Chinese hamster ovary (CHO) cell lines defective in glycosaminoglycans (GAGs) biosynthesis as well as the enzymatic removal of specific cell surface GAGs, the molecular identity of the EMR2 ligand was identified as chondroitin sulfate (CS). Thus, exogenous CS GAGs blocked the EMR2-ligand interaction in a dose-dependent manner. EMR2-CS interaction is Ca2+- and sulphation-dependent and results in cell attachment. This is the first report of a GAG ligand for the TM7 receptors extending the already vast repertoire of stimuli of the GPCR superfamily.
Introduction
The EGF-TM7 proteins are a novel group of cell surface receptors restricted to leukocytes and/or smooth muscle cells.1,2 These receptors are characterized by a unique hybrid structure consisting of tandem repeats of epidermal growth factor (EGF)–like modules coupled to a class B G protein–coupled receptor (GPCR) 7 transmembrane (TM7) domain by a glycosylated stalk region.3,4 To date, 6 members of the EGF-TM7 receptor family (EMR1, 2, 3, 4, ETL [EGF-TM7-latrophilin–related protein], and CD97) have been identified in humans, mice, or rats.5-14 Genomic analysis shows that all human EGF-TM7 genes with the exception of ETL are located within chromosome 19p13 regions and possess similar exon-intron organizations. The EGF-TM7 molecules belong to a larger protein family known as the LNB-TM7 or B2 subgroup of the class B GPCRs (Family-B G protein–coupled receptors with large extracellular N-terminal domains).3,4 All LNB-TM7 receptors possess large extracellular domains consisting of various protein modules implicated in protein-protein interactions. A dual adhesion and signaling function has been suggested for these molecules whereby the interaction of the extracellular region with other cell surfaces or extracellular matrix proteins triggers intracellular signaling through the transmembrane domain.
Consistent with this hypothesis, specific cellular ligands for the EGF-TM7 receptors have been reported.9,10,15 The well-characterized CD97-CD55 interaction is Ca2+ dependent and mediated exclusively by the EGF-like modules on CD97 and the short consensus repeat (SCR) domains on CD55.16 Interestingly, the interaction between CD97 and CD55 is phylogenetically restricted.17,18 Thus, CD55 on human erythrocytes interacts only with human, but not mouse, CD97 transfectants. Similarly, mouse but not human CD55 binds restrictively to mouse CD97 transfectants. Moreover, CD55-deficient erythrocytes isolated from patients with paroxysmal nocturnal hemoglobinuria (PNH) or the Inab phenotype failed to adhere to CD97 transfectants in vitro.15 In addition, different CD55-binding affinities have been shown for different alternatively spliced CD97 isoforms containing different numbers of the EGF-like modules.19 Alternative splicing therefore regulates the CD55-binding specificity of CD97. Recently, putative cell-surface ligands for EMR3 and EMR4 have also been found on monocyte-derived macrophages/activated neutrophils and the mouse B lymphoma cell line A20, respectively.9,10 Overall, these results indicate that the EGF-TM7 proteins function in part as cell adhesion molecules through interactions mediated by the EGF-like domains.
In addition to cell-surface proteins, cellular interactions often involve glycosaminoglycans (GAGs) or proteoglycans (PGs) that are present abundantly on cell membranes and in the extracellular matrix.20 Two major types of GAG, heparin/heparan sulfate (HS) and chondroitin sulfate (CS)/dermatan sulfate (DS), are categorized based on the composition of their amino sugars.21 More structural complexity arises through modification of the sugars by epimerization and sulphation generating subregional variability within GAGs. Such modifications often determine the specific biologic responses mediated by PGs, including the binding of growth factors, cytokines, chemokines, matrix proteins, proteases, and antimicrobial peptides.20 Cellular PGs can also serve as attachment sites for various pathogens including bacteria and viruses for their entry into host cells. Thus, GAGs and PGs have been implicated in a host of biologic processes ranging from cell adhesion, proliferation, and differentiation, to tissue repair, immune responses, and tumorgenesis.22
EMR2 is a myeloid cell–restricted member of the EGF-TM7 family that is closely related to CD97.8,23 The EGF-like domains of the full-length EMR2 protein share 97.5% sequence identity with those of CD97. Similar to CD97, distinct EMR2 protein isoforms consisting of different numbers of the EGF-like domains have been documented.8 Unlike CD97 however, EMR2 (EGF-1, 2, 5) was found to bind CD55 with a dissociation constant (KD) at least an order of magnitude lower than that of CD97 (EGF-1, 2, 5), even though they differ by only 3 amino acids.16 This indicates that the EGF-like domain-mediated interaction is highly specific and can be finely adjusted by a small number of amino acid changes on the surface of the EGF-like modules. It also suggested that additional EMR2-specific cellular ligands might exist. In this report, we describe the identification of CS as an EMR2 isoform–specific ligand and show that the specific interaction between EMR2 and CS leads to cell attachment. These data demonstrate the ability of EGF-TM7 receptors to interact with a diverse range of ligand types through alternative splicing and indicate a possible role for EMR2 in myeloid cell migration and trafficking.
Materials and methods
Materials
All chemicals and reagents were obtained from Sigma (St Louis, MO) unless otherwise specified. Cell culture media and supplements were purchased from InVitrogen. Laboratory-bred mice were housed in and provided by the animal facility at the Sir William Dunn School of Pathology under standard pathogen-free conditions.
Cell culture
All culture media were supplemented with 10% heat inactivated fetal calf serum (FCS), 2 mM l-glutamine, 50 IU/mL penicillin, and 50 μg/mL streptomycin. Wild-type and mutant Chinese hamster ovary (CHO) cells deficient in galactosyltransferase-I (PgsB-618)24 or N-acetylglucosaminyl transferase/glucuronyl transferase (PgsD-677)25 were cultured in Ham F12 medium. During sulphation inhibition assays, CHO-K1 cells were cultured for 2 days in Fischer medium supplemented with 10% dialyzed FCS and 10 mM sodium chlorate in the presence or absence of 10 mM MgSO4. Lec1 cells were cultured in minimum essential media (MEM)–Alpha with ribonucleosides and deoxyribonucleosides26,27 and ARH-77 cells transfected with various proteoglycan expression constructs were grown in RPMI-1640 supplemented with 0.3 μg/mL G418 (Gibco, Paisley, United Kingdom).28,29 CHO-K1 cells expressing transmembrane EMR21-5 and EMR21,2,5 were selected in media containing G418 (1 mg/mL) for 10 days and further enriched by flow cytometric sorting using EMR2 stalk-specific 2A1 monoclonal antibody (mAb). G418-resistant CHO-K1 cells transfected with pcDNA3.1+ vector only were used as a negative control. Monocyte-derived macrophages and primary dermal fibroblasts were cultured in RPMI-1640 medium.
Generation of biotinylated mouse Fc fusion proteins
All expression vectors were constructed on pcDNA3.1+ (InVitrogen). The EMR2/CD97 mouse Fc (fragment crystallizable) fusion proteins were produced by the modification of a previously described expression vector.9 In brief, the EGF-like domains of EMR2 and CD97 isoforms were cloned immediately upstream of a mutated mouse Fc fragment possessing a C-terminal consensus biotinylation peptide sequence, (DPNSGSLHHILDAQKMVWNHR*). The resulting constructs produced soluble EGF-like domains fused to a non–Fc-receptor-binding mouse Fc fragment (Ig2b-CH2 + 3) that could be specifically biotinylated by the E coli biotin holoenzyme synthetase BirA.30 The expression and in vitro biotinylation of mouse Fc fusion proteins were carried out as previously described.9
Cellular ligand-binding assay
To search for the putative cellular ligand of EMR2, various primary cells and cell lines were subjected to a fluorescence-activated cell sorting (FACS)–based cell-binding analysis using a well-established method for detecting protein-protein interactions.31 In brief, 10 μL avidin-coated fluorescent beads (Spherotech, Libertyville, IL) were washed twice and mixed with 1 μg biotinylated protein in Hanks balanced salt solution (HBSS) containing 0.5% bovine serum albumin (BSA) (HBSS/BSA) in a total volume of 10 μL. After incubation at 4°C for 1 hour, the beads were washed, resuspended in 10 μL HBSS/BSA and sonicated at 20% power for one minute (Sonicator; Heat Systems, Farmingdale, NY). The bead-protein complex was then added to single-cell suspensions in a 96-well plate (5 × 105 cells/50 μL beads/well) and incubated for 1 hour at 4°C. Where indicated, cells were pretreated with various enzymes for 30 minutes at 37°C before incubation with fluorescent probes: trypsin (1 mg/mL), α-chymotrypsin (1 mg/mL), dispase (1 mg/mL), heparinase I (150 mU/mL), heparinase III (50 mU/mL), chondroitinase A, B, AC, and ABC (150 mU/mL). For antibody blocking and Ca2+ dependence assays, antibodies and EGTA (ethyleneglycotetraacetic acid) were used at a final concentration of 5 μg/mL and 5 mM, respectively. For the blocking by exogenous GAGs, various GAGs (Sigma) including HS (H-9902), CS-A (C-8529), CS-B (C-3788), and CS-C (C-4384) were incubated at the indicated concentration with the probes 10 minutes before adding to cells. For cell rosetting assay, single-cell suspensions were achieved by passing cells through a 70-μmBD Falcon Cell Strainer (BD Bioscience, Oxford, United Kingdom) several times and confirmed by light microscopy examination. Cells at 1 × 106 cells/mL were mixed gently end-over-end for 1 hour at room temperature and the number of cell rosettes consisting of more than 4 cells were counted. A total of 20 fields of × 400 magnification were examined.
Tissue staining using multivalent fluorescent probes
Fresh frozen human and mouse tissue sections (5 μm) were thawed for 5 minutes, washed twice with ice cold HBSS, and then incubated with blocking buffer (Dulbecco modified Eagle medium [DMEM]/10%FCS/10% goat serum) for 20 minutes at 4°C. Fluorescent beads (20 μL) were coupled to biotinylated protein as previously described, resuspended in 200 μL blocking buffer, added to the tissue sections, and incubated at 4°C for 1 hour. The sections were washed 8 times in ice-cold HBSS solution and observed by fluorescence microscopy (Axioplan 2; Zeiss, Thornwood, NY). For double staining, tissue sections were incubated after blocking for 30 minutes at 4°C with various primary antibodies (5 μg/mL to 10 μg/mL). The slides were washed 3 times with blocking buffer before a non–fluorescein isothiocyanate (FITC)–conjugated secondary antibody (Jackson ImmunoResearch Laboratories, Luton, United Kingdom) (1:100-200 dilution) was added for 30 minutes. Tissues were finally washed in blocking buffer and stained with fluorescent beads. The staining was documented using Spot Advanced software (Diagnostic Instruments, Sterling Heights, MI).
Generation of monoclonal antibodies
1B5 and 2B1 were generated in Armenian hamsters as previously described.18 Hybridoma supernatants were screened by flow cytometry for binding of cells stably expressing EMR2/1-5 or CD97/1-5. Supernatants recognizing both transfectants were further screened using transiently transfected COS cells. Two clones (1B5 and 2B1) bound to EMR2/1-5 and CD97/1-5 but not the smaller isoforms. Both clones were then subcloned until monoclonal and stable. The hybridomas were cultured and purified using protein A sepharose (Pharmacia, Piscataway, NJ). 2A1 and CLB-CD97/1 mAbs that recognize the EMR2 stalk region and the first EGF-like domain of both CD97 and EMR2, respectively, were described elsewhere.13,15,23
Cell attachment assays
Assays were based on those described previously.32 In brief, 96-well plates (Reacti-Bind; Pierce, Rockford, IL) were coated with 100 μLof 1 μg/mL to 100 μg/mL substrate (recombinant EMR2 proteins, bovine fibronectin, or BSA) in HBSS, pH 7.0, at 4°C overnight. Plates were washed with PBS and blocked with 20 mg/mL BSA in HBSS for 1 hour at 37°C. After rinsing in PBS, 1 × 105 cells suspended in 100 μL serum-free Ham F12 medium were added to each well and incubated for 1 hour at 37°C incubator. The wells were carefully washed twice in HBSS before fixing in 2% glutaraldehyde/0.1 M cacodylate buffer, pH 7.2, for 20 minutes. After fixation, the cells were rinsed in PBS and stained with 1% methylene blue/0.1 M borate buffer for 30 minutes. After excess dye was removed the cells were lysed in ethanol/0.1 M HCl (1:1 ratio) and absorbance measured at 620 nm. For antibody-blocking experiments, protein-coated wells were incubated for 1 hour with antibodies (10 μg/mL in HBSS) at room temperature before the addition of cells.
Immunocytochemistry
Glass coverslips coated with 50 μg/mL substrates in HBSS overnight were used to perform the CHO-cell adhesion assay. After careful washing in HBSS, cells were fixed in 2% paraformaldehyde/PBS for 15 minutes at room temperature. Rhodamine-TRITC-phalloidin (Sigma) was used for total F-actin staining according to the manufacturer's instructions. Immunocytochemical analysis was carried out with a confocal laser scanning inverted microscope (MicroRadiance AG2; Zeiss).
Results
Generation of the human EMR2 protein probes and screening for EMR2 cellular ligands
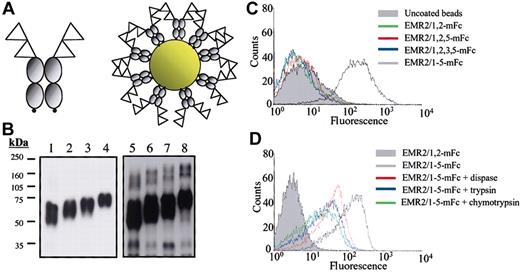
To search for potential cellular ligands for human EMR2, a sensitive assay system using multivalent probes to increase the avidity of molecular associations was used.31 In brief, expression constructs encoding recombinant EGF-like domains coupled to a mouse Fc fragment and a biotinylation signal sequence were engineered. These constructs were then expressed, purified, and biotinylated. Finally, the biotinylated proteins were coupled in a specific orientation to avidin-coated fluorescent beads (Figure 1A-B) to screen for ligand-bearing cells or tissues. As EMR2 possesses a number of naturally occurring splice variants containing different numbers and arrangements of the EGF-like domains, 4 individual probes were generated and used for ligand screening (Figure 1B).
Cell-surface ligand-binding analysis. (A) A schematic representation of the biotinylated mouse Fc fusion proteins. The EGF-like modules are represented as triangles, gray circles represent the mouse Fc region, and the small black circles indicate the biotinylation signal. (B) Western blot analysis of EMR2-mouse Fc fusion proteins. An Fc-specific antimouse immunoglobulin G (IgG) horseradish peroxidase (HRP) was used to detect the purified mouse Fc fusion proteins: EMR2/1,2 mouse Fc (lane 1), EMR2/1,2,5 mouse Fc (lane 2), EMR2/1,2,3,5 mouse Fc (lane 3), and EMR2/1,2,3,4,5 mouse Fc (lane 4). Lanes 5 to 8 were loaded with the same EMR2 mouse Fc proteins after in vitro biotinylation and probed with Extravidin-HRP. (C) FACS profile showing that CHO-K1 cells only interact with fluorescent beads coated with the largest EMR2 isoform, but not with other isoforms. (D) The ligand binding is protease sensitive, demonstrating the requirement of cell-surface proteins for the EMR2-ligand interaction.
Cell-surface ligand-binding analysis. (A) A schematic representation of the biotinylated mouse Fc fusion proteins. The EGF-like modules are represented as triangles, gray circles represent the mouse Fc region, and the small black circles indicate the biotinylation signal. (B) Western blot analysis of EMR2-mouse Fc fusion proteins. An Fc-specific antimouse immunoglobulin G (IgG) horseradish peroxidase (HRP) was used to detect the purified mouse Fc fusion proteins: EMR2/1,2 mouse Fc (lane 1), EMR2/1,2,5 mouse Fc (lane 2), EMR2/1,2,3,5 mouse Fc (lane 3), and EMR2/1,2,3,4,5 mouse Fc (lane 4). Lanes 5 to 8 were loaded with the same EMR2 mouse Fc proteins after in vitro biotinylation and probed with Extravidin-HRP. (C) FACS profile showing that CHO-K1 cells only interact with fluorescent beads coated with the largest EMR2 isoform, but not with other isoforms. (D) The ligand binding is protease sensitive, demonstrating the requirement of cell-surface proteins for the EMR2-ligand interaction.
EMR2 binds its cellular ligand in a Ca2+-dependent and isoform-specific manner
Various cell lines and primary cells of different lineages were screened using different EMR2 isoform probes by flow cytometric analysis. The full-length EMR2 splice variant (EMR2/1-5) caused a strong shift in fluorescence relative to uncoated beads when incubated with CHO-K1 cells, indicating the binding of a cell-surface ligand (Figure 1C). In contrast, all other probes containing different EMR2 splice forms (EMR2/1,2; EMR2/1,2,5; and EMR2/1,2,3,5) did not bind to CHO-K1. The ligand was confirmed to be a cellular ligand and not a serum component as cells cultured for 3 passages in a serum-free medium (Opti-MEM/CHO-s-SFMII) were still ligand positive in subsequent FACS analysis (data not shown). In addition, pretreatment of cells with proteases including trypsin, dispase, and chymotrypsin all resulted in a significant reduction in probe binding, indicating binding is dependent on cell-surface proteins (Figure 1D). As the smallest EMR2 isoform, EMR2/1,2 mouse Fc did not interact with the ligand; it was used as a negative control in all subsequent assays.
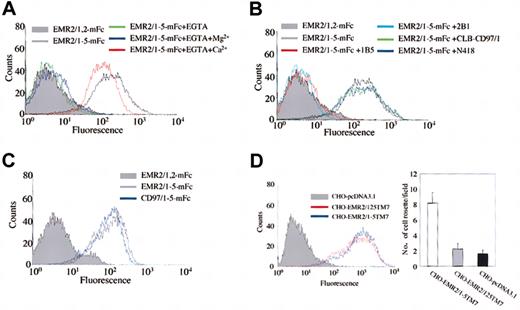
The chelation of calcium by Ca2+-binding EGF-like domains has been demonstrated to be crucial for the structural integrity and function of several other EGF-like domain-containing proteins.33 As EMR2 possesses EGF-like domains that contain Ca2+-binding consensus sequences, flow cytometric analysis was performed in the absence of Ca2+ ions to further prove the specificity of the EMR2-ligand interaction. As shown in Figure 2A, the addition of 5 mM EGTA completely ablated the binding of the EMR2/1-5 probe to CHO-K1 cells. Furthermore, the subsequent addition of 10 mM CaCl2 restored probe binding to the normal level, whereas the subsequent addition of 10 mM MgCl2 did not. This result confirms the EMR2-ligand interaction is indeed Ca2+ dependent.
Characterization of the EMR2-ligand interaction. (A) The EMR2-ligand interaction is Ca2+ dependent. (B) Preincubation with anti–EGF-like domain 4 antibodies (1B5 and 2B1) results in the ablation of the binding of multivalent EMR2 probes to CHO-K1. An anti–EGF-like domain 1 antibody (CLB-CD97/1) and a control Ab (N418) had no effect on binding. (C) FACS analysis showing that binding of CHO-K1 cells by the largest isoform of human CD97 is similar to that of EMR2. (D) Generation of stably transfected CHO-K1 cells expressing transmembrane EMR2/1-5 and EMR2/1,2,5 proteins (left panel). Expression of cell-surface EMR2 was determined by flow cytometric analysis using 2A1 mAb and an isotype-control Ab (data not shown). The formation of cell rosettes in single-cell suspensions (right panel). EMR2/1-5–expressing cells (white bar) formed more cell rosettes than did EMR2/1,2,5–expressing cells (gray bar) and pcDNA3.1-transfected cells (black bar) (n = 20). Error bars are equal to one standard deviation.
Characterization of the EMR2-ligand interaction. (A) The EMR2-ligand interaction is Ca2+ dependent. (B) Preincubation with anti–EGF-like domain 4 antibodies (1B5 and 2B1) results in the ablation of the binding of multivalent EMR2 probes to CHO-K1. An anti–EGF-like domain 1 antibody (CLB-CD97/1) and a control Ab (N418) had no effect on binding. (C) FACS analysis showing that binding of CHO-K1 cells by the largest isoform of human CD97 is similar to that of EMR2. (D) Generation of stably transfected CHO-K1 cells expressing transmembrane EMR2/1-5 and EMR2/1,2,5 proteins (left panel). Expression of cell-surface EMR2 was determined by flow cytometric analysis using 2A1 mAb and an isotype-control Ab (data not shown). The formation of cell rosettes in single-cell suspensions (right panel). EMR2/1-5–expressing cells (white bar) formed more cell rosettes than did EMR2/1,2,5–expressing cells (gray bar) and pcDNA3.1-transfected cells (black bar) (n = 20). Error bars are equal to one standard deviation.
Dissection of the ligand-binding site
The fact that EMR2/1-5 is the only naturally occurring isoform capable of binding CHO-K1 cells suggests that the fourth EGF-like domain might be an important element of the ligand-receptor interface. In an attempt to dissect the ligand-binding site on EMR2, anti-CD97 antibodies were used in blocking studies. A number of antibodies directed against the EGF-like domains of CD97 were found to crossreact with EMR2 due to the strong sequence homology between these 2 molecules.8,23 A clear blocking effect was observed with the EGF-like domain 4–specific antibodies 1B5 and 2B1 (see “Materials and methods”). In contrast, binding was not inhibited by N418, a control hamster antibody, or CLB-CD97/1, which is reactive against the EGF-like domain 1 (Figure 2B). This result indicates that the major ligand-binding site is located at the EGF-like domain 4.
Both CD97 and EMR2 share identical EGF-like domains 4 and 5. This led us to perform similar cell binding assays using probes coated with CD97/1,2,5, CD97/1,2,3,5, and CD97/1-5 mouse Fc fusion proteins. As expected, similar fluorescence shifts were observed for both full-length EMR2 and CD97 probes (Figure 2C). As with EMR2, CD97 ligand binding was shown to be Ca2+ dependent and mediated only by the full-length isoform (data not shown). The interaction can be blocked by 1B5 and 2B1, but not anti-CD55 antibodies (data not shown), indicating the involvement of the fourth EGF-like domain in the interaction. These results indicate that alternative splicing of the EGF-TM7 receptors (eg, CD97) produces isoforms capable of interacting with different cellular ligands.
As the interaction between the EMR2/1-5 probe and the cellular ligand was conducted using Fc fusion proteins containing only the EGF-like domains, it is uncertain whether they mimic the binding activity of the native proteins. To address this concern, biotinylated recombinant EMR2 probes containing the entire extracellular domain (the EGF-like domains plus the stalk), but devoid of the Fc fragment, were generated as described previously.16 These probes produced exactly the same results in the FACS-based cell binding assay as did the Fc fusion protein probes (data not shown), suggesting that the ligand-binding interaction is restricted to the EGF-like domains. This result is consistent with the previous finding showing the EGF-like domain 4 as the major ligand-binding site (Figure 2B). Next, to ascertain that the cell-surface EMR2 receptor can also mediate similar interaction, stably transfected CHO-K1 cells expressing transmembrane EMR2/1-5 and EMR2/1,2,5 proteins were generated and used in a cell rosetting assay (Figure 2D). After gentle mixing of cells in single-cell suspensions, the formation of cell rosettes was evaluated by light microscopy. As shown in Figure 2D, cells expressing EMR2/1-5 formed significantly more cell rosettes than did the pcDNA3.1-transfected cells and cells expressing EMR2/1,2,5. Taken together, these results confirm that the ligand binding mediated by the EMR2 EGF-like domains mimics the physiologic interaction between the cell-surface EMR2 protein and its cellular ligand.
The EMR2 ligand is expressed by many adherent cells and is conserved across species
Ligand screening by FACS was performed on a number of cell lines and primary cells (Table 1). Of the primary cells tested, only dermal fibroblasts and monocyte-derived macrophages expressed detectable levels of ligand. All nonadherent cells tested failed to bind the multivalent probes, whereas many of the adherent cell lines from a range of species bound strongly, in an EMR2/1-5 isoform and Ca2+-dependent manner, suggesting the ligand is abundantly expressed and conserved across species.
Primary cells and cell lines analyzed for the presence of the cellular ligand specific to the EMR2/1-5 isoform
Primary cells . | EMR2-ligand binding affinity* . |
|---|---|
| PB lymphocytes, B + T† | +/- |
| Monocytes | +/- |
| Monocyte-derived Mφs, days 1-5 | ++ |
| Neutrophils | - |
| Erythrocytes | - |
| Dermal fibroblasts | ++ |
| Cell lines | |
| HL-60‡ | - |
| THP-1‡ | - |
| MonoMac6‡ | - |
| U937‡ | - |
| K562‡ | - |
| HEL‡ | - |
| Daudi | - |
| H9 | - |
| Jurkat | - |
| A20 | - |
| HEK293T | ++++ |
| Hep3B | + |
| HepG2 | +/- |
| HeLa | +++ |
| NIH3T3 | ++++ |
| RAW | + |
| COS-7 | ++++ |
| CHO-K1 | ++++ |
Primary cells . | EMR2-ligand binding affinity* . |
|---|---|
| PB lymphocytes, B + T† | +/- |
| Monocytes | +/- |
| Monocyte-derived Mφs, days 1-5 | ++ |
| Neutrophils | - |
| Erythrocytes | - |
| Dermal fibroblasts | ++ |
| Cell lines | |
| HL-60‡ | - |
| THP-1‡ | - |
| MonoMac6‡ | - |
| U937‡ | - |
| K562‡ | - |
| HEL‡ | - |
| Daudi | - |
| H9 | - |
| Jurkat | - |
| A20 | - |
| HEK293T | ++++ |
| Hep3B | + |
| HepG2 | +/- |
| HeLa | +++ |
| NIH3T3 | ++++ |
| RAW | + |
| COS-7 | ++++ |
| CHO-K1 | ++++ |
The plus (+) and minus (-) signs indicate relative fluorescence intensity.
Determined by the fluorescence intensity of the FACS-based assay.
Cells were treated with or without PHA-L (2 μg/mL for 2, 6, and 24 hours).
Cells were treated with or without PMA (10 ng/mL for 2 days).
The EMR2 ligand is found within areas of connective tissue
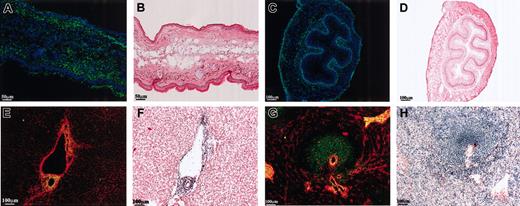
In an attempt to characterize the ligand further, its in vivo distribution was investigated. The multivalent fluorescent beads were used to probe both human and mouse frozen tissue sections due to the conserved expression of the ligand. Figure 3A shows a mouse ear skin section stained with the EMR2/1-5 probe (green) and nuclear staining (blue); beads are clearly seen binding to the epidermis, dermis, and underlying connective tissue. Extensive EMR2 ligand–positive staining was also observed within the mucosa, submucosa, and adventitia of mouse esophagus (Figure 3C). Figure 3B,D shows adjacent sections stained with hematoxylin and eosin for comparison. Probes containing the other EMR2 isoforms and mouse Fc probe alone were used as staining controls and showed no binding for all tissues mentioned (data not shown). As with flow cytometric results, binding of EMR2 to the tissues staining was also Ca2+ dependent and blocked by 1B5 and 2B1 antibodies (data not shown). Within all the tissues tested, the distribution of the EMR2 ligand was consistent with that of a connective tissue or an extracellular matrix (ECM)–associated protein. To further demonstrate this, double staining using anticollagen I (red) and EMR2-coated beads (green) was performed on human tissue samples. Figure 3E,G shows double staining of collagen and EMR2/1-5 beads on human liver and spleen sections. As with mouse liver, ligand staining occurred around large vessels such as branches of the portal vein and bile ducts of human liver. Interestingly, the human spleen staining differed from that of the mouse spleen as strongly ligand-positive areas were observed in the white pulp of the splenic nodes as well as in areas of connective tissue. The tissue staining results from both mouse and human tissues are summarized in Table 2.
Tissue distribution of EMR2 ligand. Multivalent probes (green) were used to examine the distribution of the EMR2 ligand in human and mouse tissues. Double staining was performed with either Hoechst stain (blue) to highlight nuclei or an antihuman collagen I antibody (red). Adjacent sections were stained with hematoxylin and eosin. (A-B) Mouse skin. (C-D) Mouse esophagus. (E-F) Human liver. (G-H) Human spleen.
Tissue distribution of EMR2 ligand. Multivalent probes (green) were used to examine the distribution of the EMR2 ligand in human and mouse tissues. Double staining was performed with either Hoechst stain (blue) to highlight nuclei or an antihuman collagen I antibody (red). Adjacent sections were stained with hematoxylin and eosin. (A-B) Mouse skin. (C-D) Mouse esophagus. (E-F) Human liver. (G-H) Human spleen.
The distribution of the EMR2/1-5 ligand in mouse and human tissues
Tissues . | Reactivity . |
|---|---|
| Brain | - |
| Heart | + |
| Kidney* | + |
| Skin* | +++ |
| Liver* | +/- |
| Spleen* | + |
| Thymus | +/- |
| Lymph node | - |
| Lung* | - |
| Small intestine | ++ |
| Smooth muscle | +++ |
| Tendon | +++ |
| Uterus | +++ |
| Ovary | +++ |
| Testis | +++ |
| Pancreas | ++ |
| Esophagus | ++ |
Tissues . | Reactivity . |
|---|---|
| Brain | - |
| Heart | + |
| Kidney* | + |
| Skin* | +++ |
| Liver* | +/- |
| Spleen* | + |
| Thymus | +/- |
| Lymph node | - |
| Lung* | - |
| Small intestine | ++ |
| Smooth muscle | +++ |
| Tendon | +++ |
| Uterus | +++ |
| Ovary | +++ |
| Testis | +++ |
| Pancreas | ++ |
| Esophagus | ++ |
The plus (+) and minus (-) signs indicate relative binding of EMR2/1-5 probe.
Confirmed with human tissues.
EMR2 mediates cell attachment
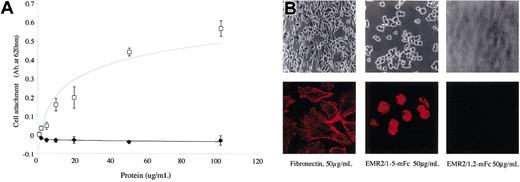
To investigate the potential downstream effects of the EMR2-ligand interaction, cell attachment assays were performed. Consistent with the FACS data, EMR2/1-5 was found to mediate CHO-K1 attachment (Figure 4A); cell attachment by EMR2/1-5 was concentration- and Ca2+-dependent and blocked by 1B5 and 2B1 antibodies (data not shown). Cells plated on an EMR2/1,2 mouse Fc–coated surface did not bind above background levels (Figure 4A). Observation by light microscopy showed that cells attached and spread within 1 hour when plated onto fibronectin (Figure 4B). In contrast, cells incubated on EMR2/1-5 attached but did not spread and had a rounded morphology (Figure 4B). Confocal microscopy examination indicated that cells incubated on EMR2/1-5 did not activate actin filaments and induce concomitant stress fiber formation as shown for cells cultured on fibronectin (Figure 4B).
EMR2/1-5 mediates cell attachment. (A) Graph showing the effects of EMR2 protein concentration on cell attachment. □ and ♦ denote EMR2/1-5 mouse Fc and EMR2/1,2 mouse Fc proteins, respectively (n = 3). Error bars are equal to one standard deviation. (B) Top row: morphology of attached CHO-K1 cells. Cells spread when incubated with fibronectin for 60 minutes. However, cells incubated only with EMR2/1-5 mouse Fc attach. Original magnification, × 400. Lower panels: confocal microscopy showing actin staining of cells attached to either fibronectin or different EMR2 protein isoforms. Original magnification, × 600.
EMR2/1-5 mediates cell attachment. (A) Graph showing the effects of EMR2 protein concentration on cell attachment. □ and ♦ denote EMR2/1-5 mouse Fc and EMR2/1,2 mouse Fc proteins, respectively (n = 3). Error bars are equal to one standard deviation. (B) Top row: morphology of attached CHO-K1 cells. Cells spread when incubated with fibronectin for 60 minutes. However, cells incubated only with EMR2/1-5 mouse Fc attach. Original magnification, × 400. Lower panels: confocal microscopy showing actin staining of cells attached to either fibronectin or different EMR2 protein isoforms. Original magnification, × 600.
EMR2 binds to cell-surface proteoglycans
The flow cytometric data and tissue distribution strongly implied that the EMR2 ligand was a component of the ECM. The ECM is known to be composed of 3 major components: glycoproteins, proteoglycans, and collagens. In order to demonstrate or exclude these molecules as potential candidates, a number of CHO cell mutants were analyzed. CHO-derived Lec1 cells27 lack GlcNAc glycosyl transferase, which results in N-linked sugar biosynthesis being blocked at the Man5-GlcNAc2-Asn intermediate stage. FACS analysis showed that Lec1 cells bind EMR2 probes to a similar extent as CHO-K1 cells, indicating that N-linked glycosylation is not important for EMR2 binding (Figure 5A). To test whether the ligand is a GAG side chain of a proteoglycan, 2 CHO-derived cell lines, PgsB-618 and PgsD-677, were analyzed.24,25 PgsB-618 cells are deficient in galactosyl transferase-I, the enzyme involved in the first step of GAG side chain biosynthesis. As a consequence, PgsB-618 has a much reduced expression of GAGs on the cell surface. Surprisingly, PgsB-618 cells were completely negative for the EMR2 ligand activity (Figure 5A), indicating that the ligand is either a GAG moiety or a protein/GAG complex of a proteoglycan. PgsD-677 is deficient in N-acetylglucosaminyl transferase/glucuronyl transferase and therefore lacks all heparan sulfate GAG chains. Figure 5A shows PgsD-677 is ligand positive, indicating the ligand is not heparan sulfate GAG. In line with the flow cytometric analysis, similar results were obtained using a cell attachment assay. Thus, CHO-K1 and PgsD-677 cells bound to EMR2/1-5 mouse Fc–coated plates, whereas PgsB-618 cells did not (Figure 5B). More PgsD-677 cells bound to EMR2/1-5 mouse Fc–coated plates than wild-type CHO-K1 cells, presumably due to the higher level of cell-surface CS GAG on PgsD-677.25 As expected, all 3 cell lines bound to fibronectin-coated plates equally well and EMR2/1,2 mouse Fc and BSA did not cause any cell attachment (Figure 5B).
Dissection of the EMR2 ligand. (A) FACS analysis of CHO cell mutants shows that EMR2 binds a CS-bearing proteoglycan. PgsB-618 lacks all GAG side chains, PgsD-677 lacks heparan sulfate side chains, and Lec1 cells lack N-linked carbohydrates. (B) Cell attachment assays using BSA (□), fibronectin (▧), EMR2/1-5 mouse Fc (⊞), and EMR2/1,2 mouse Fc (▪). Each point represents the mean ± SEM of triplicate wells. The experiments were performed 3 times with similar results. (C) Enzymatic digestion of GAG side chains show that EMR2 binds to a CS-B– or CS-C–bearing proteoglycan. (D) FACS analysis of CHO-K1 cells treated with 10 mM sodium chlorate or 10 mM sodium chlorate and 10 mM magnesium sulfate. (E) The EMR2-ligand interaction is blocked by exogenous GAGs. Efficient blocking was observed when incubated with CS-B (♦) and CS-C (▵), but not HS (▪) or CS-A (□). Note that the blocking effect is dose dependent. The figure is a representative of 3 independent experiments with similar results.
Dissection of the EMR2 ligand. (A) FACS analysis of CHO cell mutants shows that EMR2 binds a CS-bearing proteoglycan. PgsB-618 lacks all GAG side chains, PgsD-677 lacks heparan sulfate side chains, and Lec1 cells lack N-linked carbohydrates. (B) Cell attachment assays using BSA (□), fibronectin (▧), EMR2/1-5 mouse Fc (⊞), and EMR2/1,2 mouse Fc (▪). Each point represents the mean ± SEM of triplicate wells. The experiments were performed 3 times with similar results. (C) Enzymatic digestion of GAG side chains show that EMR2 binds to a CS-B– or CS-C–bearing proteoglycan. (D) FACS analysis of CHO-K1 cells treated with 10 mM sodium chlorate or 10 mM sodium chlorate and 10 mM magnesium sulfate. (E) The EMR2-ligand interaction is blocked by exogenous GAGs. Efficient blocking was observed when incubated with CS-B (♦) and CS-C (▵), but not HS (▪) or CS-A (□). Note that the blocking effect is dose dependent. The figure is a representative of 3 independent experiments with similar results.
EMR2 specifically binds to sulphated CS side chains
Proteoglycans are known to contain heterogeneous GAG subtypes with differential chain-length and different degrees of sulphation and epimerization. To investigate which specific GAG subtype is the potential EMR2 ligand, cellular GAG side chains were removed by specific glycosidases. Figure 5C shows that treatment of cells with chondroitinase A and heparinase I and III has little effect, whereas chondroitinase B or ABC treatment completely ablates binding of the EMR2 probes. Chondroitinase AC treatment partially reduces the binding of probes. These results demonstrate that the cellular ligand is likely to consist of CS-B (DS) or CS-C side chains, or both. The sulphation of sugar moieties has been shown to be crucial for ligand specificity and binding in many protein-GAG interactions.34,35 To determine whether this was also true for the EMR2-ligand interaction, CHO-K1 cells were grown in sulfate-free medium in the presence of sodium chlorate, which competes with sulfate for sulphotransferase enzymes and therefore blocks the sulphation of new GAG chains. As with chondroitinase B digestion, this treatment completely abolished binding (Figure 5D). On the other hand, cells cultured in the presence of sodium chlorate supplemented with magnesium sulfate expressed the same level of ligand as did cells cultured in normal media (Figure 5D). Finally, to confirm the specificity of the EMR2-CS ligand interaction, various soluble GAGs were added in the binding assay to block the interaction. As shown in Figure 5E, the addition of exogenous CS-B and CS-C inhibited the interaction in a dose-dependent manner, whereas the addition of HS and CS-A had little effect, even at high concentrations. Together these data suggest that the binding of EMR2 probes to cells is mediated predominantly by sulphated cell-surface CS-B or CS-C side chains.
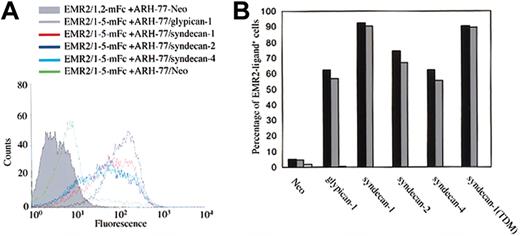
Two major classes of cell-surface proteoglycans are the glypican and syndecan families. To define which cell-surface PG member contains the specific CS ligand for EMR2, stable transfectants of the B lymphoblastoid ARH-77 cell line expressing either syndecan-1, 2, 4, glypican 1, or an HS-negative syndecan-1 mutant (syndecan-1/TDM) were used.28,29 Control cells expressing a neomycin resistance gene had little cell-surface PG and were found to be ligand negative (Figure 6A). Surprisingly, however, all the other transfectants were ligand positive, suggesting CS decoration on the various PG core proteins (Figure 6A). The presence of CS side chains on syndecan molecules has been previously reported36,37 ; however, it is not known whether glypican 1 can carry any CS side chains. To confirm that the interaction between the ARH-77 transfectants and EMR2 probes is indeed mediated by CS, cells were treated with glycosidases. Again, chondroitinase ABC, but not heparinase I+III, treatment completely abolished the binding of probes to the ARH-77 transfectants (Figure 6B). Furthermore, the addition of exogenous CS-B and CS-C but not HS or CS-A blocked the binding of probes to cells (data not shown). These results clearly indicate that the various PGs expressed on ARH-77 stable transfectants contain CS side chain ligands for EMR2.
EMR2-CS interaction is PG core protein–independent. (A) FACS analysis of stably transfected ARH-77 cells probed with EMR2 multivalent fluorescent beads. ARH-77 cells expressing an HS-negative syndecan-1 mutant (syndecan-1TDM) show a similar binding activity (data not shown). (B) Enzymatic digestion of GAG side chains show that all cell-surface PGs expressed on stably transfected ARH-77 cells contain a chondoitinase ABC–sensitive ligand for EMR2. Black bars represent cells with no treatment, gray bars represent heparinase I+III treatment, and white bars represent chondoritinase ABC treatment. The figure is a representative of 3 independent experiments with similar results.
EMR2-CS interaction is PG core protein–independent. (A) FACS analysis of stably transfected ARH-77 cells probed with EMR2 multivalent fluorescent beads. ARH-77 cells expressing an HS-negative syndecan-1 mutant (syndecan-1TDM) show a similar binding activity (data not shown). (B) Enzymatic digestion of GAG side chains show that all cell-surface PGs expressed on stably transfected ARH-77 cells contain a chondoitinase ABC–sensitive ligand for EMR2. Black bars represent cells with no treatment, gray bars represent heparinase I+III treatment, and white bars represent chondoritinase ABC treatment. The figure is a representative of 3 independent experiments with similar results.
Discussion
The GPCR TM7 domains have been shown to mediate the signal transduction of an extensive array of exogenous stimuli including hormones, cytokines, peptides, amino acid derivatives, ions, neuro-transmitters, and sensory stimuli such as photons, taste, and odorants.38-40 Here, we report the identification of a GAG ligand for EMR2/CD97, extending the range of the ligand types for both the EGF-like domains and the GPCRs. The EMR2/CD97–CS interaction is mediated specifically by the largest isoform of human EMR2 and CD97 receptors in a Ca2+- and sulphation-dependent fashion and results in cell attachment (Figures 1, 2, 4, 5). These results have further confirmed the cellular adhesion function of the EGF-TM7 receptors. Many other LNB-TM7 molecules are also thought to mediate cellular interactions through their large extracellular domains.3,4 However, identification of their endogenous ligands has remained largely elusive, hindering their functional analysis. Adaptations of the experimental system described here should facilitate ligand identification and shed light on the physiologic roles of these intriguing cell-surface receptors.
The Ca2+-dependent nature of the EMR2-CS ligand interaction is consistent with the role of Ca2+ in the biologic functions of other EGF-like domain–containing proteins.33 The Ca2+-binding (cb) EGF-like domain is a common structural module used by cell-surface proteins and can be found in extracellular proteins involved in cellular adhesion, blood coagulation, receptor-ligand interactions, extracellular matrix structure, and determination of cell fate.41 The EGF-like domains can interact with other EGF-like domains as shown in the Notch-Delta interaction42,43 or with other protein modules such as short consensus repeats (SCR) of the complement control proteins as demonstrated by the CD97-CD55 interaction.15,16 NMR studies have shown the Ca2+ ion to be important in maintaining the conformational rigidity and binding surfaces of cbEFG-like domains.44 The mapping of the major binding site to the cbEGF-like domain 4 suggests that the EMR2-CS interaction is mediated either by the chelated Ca2+ ion or the intramolecular loops on the surface of the EGF-like domain 4 which are stabilized by the chelated Ca2+ ion (Figure 2). As previous studies have shown that alteration of a small number of residues within the EGF-like domains is able to significantly affect the specificity and affinity of ligand binding,16 it is likely that the different EGF-like domains of other EGF-TM7 molecules are able to bind a number of ligand types. Moreover, the identification of dual ligands (CD55 and CS) for different CD97 receptor isoforms indicates that differential splicing of the same gene can allow the binding of different cellular ligands. It is interesting to note that while the CD97-CD55 interaction is species restricted, the CD97/EMR2–CS interaction is conserved across species. Although the functional significance of such a disparity is unknown, it can possibly be explained by the fact that CS side chain ligands are ubiquitous carbohydrate structures, whereas CD55 is a cellular protein ligand, which relies on specific protein interfaces for interacting with other proteins.
The human CD97-CD55 interaction has been characterized as a low-affinity and rapid off-rate reaction, which is typical of the protein-protein interaction mediated by the majority of leukocyte cell-surface receptors. The EMR2/CD97–CS interaction is likely to be of similar characterization based on the following observation. First, the binding of EMR2 probes to cells was most evident when used as multivalent forms by coupling to avidin-coated beads; soluble EMR2 probes generated only minimal binding (data not shown). Second, the blocking effect by exogenous GAGs required relatively high concentration (Figure 5E). The enzymatic digestion studies and the blocking effect by exogenous GAGs have clearly established that the cellular ligand for the largest EMR2 and CD97 isoform is sulphated CS GAG (Figures 5 and 6); however, the precise structure of the CS ligand remains elusive. Of note, it is not known why IdoA-GalNAc containing GAGs (CS-B) and GlcA-GalNAc-6-sulfate (CS-C) are active, while IdoA-containing HS and 4-sulfated CS-A are inactive in blocking the interaction. Future biophysic analysis of the EMR2/CD97–CS interaction should answer these questions.
The PG core protein-independent nature of the EMR2/CD97–CS interaction is intriguing and suggests that EMR2/CD97 is capable of interacting with a wide range of CS-containing PGs (Figure 5E). Although the syndecan molecules have been shown to be a hybrid PG that can carry both HS and CS chains,36,37 the same has not been demonstrated for the glypicans. Our data clearly indicate that these PGs overexpressed in the stably transfected ARH-77 cell lines were modified by CS-type GAG (Figure 6). It will be interesting to investigate whether this is a general feature or just restricted to the transfected ARH-77 cells. The epimerization and sulphation reactions of HS/DS synthesis have been shown to be tightly regulated in a tissue- and cell-type specific fashion, generating complex subregional heterogeneity.21 The restricted tissue distribution patterns of the CS ligand for EMR2 and the sulphation-dependent interaction reflect the heterogeneous nature of the CS side chains and may represent a normal monocyte/macrophage tissue homing or recruitment mechanism in vivo. On the other hand, DS has also been found to be associated with the modification of resistance to infectious diseases and wound repair.45 Thus, DS is the major soluble GAG present in human wound tissues, where its function includes promotion of the activity of FGF-246 and the activation of endothelial leukocyte adhesion through stimulation of ICAM-1.47 Due to the restricted expression of EMR2/CD97 in myeloid and lymphoid cells, one can speculate that specific CS found in areas of tissue injury may have an important role in the recruitment of and the functional regulation of leukocytes expressing surface EMR2/CD97 during acute inflammation. CSPGs are also important during cholesterol deposition and foam-cell formation in atherogenesis.48 The function of EMR2/CD97 expressing macrophages binding to CS in atherosclerotic lesions would be an interesting area of study. Finally, Decorin, another DS-containing PG, is markedly increased in the tumor stroma of colon cancer.49 The altered composition of proteoglycans in the stroma of invasive tumors may be involved in the attraction or function of tumor-associated leukocytes resulting in important pro- or anti-tumor effects.
Future structural analysis of the CS ligand, such as the minimum length of the disaccharide repeats and the extent of epimerization and sulphation as well as studies of signal transduction following ligand interaction should yield crucial structural and functional information for this unique receptor-GAG interaction.
Prepublished online as Blood First Edition Paper, June 26, 2003; DOI 10.1182/blood-2002-11-3540.
Supported by grants from the British Heart Foundation (PG/02/144 to H.-H.L.), Wellcome Trust (G.-W.C. and M.S.), The Netherlands Organisation for Scientific Research (M.J.K.), and the Medical Research Council, United Kingdom (S.G.). J.Q.D. is the recipient of an Oxford Nuffield Medical Fellowship. H.-H.L. was supported in part by a research grant from CELLTECH R&D. J.H. is a fellow of the Royal Netherlands Academy of Arts and Sciences.
M.S. and H.-H.L. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors would like to thank Liz Darley for the excellent technical support for tissue histology. Dr Phil Taylor is thanked for critical reading of the manuscript.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal