Abstract
One of the most studied secreted phospholipases A2 (sPLA2), the group IIA sPLA2, is found at high levels in inflammatory fluids of patients with autoimmune diseases. A characteristic of group IIA sPLA2 is its preference for negatively charged phospholipids, which become exposed on the extracellular leaflet of apoptotic cell membranes. We recently showed that low molecular weight heparan sulfate proteoglycans (HSPGs) and uncharacterized detergent-insoluble binding site(s) contribute to the enhanced binding of human group IIA PLA2 (hGIIA) to apoptotic human T cells. Using matrix-assisted laser desorption/ionization time of flight (MALDI-TOF) mass spectrometry we now identify vimentin as the major HSPG-independent binding protein of hGIIA on apoptotic primary T lymphocytes. Vimentin is partially exposed on the surface of apoptotic T cells and binds hGIIA via its rod domain in a calcium-independent manner. Studies with hGIIA mutants showed that specific motifs in the interfacial binding surface are involved in the interaction with vimentin. The sPLA2 inhibitor LY311727, but not heparin, inhibited this interaction. In contrast, heparin but not LY311727 abrogated the binding of hGIIA to cellular HSPGs. Importantly, vimentin does not inhibit the catalytic activity of hGIIA. Altogether, the results show that vimentin, in conjunction with HSPGs, contributes to the enhanced binding of hGIIA to apoptotic T cells.
Introduction
Phospholipases A2 (PLA2) hydrolyze membrane phospholipids to produce free fatty acids and lysophospholipids.1,2 PLA2 provide precursors for the biosynthesis of proinflammatory lipid mediators such as prostaglandins, leukotrienes, and lipoxins when the hydrolyzed fatty acid is arachidonic acid (AA) and platelet-activating factor when the lysophospholipid is 1-alkyl-2-lyso-phosphatidylcholine. Several high molecular weight intracellular mammalian PLA2 and a family of 14- to 19-kDa PLA2, referred to as secretory PLA2 (sPLA2), have been identified.2,3 Mammalian sPLA2 are classified into 10 different groups.3,4
sPLA2 can interact with external cell membranes through direct binding to phospholipids or specific cell surface receptors.3,5,6 The M-type receptor present on skeletal muscle7 and human neutrophils8 belongs to the superfamily of C-type lectins and binds with high affinity to the snake venom sPLA2 OS1 and OS2, pancreatic group IB PLA2, and rabbit and mouse group IIA PLA2 but not to bee venom group III PLA2 and very weakly to human group IIA PLA2 (hGIIA).7,9-13 The N-type receptors identified in rat brain have high affinity for OS2 and bee venom group III PLA2.14 Venom and mammalian sPLA2 also bind to a number of cell soluble proteins, such as pentraxins,15 reticulocalbins,16 calmodulin,17 heparin, and to the serum coagulation factor Xa.18
Several mammalian sPLA2 such as group II and V enzymes also bind to heparan sulfate proteoglycans (HSPGs) like glypican-119 and decorin.20 hGIIA is found in high concentrations in the circulation of patients with acute pancreatitis and certain cancers and in the synovial fluid from patients with rheumatoid arthritis.1,2,21-23 hGIIA has been proposed to release AA for the generation of prostaglandins in certain conditions.19,24-31 The binding of hGIIA to HSPGs is required for exogenous hGIIA-catalyzed AA release from apoptotic human T cells and from HEK-293 cells overexpressing hGIIA.25,32-35 Binding to HSPGs has also been linked to sPLA2 activation following internalization and targeting to nuclear membranes or caveolae.19,36
T cells are central to the specific immune response, and following their activation they are eliminated by a Fas-mediated cell death program.37 The presence of activated T cells along with high concentrations of hGIIA in the inflamed synovium of rheumatoid arthritis patients is a hallmark of the disease, and apoptosis is an important mechanism for their elimination and the eventual resolution of inflammation.37,38 The increased susceptibility of apoptotic cells to the action of hGIIA compared with their nonapoptotic counterparts was initially attributed to the enzyme's affinity for anionic phospholipids, which become exposed on the cell surface following the associated loss of membrane asymmetry. However, the susceptibility of apoptotic S49 lymphoma cells to venom group II PLA2 activity occurs prior to the loss of membrane asymmetry,39 while the sensitivity of early apoptotic human T cells and scramblase-transfected cells to the action of hGIIA is dependent on the heparanoid binding site,32,40 suggesting that factors other than exposed anionic phospholipids are responsible for the sensitivity to group IIA sPLA2.
We recently observed that primary human T cells induced to undergo apoptosis in response to activation of the Fas receptor express, in addition to HSPGs, unidentified hGIIA binding sites that are not present on the surface of nonapoptotic lymphocytes. Using a proteomic approach we report here the identification of the cytoskeletal protein vimentin as a novel protein interacting with hGIIA on apoptotic human T cells. We show that this intracellular protein is externalized during apoptosis and found that hGIIA binds to the central core (rod domain) of vimentin.
Materials and methods
Reagents
Heparin 1-A (H3393) was purchased from Sigma-Aldrich Canada (Oakville, ON, Canada). Purified vimentin from bovine lens was from Maine Biotechnology Services (MBS; Portland, ME). RPMI 1640 cell culture media, fetal bovine serum (FBS), Ficoll type 400 (density, 1.077 g/mL), phosphate-buffered saline (PBS), and Hanks balanced salt solution (HBSS) were obtained from Wisent (Saint-Bruno, QC, Canada). Essential fatty acid–free bovine serum albumin (BSA) was purchased from ICN Biomedical (Costa Mesa, CA). Dithiothreitol (DTT) was from Roche (Laval, Québec, QC, Canada). Recombinant hGIIA, human vimentin cDNA in pBlueScript (American Type Culture Collection [ATCC], Manassas, VA; ATCC no. 750603), and the sPLA2 inhibitor LY311727 were generous gifts from Dr Alfred Fonteh (Huntington Medical Research Institutes, Pasadena, CA), Dr Michel Vincent (Université Laval, Québec, QC, Canada), and Dr E. Michelich (Ely Lilly, Indianapolis, IN), respectively. Recombinant mutants of hGIIA were generous gifts from Dr Gerard Lambeau (Institut de Pharmacologie Moléculaire et Cellulaire, Valbonne, France) and Dr Michael H. Gelb (University of Washington, Seattle).41,42
Antibodies
The OKT3 antibody (Ab) was a gift from Dr Walid Mourad (CHUL, Québec, QC, Canada). The anti-CD95 (clone CH-11) was from Upstate Biotechnology (Lake Placid, NY). Polyclonal and monoclonal antibodies (mAbs) to hGIIA were obtained from Cayman Chemical (Ann Arbor, MI). The antiheparan sulfate proteoglycan mAb 10E4 was from Biolynx (Brockville, ON). Horseradish peroxidase (HRP)–conjugated goat antrabbit immunoglobulin G (IgG) and HRP-conjugated goat antimouse IgG were from BioCan Scientific (Mississauga, ON, Canada). The goat polyclonal Ab V4630 to vimentin was from Sigma-Aldrich Canada. The mAb antivimentin clone 3B4 was from MBS. The rabbit polyclonal Ab H84 and the mAb V9 raised against vimentin, fluorescein isothiocyanate (FITC)–conjugated mouse antirabbit IgG, HRP-conjugated goat antimouse and mouse antigoat IgG were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit, mouse, and goat IgG were from Cedarlane (Hornby, ON, Canada).
Lymphocyte preparation, incubation, and induction of apoptosis
Human lymphocytes were isolated from heparinized venous blood collected from healthy volunteers who had taken no medication in the previous 48 hours. Lymphocytes were purified on Ficoll cushions as previously described.32 Lymphocytes (106 cells per milliliter) were cultured in RPMI containing 10% FBS and the OKT3 mAb (1 μg/mL) for 4 days at 37°C in a 5% CO2 atmosphere. Interleukin-2 (20 U/mL) was added after one day of cell culture. Fas-mediated apoptosis was induced by adding 0.5 μg/mL of the anti-CD95 mAb to 200 μL of cultured lymphocytes for the final 18 hours of incubation.
Detection of HSPGs and of lymphocyte-associated hGIIA
For the detection of HSPGs, cells were boiled in Laemmli sample buffer43 and the proteins (75 μg) were separated on 15% sodium dodecyl sulfate (SDS)–polyacrylamide gels and transferred to polyvinylidene fluoride (PVDF) membranes. Membranes were blocked 30 minutes at room temperature (RT) with 5% milk proteins in Tris-buffered saline (TBS)–Tween (190 mM NaCl, 0.15% Tween 20, 25 mM Tris [tris(hydroxymethyl)aminomethane]–HCl, pH 7.6), washed in TBS-Tween, and incubated with the anti-HSPG mAb (2 μg/mL) for 18 hours at 4°C. Membranes were washed 6 times in TBS-Tween and incubated with an HRP-linked goat antimouse IgM (1:15 000). After 6 washes with TBS-Tween the signals were revealed using enhanced chemiluminescence (ECL) Plus (Mandel-NEN Life Science Products, Guelph, ON, Canada).
To monitor the association of hGIIA with cells, hGIIA (0.5 μg) was incubated with lymphocytes (2 × 105) for 15 minutes on ice. Cells were washed twice in PBS, boiled in Laemmli sample buffer, and proteins were separated on 15% SDS–polyacrylamide gels. Samples were transferred to PVDF membranes, and hGIIA was detected using the polyclonal hGIIA Ab (1:1000 dilution) as previously described.32
Hypotonic, hypertonic lysis of human T cells and coprecipitation of hGIIA and its ligands
Cell lysis was performed using a differential solubilization protocol.44 Living or anti-Fas–treated lymphocytes (3 × 107) were incubated with hGIIA (15 μg/mL), washed 3 times in PBS, and lysed in hypotonic lysis buffer (Hypo LB: 0.1% Nonidet P-40 [NP-40], 20 mM Tris-HCl [pH 7.5], 10 mM NaCl, 1 mM EDTA [ethylenediaminetetraacetic acid], 10 μg/mL aprotinin, 10 μg/mL leupeptin, and 2 mM phenylmethylsulfonyl fluoride [PMSF]) for 5 minutes at 4°C. Samples were centrifuged (600g, 10 minutes, 4°C), the resulting supernatant (Hypo LB–soluble fraction) was removed, and the pellets containing the Hypo LB–insoluble proteins were resuspended by sonication in hypertonic lysis buffer (Hyper LB: 1% NP-40, 1% Triton X-100, 20 mM Tris-HCl [pH 7.5], 400 mM NaCl, 1 mM EDTA, 10 μg/mL aprotinin, 10 μg/mL leupeptin, and 2 mM PMSF) and centrifuged (10 000g, 10 minutes, 4°C). The resulting supernatant (Hypo LB–insoluble/Hyper LB–soluble) and pellets and the Hypo LB–soluble fraction were boiled in Laemmli sample buffer, and hGIIA or HSPGs were detected as described above. For the coprecipitation of hGIIA and its binding proteins, the Hypo LB–soluble and the Hypo LB–insoluble/Hyper LB–soluble supernatants were adjusted to an isotonic salt concentration (buffer B: final concentration 137 mM NaCl, 0.75% Triton X-100, 0.75% NP-40, 1 mM EDTA, and 20 mM Tris-HCl, pH 7.5) and precleared with protein G–Sepharose beads (Amersham, Baie d'Urfé, QC) at 4°C for 45 minutes. The beads were incubated (1 hour) with 50 μg hGIIA mAb and mixed with the supernatants (2.5 hours, 4°C). After 3 washes in buffer B, the beads were boiled in Laemmli sample buffer and proteins separated on 7.5% SDS–polyacrylamide gels. Proteins were visualized with Sypro Ruby.
MALDI-TOF analysis and database searching
In-gel protein digestion was performed on a MassPrep liquid handling station (Micromass, Beverly, MA) using sequencing grade trypsin (Promega, Madison, WI). Extracted peptides were lyophilized and resuspended in 0.1% trifluoroacetic acid (TFA). The matrix used for matrix-assisted laser desorption/ionization (MALDI) analysis was α-cyano-4-hydroxycinnamic acid (20 mg/mL in 50% acetonitrile, 0.1% TFA). Equal volumes of peptide and matrix solution were mixed and spotted on a stainless steel MALDI sample plate. The sample/matrix solution was allowed to air dry and was washed 3 times with 0.1% TFA. MALDI–time of flight (TOF) spectra were acquired on a Voyager-DE PRO Biospectrometry Workstation (Applied Biosystems, Framingham, MA) and analyzed using the DataExplorer software version 4.0 (Applied Biosystems, London, ON). The instrument was operated in the positive-ion reflector delayed-extraction mode. The ProFound program (version 4.10.5, The Rockefeller University, http://prowl.rockefeller.edu/cgi-bin/ProFound) was used to search the nonredundant National Center for Biotechnology Information (NCBI) protein database for matching peptide mass fingerprints. Search criteria allowed a maximum of one missed cleavage by trypsin, complete carboxamidomethylation of cysteine, partial methionine oxidation, and mass deviation smaller than 60 ppm.
Flow cytometry staining for vimentin
Cells treated with the anti-CD95 Ab were incubated with antivimentin Abs or isotype Abs (50 μg/mL) for 30 minutes on ice. Samples were washed twice and incubated with an FITC-conjugated Ab for 30 minutes on ice. The cell suspensions were then washed twice prior to flow cytometry analyses. Living or apoptotic cells were gated (minimum of 15 000 cells per gate) according to the cell size and scatter.45-47
Preparation of recombinant vimentin domains
Head, rod, and tail domains of vimentin were prepared by polymerase chain reaction (PCR) amplification using the Pwo polymerase (Roche) and the full-length cDNA for human vimentin as a template. The primers used were 5-GGAATTCATGTCCACCAGGTCCGTG-3 and 5-CACAAGCTTGCGGGTGTTCTTGAACTC-3 for the head domain (amino acids 1 to 95); 5-AGGAATTCGAGTTCAAGAACACCCGC-3 and 5-CCAAGCTTCCCTTCCAGCAGCTTCCT-3 for the rod domain (amino acids 96 to 406); and 5-AGGGATCCAAGAGGAGAGCAGGATTTCT-3 and 5-GAAGCTTGTCAAGGTCATCGTGATGC-3 for the tail domain (amino acids 406 to 466) of vimentin. The amplified fragments were digested (EcoRI and HindIII for head and rod domains and BamHI and HindIII for the tail domain) and cloned into pET21b (Stratagene, La Jolla, CA). Rosetta (DE3) bacteria were transformed with the plasmids, and protein expression was induced with 1 mM isopropyl β-D-thiogalactopyranoside. The His6-tagged proteins were purified under denaturing conditions using Ni-NTA columns (Qiagen, Mississauga, ON, Canada).
Far-Western analysis of hGIIA binding to vimentin
Vimentin (98% pure) was spotted on PVDF membranes using a Bio-Dot SF (Bio-Rad Laboratories, Mississauga, ON). Membranes were blocked for 1 hour with 5% milk proteins in TBS-Tween and were incubated for 16 hours at 4°C with 0.1 μg/mL wild-type (WT) or mutant hGIIA in TBS-Tween in the presence or the absence of 50 μg/mL heparin, 10 μM LY311727, or 10 mM DTT. hGIIA was detected by immunoblotting using the polyclonal anti-hGIIA Ab (1:1000) and an HRP-linked goat antirabbit IgG (1:20 000) and the signal revealed using ECL. The various hGIIA mutants were equally detected by this polyclonal Ab. To map the hGIIA binding site on vimentin, the head, rod, and tail domains (20 μg) were separated on 12% SDS–polyacrylamide gels under nonreducing conditions48 and transferred on PVDF membranes. Membranes were blocked with 5% milk proteins in TBS-Tween, incubated 16 hours at 4°C with 0.1 μg/mL hGIIA, and hGIIA was detected by immunoblotting.
Interaction of hGIIA with HSPGs
hGIIA (5 μg) was incubated at RT in 100 μL binding solution (100 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], 137 mM NaCl, 1 mM CaCl2) alone or in the presence of 500 μg heparin or 100 mM DTT. After 30 minutes, 900 μL binding solution was added and the sample was injected onto 1 mL HiTrap affinity columns (Amersham). These affinity columns are filled with beads covalently coupled to sulfated glucosaminoglycans extracted from porcine intestinal mucosa. Columns were washed with 6 mL binding solution; the flow through, containing unbound hGIIA, was collected; and 100 μL underwent SDS–polyacrylamide gel electrophoresis (SDS-PAGE) on a 15% polyacrylamide gel. hGIIA was detected by immunoblotting as described above.
hGIIA catalytic activity on immobilized vimentin
hGIIA catalytic activity was measured in high-binding 96-well microtiter costar plates (Corning, Corning, NY) using N-((6-(2,4-dinitrophenyl)amino)hexanoyl)-2-(4,4-difluoro-5,7-dimethyl-4-bora-3a,4a-diaza-s-indacene-3-pentanoyl)-1-hexadecanoyl-sn-glycero-3-phospho-ethanolamine (PED6) (Molecular Probes, Eugene, OR) as a substrate; PLA2-mediated hydrolysis of this substrate generates a fluorescent product that allows the direct measurement of catalytic activity. Wells were coated with vimentin or the rod domain (100 μL, 50 μg/mL) for 3 hours, washed 3 times with PBS, and blocked with 5% milk proteins in PBS prior to addition of 10 ng hGIIA in 100 μL (18 hours, 4°C). As a control for nonspecific hGIIA binding, uncoated wells were blocked with 5% milk proteins in PBS prior to incubation with 10 ng hGIIA. As a positive control, wells were incubated with 10 ng hGIIA and were then blocked with 5% milk protein solution. Wells were then washed 3 times in PBS, and hGIIA catalytic activity was monitored by adding 30 μM PED6 in 100 mM HEPES, pH 7.5, with 1 mM CaCl2 and 1 mg/mL BSA. Fluorescence emission (excitation, 500 nm; emission, 512 nm) was monitored using a Bio-Tek FL600 microplate fluorescence reader (ESBE Scientific, Montréal, QC, Canada). The specific activity was determined by dividing the fluorescence (Δ512, arbitrary units) by the quantity of hGIIA (nanograms per milliliter) in each well. The quantity of hGIIA in wells was determined by adding the polyclonal anti-hGIIA (1:500) for 1 hour. Wells were washed 3 times, incubated with the HRP-linked goat antirabbit IgG (1:20 000) for 1 hour, and washed 3 times prior to addition of 100 μL citrate-phosphate buffer (4 mM citric acid, 4mMNa2HPO4, pH 4) containing 0.5 mg/mL 2,2-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) and 0.0002% H2O2. The plate was incubated 30 minutes at RT under agitation, and the amounts of hGIIA were determined by reading the absorbance at 405 nm.
Results
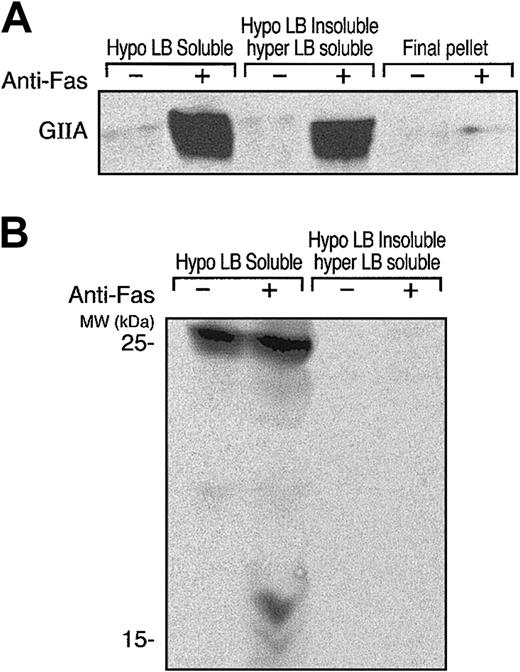
The affinity of group IIA PLA2 for negatively charged phospholipids41,49 was proposed to explain the increased binding of group IIA PLA2 to apoptotic cells in which a phosphatidylserine (PS) flip to the exterior leaf of the plasma membrane occurs. However, expression of venom group II PLA2 binding sites other than anionic phospholipids during apoptosis has been suggested.39 In fact, we recently showed that hGIIA binds to apoptotic human T cells via interactions with HSPGs and other newly exposed binding sites on the cell surface during apoptosis.32 Differential cell solubilization experiments indicated that the HSPGs were associated with a detergent (NP-40)–soluble fraction while other hGIIA ligands were present in a detergent-insoluble fraction of apoptotic lymphocytes. As shown in Figure 1A, hGIIA had a significantly increased affinity for anti-Fas–treated human T cells compared with untreated cells, and the bound hGIIA fractionated into the Hypo LB–soluble and Hypo LB–insoluble/Hyper LB–soluble fractions but not into the Hyper LB–insoluble fraction. Consistent with previous observations that heparin has no effect on the binding of hGIIA with the Hypo LB–insoluble fraction,32 Figure 1B shows that none of the HSPG isoforms detected with mAb 10E4 in the Hypo LB–soluble fraction, including the 17-kDa form that binds to hGIIA, were present in the Hypo LB–insoluble/Hyper LB–soluble fraction.
hGIIA and HSPGs localize to soluble fractions of sequentially lysed apoptotic T cells. hGIIA was incubated with control or anti-Fas–treated human T cells for 15 minutes and washed. Cells were then lysed sequentially with hypotonic and hypertonic lysis buffers. Soluble fractions and the pellet from cells treated with hypertonic lysis buffer were prepared as described in “Materials and methods.” Proteins in the samples were separated by SDS-PAGE and transferred to PVDF membranes. The membranes were probed with anti-hGIIA (A) or anti-HSPG antibodies (B) as described in “Materials and methods.”
hGIIA and HSPGs localize to soluble fractions of sequentially lysed apoptotic T cells. hGIIA was incubated with control or anti-Fas–treated human T cells for 15 minutes and washed. Cells were then lysed sequentially with hypotonic and hypertonic lysis buffers. Soluble fractions and the pellet from cells treated with hypertonic lysis buffer were prepared as described in “Materials and methods.” Proteins in the samples were separated by SDS-PAGE and transferred to PVDF membranes. The membranes were probed with anti-hGIIA (A) or anti-HSPG antibodies (B) as described in “Materials and methods.”
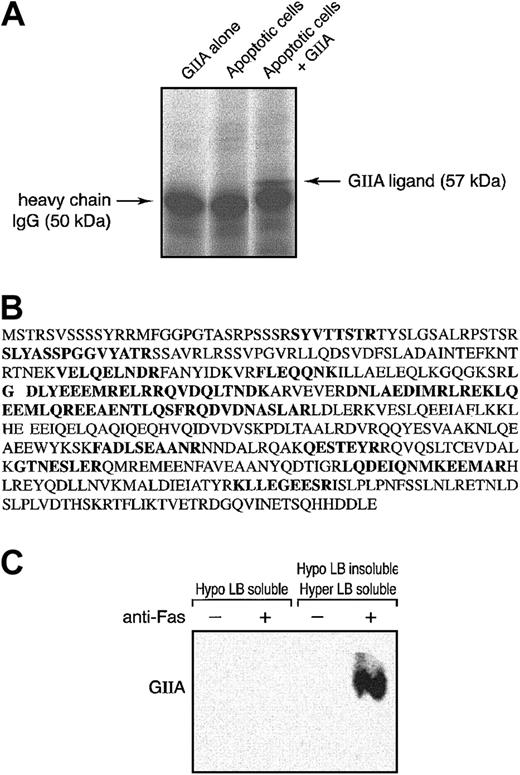
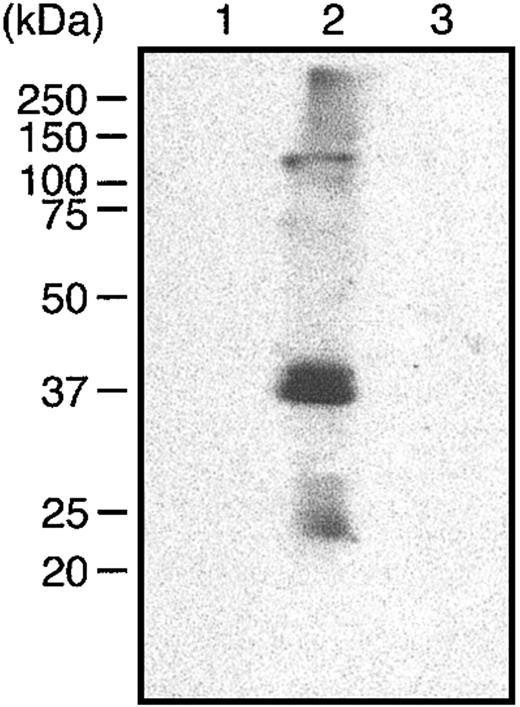
To identify the hGIIA ligands in the Hypo LB–insoluble/Hyper LB–soluble fraction, anti-Fas–treated cells were incubated with or without exogenous hGIIA, and hGIIA associated with the Hypo LB–insoluble/Hyper LB–soluble fraction was immunoprecipitated. Immune complexes were subjected to SDS-PAGE, and proteins were stained using Sypro Ruby. Figure 2A shows that a 57-kDa protein was coprecipitated from the Hypo LB–insoluble/Hyper LB–soluble fraction of apoptotic cells that had been incubated with hGIIA. This 57-kDa protein was analyzed by MALDI–TOF–mass spectrometry (MALDI-TOF-MS), and a search in the nonredundant NCBI protein database for matching peptide mass fingerprints revealed that 16 peptides were unique to human vimentin (Figure 2B). The association of hGIIA with vimentin was further confirmed by the immunoprecipitation of vimentin from the Hypo LB–soluble and the Hypo LB–insoluble/Hyper LB–soluble fractions of cells that had been incubated with hGIIA. Figure 2C shows that hGIIA was coprecipitated with vimentin from the Hypo LB–insoluble/Hyper LB–soluble lysates of anti-Fas–treated cells but not from the Hypo LB–soluble fraction or from lysates of cells that had not been activated with the anti-Fas mAbs.
hGIIA coprecipitates a 57-kDa protein, vimentin, from the Hypo LB–insoluble/Hyper LB–soluble fraction of apoptotic T cells. (A) Apoptotic human T cells were incubated with or without hGIIA and lysed as described in “Materials and methods.” hGIIA was immunoprecipitated from the Hypo LB–insoluble/Hyper LB–soluble fraction. The immune complexes were separated by SDS-PAGE and revealed using Sypro Ruby. Pure hGIIA not incubated with T cells was also immunoprecipitated using the same procedures. (B) The 57-kDa band was collected, digested, and analyzed by MALDI-TOF-MS. The amino acid sequences of the 16 peptides identified by MALDI-TOF-MS are shown in bold. (C) Cells treated or not with an anti-Fas antibody were incubated with hGIIA as described in “Materials and methods.” Cells were sequentially lysed, and vimentin was immunoprecipitated from the soluble fractions with the antibody V4630. Proteins were separated by SDS-PAGE and transferred to PVDF membranes. The membranes were probed with the hGIIA antibody as described in “Materials and methods.”
hGIIA coprecipitates a 57-kDa protein, vimentin, from the Hypo LB–insoluble/Hyper LB–soluble fraction of apoptotic T cells. (A) Apoptotic human T cells were incubated with or without hGIIA and lysed as described in “Materials and methods.” hGIIA was immunoprecipitated from the Hypo LB–insoluble/Hyper LB–soluble fraction. The immune complexes were separated by SDS-PAGE and revealed using Sypro Ruby. Pure hGIIA not incubated with T cells was also immunoprecipitated using the same procedures. (B) The 57-kDa band was collected, digested, and analyzed by MALDI-TOF-MS. The amino acid sequences of the 16 peptides identified by MALDI-TOF-MS are shown in bold. (C) Cells treated or not with an anti-Fas antibody were incubated with hGIIA as described in “Materials and methods.” Cells were sequentially lysed, and vimentin was immunoprecipitated from the soluble fractions with the antibody V4630. Proteins were separated by SDS-PAGE and transferred to PVDF membranes. The membranes were probed with the hGIIA antibody as described in “Materials and methods.”
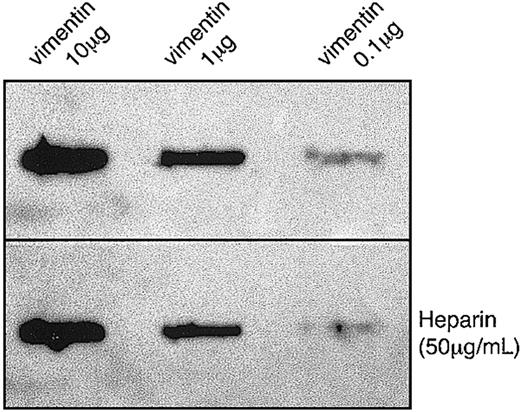
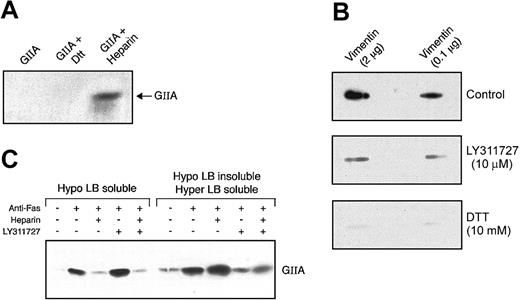
Vimentin was recently shown to form complexes with type 1 plasminogen activator inhibitor (PAI-1) through an association with vitronectin.50 To evaluate whether the binding of hGIIA to vimentin was direct or indirect, a far-Western binding technique was utilized. Figure 3 shows that hGIIA binds to pure vimentin immobilized on a PVDF membrane in the presence or the absence of heparin, indicating that the interaction of hGIIA with vimentin does not require an adaptor molecule and that, unlike binding to HSPGs, heparin does not affect the binding to vimentin. Human M-type sPLA2 receptor binds strongly to group IB PLA2, poorly to hGIIA, and does not bind to group III PLA2.7,9-12,14 Therefore, the ability of group III PLA2 and group IB PLA2 to compete with hGIIA (0.1 μg/mL) for binding to vimentin was also evaluated. The binding of hGIIA to immobilized vimentin was not affected by the addition of other PLA2 (up to 10 μg/mL) to the incubation medium, suggesting that other sPLA2 are poor ligands of vimentin and/or do not compete with hGIIA for binding to vimentin (data not shown).
hGIIA binding to purified vimentin is not inhibited by heparin. Indicated amounts of vimentin were spotted on a PVDF membrane and incubated with hGIIA (0.1 μg/mL) in the absence (top panel) or the presence (bottom panel) of heparin (50 μg/mL). The membrane was washed and probed with an anti-hGIIA antibody as described in “Materials and methods.”
hGIIA binding to purified vimentin is not inhibited by heparin. Indicated amounts of vimentin were spotted on a PVDF membrane and incubated with hGIIA (0.1 μg/mL) in the absence (top panel) or the presence (bottom panel) of heparin (50 μg/mL). The membrane was washed and probed with an anti-hGIIA antibody as described in “Materials and methods.”
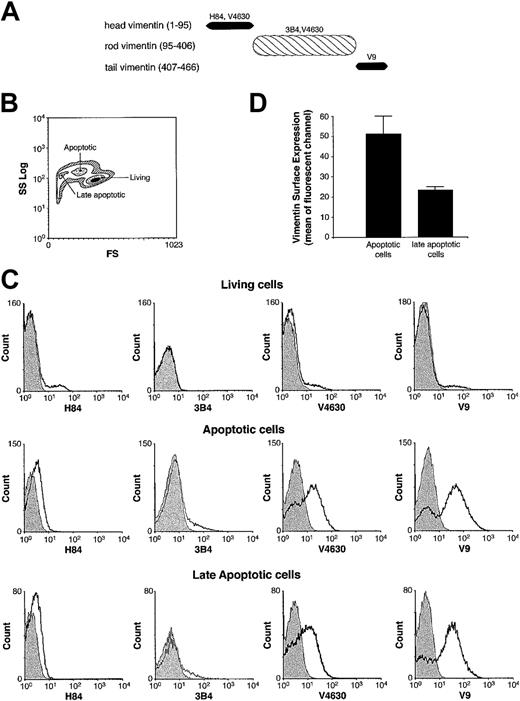
Vimentin is composed of 3 different domains.51 To localize the hGIIA binding site on vimentin, domains comprising the first 95 amino acids from the N-terminal domain (head domain), the central core of vimentin composed of a helical structure (rod domain, amino acids 96 to 406), and the carboxy-terminal amino acids 406 to 466 (tail domain) (Figure 4A) were expressed as C-terminal His6-tagged fusion proteins. Four antibodies, a rabbit polyclonal against the first 84 amino acids of vimentin (H84), a goat polyclonal (V4630), and 2 mouse monoclonal Abs (3B4 and V9) against vimentin, were evaluated by immunoblotting for their ability to recognize the 3 vimentin domains. We determined that the head domain was recognized by H84 and V4630, the rod domain was recognized by V4630 and 3B4, and the tail domain was recognized by V9 (data not shown).
Domain specificity of 4 antivimentin antibodies and their affinity for human T-cell surface. (A) Head, rod, and tail domains of human vimentin, with apparent molecular weights of 12, 37, and 8 kDa, respectively, are shown with their antibody specificity. (B) Cell-size scatter of T cells treated with an anti-Fas antibody for 18 hours. Regions corresponding to living, apoptotic, and late apoptotic cells are identified according to cell size (FS) and granularity (SS). (C) Cells were incubated with indicated antivimentin antibodies or an isotype control (shadow area) and then incubated with the corresponding FITC-conjugated secondary antibodies. A minimum of 15 000 cells per gate were analyzed by flow cytometry. (D) Cells were stained with the antivimentin antibody V4630, incubated with the secondary FITC-coupled antigoat antibody, and analyzed by flow cytometry. The mean ± SEM fluorescent channel of early apoptotic or late apoptotic cells from 3 independent experiments is shown.
Domain specificity of 4 antivimentin antibodies and their affinity for human T-cell surface. (A) Head, rod, and tail domains of human vimentin, with apparent molecular weights of 12, 37, and 8 kDa, respectively, are shown with their antibody specificity. (B) Cell-size scatter of T cells treated with an anti-Fas antibody for 18 hours. Regions corresponding to living, apoptotic, and late apoptotic cells are identified according to cell size (FS) and granularity (SS). (C) Cells were incubated with indicated antivimentin antibodies or an isotype control (shadow area) and then incubated with the corresponding FITC-conjugated secondary antibodies. A minimum of 15 000 cells per gate were analyzed by flow cytometry. (D) Cells were stained with the antivimentin antibody V4630, incubated with the secondary FITC-coupled antigoat antibody, and analyzed by flow cytometry. The mean ± SEM fluorescent channel of early apoptotic or late apoptotic cells from 3 independent experiments is shown.
The head domain of vimentin has been shown to be externalized following platelet activation.50 Because vimentin is likely to be externalized in apoptotic lymphocytes, we analyzed by flow cytometry which domains may be exposed on the cell surface for interaction with exogenous hGIIA. During Fas-induced apoptosis, cells become smaller and more granular, allowing the discrimination between living, early, or late apoptotic cells (Figure 4B).45-47 Figure 4C shows that none of the 4 antivimentin Abs were able to stain living cells, indicating that vimentin was not exposed on the cell surface. However, apoptotic cells were stained by the V4630 Ab, which recognizes epitopes on the head and rod domains, and by the V9 mAb, which is specific for the tail domain. In contrast, apoptotic cells were not stained by the polyclonal H84 Ab directed against the vimentin head domain or the monoclonal 3B4 recognizing the vimentin C-terminal coiled coil motif52 of the rod domain. These results suggest that both rod, at least in part, and tail domains, but not the head domain of vimentin, are exposed on the surface of apoptotic T cells. More vimentin was detected on the surface of early apoptotic cells compared with late apoptotic cells (Figure 4D). Because hGIIA was previously shown to preferentially bind to early apoptotic rather than late apoptotic T cells,32 the result suggests that vimentin could be the ligand responsible for the increased hGIIA binding to early apoptotic cells. To further map the hGIIA binding site on vimentin, a far-Western experiment using the head, rod, and the tail domains was performed. Figure 5 shows that hGIIA only binds to the rod domain. The binding of hGIIA to vimentin rod was not inhibited by the polyclonal V4630 antibody or by the 3B4 mAb against the C-terminal coiled coil 2B motif (not shown). Taken together, these results are consistent with the concept that both the tail and rod domains of vimentin are exposed on the surface of Fas-induced apoptotic T cells, thereby allowing exogenous hGIIA to bind to these cells via interactions with the rod domain of vimentin.
Far-Western analysis of the hGIIA binding domain on vimentin. Vimentin head (1), rod (2), and tail (3) domains were separated by SDS-PAGE and transferred to a PVDF membrane. The membrane was incubated with hGIIA (0.1 μg/mL) and probed with the hGIIA antibody as described in “Materials and methods.”
Far-Western analysis of the hGIIA binding domain on vimentin. Vimentin head (1), rod (2), and tail (3) domains were separated by SDS-PAGE and transferred to a PVDF membrane. The membrane was incubated with hGIIA (0.1 μg/mL) and probed with the hGIIA antibody as described in “Materials and methods.”
hGIIA contains 7 disulfide bridges, one of them specific to group II enzymes. Because hGIIA is composed of positively charged clusters potentially involved in its interaction with HSPGs, DTT treatment may not affect hGIIA binding to HSPGs. Figure 6A supports this hypothesis, indicating that the structure of hGIIA maintained by disulfide bridges is not essential for binding HSPGs under physiologic salt concentrations. To determine whether the structure of hGIIA was required for its interaction with vimentin, the impact of DTT on hGIIA binding to immobilized vimentin was analyzed. Figure 6B demonstrates that reducing the disulfide bridges of hGIIA with DTT inhibits its binding to vimentin, suggesting that hGIIA must maintain its tertiary structure to bind vimentin.
Inhibitors of hGIIA that block the binding of hGIIA to vimentin and HSPGs. (A) hGIIA preincubated or not with DTT (100 mM) or heparin (500 μg/mL) for 30 minutes at RT was injected into 1 mL columns containing heparan sulfates as described in “Materials and methods.” The columns were then washed, and the eluate was analyzed for hGIIA by immunoblotting as described in the “Materials and methods.” (B) Indicated amounts of vimentin were spotted on PVDF membranes. hGIIA (0.1 μg/mL) was preincubated or not with 10 mM DTT or 10 μM LY311727 at RT for 30 minutes and then incubated with immobilized vimentin on PVDF membranes in the presence or the absence of DTT or LY311727. The membrane was then probed with the hGIIA antibody as described in “Materials and methods.” (C) hGIIA was preincubated or not with LY311727 (10 μM) for 30 minutes at RT and then incubated with control or Fas-treated cells in the presence or the absence of heparin (50 μg/mL). Cell suspensions were sequentially lysed, and proteins in the Hypo LB–soluble and Hypo LB–insoluble/Hyper LB–soluble fractions were separated by SDS-PAGE. The samples were transferred to a PVDF membrane and probed with the hGIIA antibody as described in “Materials and methods.”
Inhibitors of hGIIA that block the binding of hGIIA to vimentin and HSPGs. (A) hGIIA preincubated or not with DTT (100 mM) or heparin (500 μg/mL) for 30 minutes at RT was injected into 1 mL columns containing heparan sulfates as described in “Materials and methods.” The columns were then washed, and the eluate was analyzed for hGIIA by immunoblotting as described in the “Materials and methods.” (B) Indicated amounts of vimentin were spotted on PVDF membranes. hGIIA (0.1 μg/mL) was preincubated or not with 10 mM DTT or 10 μM LY311727 at RT for 30 minutes and then incubated with immobilized vimentin on PVDF membranes in the presence or the absence of DTT or LY311727. The membrane was then probed with the hGIIA antibody as described in “Materials and methods.” (C) hGIIA was preincubated or not with LY311727 (10 μM) for 30 minutes at RT and then incubated with control or Fas-treated cells in the presence or the absence of heparin (50 μg/mL). Cell suspensions were sequentially lysed, and proteins in the Hypo LB–soluble and Hypo LB–insoluble/Hyper LB–soluble fractions were separated by SDS-PAGE. The samples were transferred to a PVDF membrane and probed with the hGIIA antibody as described in “Materials and methods.”
We recently reported that the binding of hGIIA to HSPGs on the surface of apoptotic cells was inhibited by heparin, an antibody directed against heparan sulfate and by heparinase III.32 hGIIA binding to immobilized vimentin was not affected by heparin (Figure 3) but was significantly diminished by the specific sPLA2 inhibitor LY311727, suggesting that epitopes near or at the active site of hGIIA are involved in the interaction with vimentin (Figure 6B). Consistent with this observation, the levels of hGIIA recovered in the Hypo LB–soluble fraction were not reduced when apoptotic lymphocytes were incubated with hGIIA and LY311727 prior to cell lysis, but those in the Hypo LB–insoluble/Hyper LB–soluble fraction that contains vimentin were significantly diminished (Figure 6C).
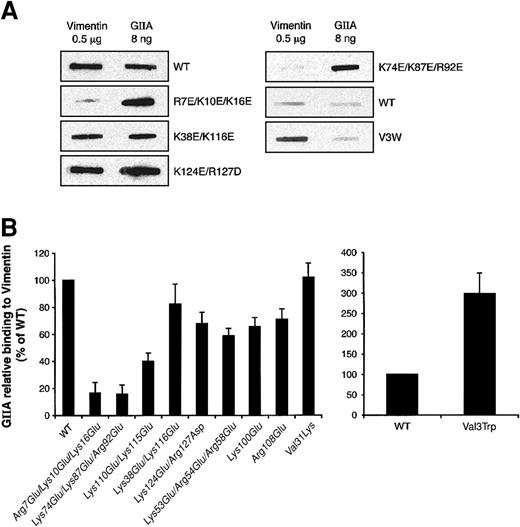
Vimentin contains basic and acidic areas that could form electrostatic interactions with other proteins. While the head domain is very basic, the tail domain contains an excess of acidic amino acids.51 Moreover, hGIIA is a positively charged protein with 23 lysines and arginines but only 8 glutamic and aspartic acid residues.41 Charge reversal of basic residues on the interfacial binding surface (IBS) of hGIIA decreases its ability to bind the soluble coagulation factor Xa.53 Because the specific hGIIA inhibitor LY311727 prevents vimentin binding and the catalytic site is proposed to be in the IBS,54 it is likely that this region is involved in the interaction with vimentin. To further ascertain the vimentin binding site on hGIIA, the binding to immobilized vimentin of several hGIIA mutants with charge reversal of positive clusters suggested to be located inside (Arg7Glu/Lys10Glu/Lys16Glu, Lys74Glu/Lys87Glu/Arg92Glu, Lys38Glu/Lys116Glu, Lys110Glu/Lys115Glu, and Lys124Glu/Arg127Asp) or outside (Lys53Glu/Arg54Glu/Arg58Glu, Lys100Glu, and Arg108Glu) the putative IBS42 was measured. Figure 7A-B shows that only the Arg7Glu/Lys10Glu/Lys16Glu, Lys74Glu/Lys87Glu/Arg92Glu, and Lys110Glu/Lys115Glu mutants had considerably decreased binding to vimentin compared with WT hGIIA. The Lys124Glu/Arg127Asp and Lys38Glu/Lys116Glu mutants still efficiently bound to vimentin even though these mutations are also located in the IBS. Interestingly, the Val3Trp mutant within the IBS, which has a 250-fold increased activity on phosphatidylcholine (PC) vesicles compared with WT hGIIA,55 showed increased binding to vimentin (Figure 7B).
Vimentin binding to hGIIA mutants. (A) hGIIA (WT) or its mutants (0.1 μg/mL) were incubated with PVDF membranes to which 0.5 μg vimentin was immobilized. As positive controls, hGIIA or its mutants (8 ng) were also immobilized onto PVDF membranes. hGIIA and its mutants were then detected by immunoblotting as described in “Materials and methods.” Decreased (left panels) and increased (right panels) binding of hGIIA mutants to vimentin is shown. (B) The ratio between the densitometric quantification of the hGIIA bound to 0.5 μg vimentin and that of 8 ng of the corresponding immobilized hGIIA represents the relative binding of hGIIA to vimentin. Results are presented as percentage of WT and are the means ± SEM of 4 different experiments.
Vimentin binding to hGIIA mutants. (A) hGIIA (WT) or its mutants (0.1 μg/mL) were incubated with PVDF membranes to which 0.5 μg vimentin was immobilized. As positive controls, hGIIA or its mutants (8 ng) were also immobilized onto PVDF membranes. hGIIA and its mutants were then detected by immunoblotting as described in “Materials and methods.” Decreased (left panels) and increased (right panels) binding of hGIIA mutants to vimentin is shown. (B) The ratio between the densitometric quantification of the hGIIA bound to 0.5 μg vimentin and that of 8 ng of the corresponding immobilized hGIIA represents the relative binding of hGIIA to vimentin. Results are presented as percentage of WT and are the means ± SEM of 4 different experiments.
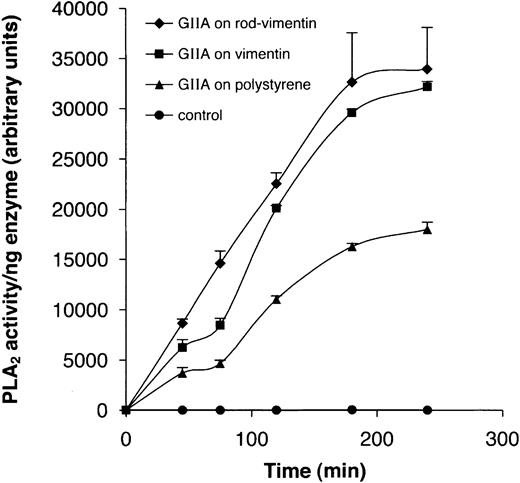
Our finding that vimentin possibly interacts with residues near or at the active site of hGIIA raised the hypothesis that this interaction would impact on the enzyme's hydrolytic activity. To evaluate the effect of this interaction on its enzymatic activity, hGIIA was bound to the bottom of wells that had been precoated or not with either pure vimentin or the rod domain. Figure 8 shows that the activity of hGIIA bound to vimentin or the rod domain in coated wells was enhanced compared with that of the enzyme bound to uncoated polystyrene wells. These results suggest that the interaction of hGIIA with vimentin could possibly have an anchoring or docking function that enables or enhances its catalytic activity toward cellular phospholipids.
Vimentin enhances the catalytic activity of hGIIA. hGIIA (0.1 μg/mL) was incubated with vimentin or rod-vimentin immobilized to the bottom of 96-well microtiter Costar plates or was directly coated to the bottom of the polystyrene wells. In controls, untreated wells were precoated with 5% milk proteins prior to incubation with hGIIA. Catalytic activity was monitored using the PED6 fluorescent phospholipid analog as described in “Materials and methods.”
Vimentin enhances the catalytic activity of hGIIA. hGIIA (0.1 μg/mL) was incubated with vimentin or rod-vimentin immobilized to the bottom of 96-well microtiter Costar plates or was directly coated to the bottom of the polystyrene wells. In controls, untreated wells were precoated with 5% milk proteins prior to incubation with hGIIA. Catalytic activity was monitored using the PED6 fluorescent phospholipid analog as described in “Materials and methods.”
Discussion
T lymphocytes play an important role in the pathogenesis of inflammatory disorders, which are characterized by the recruitment of activated T cells and neutrophils to inflamed sites. Apoptosis and the subsequent ingestion of apoptotic cells by macrophages is the major mechanism for clearing inflammatory cells leading to the resolution of inflammation.37,38 FasL/Fas-mediated programmed cell death is essential in regulating the life span of reactive T cells and may represent a negative feedback loop accelerating the resolution of inflammation by eliminating leukocytes by apoptosis.
Large amounts of group IIA PLA2 are found in the inflammatory exudates of animal models of inflammation56-58 and in the synovial fluids and plasma of patients with rheumatoid arthritis and septic shock,1,2,21 respectively, suggesting that group IIA PLA2 plays an important role in inflammatory diseases and host defense. We recently demonstrated that hGIIA has increased affinity for apoptotic T cells.32 Group IIA PLA2 prefers anionic phospholipids such as PS for interfacial binding and catalytic activity, and the exposure of PS on the external surface of the plasma membrane during apoptosis has been suggested to render apoptotic cells more susceptible to the action of hGIIA. Indeed, overexpression of the phospholipid scramblase, which promotes the flip of PS on the extracellular leaflet of the plasma membrane, renders the cells more susceptible to the action of hGIIA.40 However, the increased susceptibility of S49 lymphoma cell membranes to hydrolysis by venom group II PLA2 happens well before the loss of membrane asymmetry,39 suggesting that other binding sites may be expressed on early apoptotic cells. Accordingly, we recently showed that apoptotic human T cells express HSPGs as well as uncharacterized heparanoid-independent binding site(s) for hGIIA.32
In the present study, a 57-kDa protein that interacted with hGIIAwas affinity purified from the Hypo LB–insoluble fraction of apoptotic T lymphocytes. The MALDI-TOF-MS spectrum revealed the hGIIA binding partner was identical to vimentin, a cytoskeletal protein. Vimentin is the most widely expressed type III intermediate filament protein and shares more than 70% of sequence identity with desmin (53 to 54 kDa), the glial fibrillary acidic protein (GFAP) (50 kDa), and peripherin (57 kDa).59-61 Intermediate filaments associate with the nuclear envelope via their tail domain and to the plasma membrane via the positively charged head domain.62-64 Their possible cellular functions are to support the transport of specific populations of vesicles and to maintain organelle structures.65-70
Although vimentin is an intracellular protein, it was recently shown to be externalized during platelet activation.50 Exposure of vimentin during T-cell apoptosis and platelet activation appears to be different because the tail domain remains inaccessible to the V9 (antitail) antibody during platelet activation,50 while the tail and the rod but not the head are exposed on apoptotic T cells. We localized the hGIIA binding site to the rod domain of vimentin; however, this domain does not appear to be completely exposed because an antibody specific for the coiled coil 2B motif, near the C-terminal of the rod domain, did not stain apoptotic T cells. Several lines of evidence suggest that the tertiary structure and amino acids located within the IBS of hGIIA are required for its interaction with vimentin. Firstly, hGIIA binding to immobilized vimentin, but not to HSPGs, is sensitive to DTT, which disrupts disulfide bonds.71 Additionally, LY311727, which binds the active site of hGIIA located within the IBS resulting in significant structural modifications of the protein in the α helix in the amino terminus,72 eliminated hGIIA binding to vimentin. This suggests that the structural displacements induced by LY311727 near the active slot of hGIIA are sufficient to decrease its interaction with vimentin. Finally, hGIIA mutants with charge reversal in the IBS showed the most dramatic change in affinity for vimentin.
The studies with mutants indicate that anionic phospholipid vesicles and vimentin appear to interact with hGIIA at common sites because it was previously shown that the Arg7Glu/Lys10Glu/Lys16Glu and Lys74Glu/Lys87Glu/Arg92Glu mutants also show substantially decreased binding (290- and 200-fold, respectively) to anionic vesicles compared with WT hGIIA.41 The amphilic indole side chain of tryptophan is also associated with the capacity of lipases, including hGIIA, to interact with interfaces.73 Indeed, many venom sPLA2 have tryptophan residues in the N-terminal region within the IBS.74 Interestingly, the replacement of valine 3 by tryptophan within the IBS increased the affinity of hGIIA for vimentin by 3-fold. This is again consistent with a motif in the IBS being involved in vimentin interaction.Altogether, these observations suggest that hGIIA-vimentin interactions could mimic hGIIA-interface interactions, which are associated with increased enzymatic activity. The enhanced enzymatic activity measured when hGIIA was associated with immobilized vimentin would support that concept; however, whether interactions with vimentin on the apoptotic cell are associated with the observed release of cellular AA remains to be shown.
Although the interaction of vimentin with the IBS suggests that vimentin may play a role in hGIIA-mediated cellular AA release, the observation that heparin and heparinase III, which have no effect on the binding of hGIIA to vimentin, are very potent inhibitors of cellular AA release implicates HSPGs as activating ligands.32 While we cannot exclude the possibility that vimentin merely provides an extension arm to increase the accessibility of the substrate to its active site, it is tempting to speculate that the simultaneous interaction of hGIIA with vimentin and HSPGs, which are both expressed on the surface of early apoptotic T cells, may enhance the hydrolytic activity of hGIIA toward cellular phospholipids. It was not possible to study the impact of purified vimentin or recombinant rod domain on hGIIA-mediated cellular release of AA because they are only soluble in 6M urea, conditions that are not compatible with a cell-based activity assay. However, studies using vimentin-positive and -negative cell lines could possibly be used to determine whether or not vimentin directly impacts on hGIIA hydrolysis of cellular phospholipids.
hGIIA is not the first enzyme involved in AA metabolism known to bind to cytoskeletal proteins. The 5-lipoxygenase interacts with actin filaments via an adaptor, the coactosin-like protein,75 and the C2 domain of mouse cytosolic PLA2 was reported to bind to the head domain of vimentin76 inaCa2+-dependent manner. In contrast, the interaction of hGIIA with vimentin did not require Ca2+, because both the immunoprecipitation and the in vitro binding assay were performed without Ca2+ and/or in the presence of the Ca2+ chelator EDTA. A role for the microfilament network in the regulation of AA release and metabolism is suggested by the increased synthesis of prostaglandins following disruption of the actin cytoskeleton with cytochalasin D or latrunculin77 ; however, the mechanisms by which these proteins regulate AA metabolism have not been elucidated.
The interaction of hGIIA with vimentin may also play a role in the intracellular localization of hGIIA. Plasma membrane domains like caveolae and lipid rafts78 contain intermediate filament proteins including vimentin79 and are enriched in glycosylphosphatidylinositol (GPI)–anchored proteins such as the HSPG glypican.80 Interestingly, glypican has been shown to recruit hGIIA into caveolae-like compartments of cells.19 Although it is not known whether vimentin and HSPGs colocalize in these membrane microdomains, these proteins may play a role in the intracellular distribution of hGIIA in activated cells.19,81 The interaction of hGIIA with vimentin in conjunction with the crucial role of HSPG for its activity could provide regulatory mechanisms for its activation and its intracellular colocalization with proteins involved in the metabolism of AA.
Following the initial observations that autoantibodies from patients with autoimmune diseases could bind to apoptotic cells,82,83 it was hypothesized that posttranslational modifications or the relocalization of some proteins during apoptosis could be involved in autoimmunity. To determine whether apoptotic cells could be a reservoir of autoantigens, Gensler et al injected apoptotic Jurkat T cells into BALB/c mice and obtained monoclonal antibodies against vimentin.84 Because vimentin is a known autoantigen— because antivimentin antibodies are found in the sera from patients with rheumatoid arthritis,85 myocarditis,86 and systemic lupus erythematosus (SLE)87-89 —these studies collectively raised the possibility of vimentin externalization during apoptosis. The present study confirms that the tail and rod domains of vimentin are exposed to the extracellular space in early apoptotic cells. Whether the interaction of hGIIA with externalized vimentin plays a role in the progression of autoimmune diseases is unknown; however, future studies are needed to evaluate the role of hGIIA binding to vimentin in pathophysiological conditions.
Prepublished online as Blood First Edition Paper, June 26, 2003; DOI 10.1182/blood-2002-12-3702.
Supported by grants from the Canadian Institute of Health Research (M.E.S. and S.G.B.). E.B. is the recipient of a scholarship from Le Fonds de la Recherche en Santé du Québec.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors are thankful to Dr Alfred N. Fonteh of the Huntington Medical Research Institutes, Dr Gerard Lambeau from the Institut de Pharmacologie Moléculaire et Cellulaire, and Dr Michael H. Gelb from the University of Washington for their helpful comments.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal