Abstract
Recent suppressive subtractive hybridization analysis on human atherosclerotic plaque-derived RNA revealed genes upregulated in plaques with a thrombus versus stable plaques. Clone SSH6, containing part of a putative open reading frame of an unknown protein, was further investigated. Full-length cDNA, coding for a 473–amino acid (aa) protein, was identified in a vascular smooth muscle cell (SMC) cDNA library. Bioinformatics suggested the presence of multiple SSH6 variants due to alternative splicing of exon 3. Multiple-tissue Northern blot analysis demonstrated a differential expression pattern of these variants, as a ubiquitously expressed SSH6 mRNA missing exon 3, was detected apart from a putative vascular SMC–specific form containing exon 3. Western blot analysis indicated a ubiquitous 35-kDa protein (SSH6-β), in addition to a 45-kDa protein (vasculin), detected in the vascular wall and in plasma. Analysis of arteries displaying various stages of atherosclerosis indicated that the vasculin/SSH6-β ratio increases throughout atherogenesis. Immunohistochemical analysis demonstrated cytoplasmic expression of SSH6 gene products in macrophages, endothelial cells, and SMCs. In summary, we identified a novel mRNA/protein, vasculin, in the arterial wall and plasma. The regulated expression of vasculin in plaques suggests a role in atherogenesis. Moreover, its presence in plasma opens perspectives for vasculin as a marker for atherosclerosis.
Introduction
In the past few years, various large-scale gene expression studies have been performed in order to identify genes that are involved in atherosclerosis.1-9 In these studies a variety of techniques has been applied including cDNA expression arrays, differential display, RNA subtraction methods, serial analysis of gene expression (SAGE), and signature pyrosequencing, which have been performed both on whole-mount material and cell lines reported to be involved in atherosclerosis. Most of these studies focused on genes known to be involved in processes related to atherosclerosis such as inflammation,10 apoptosis,11 matrix turnover,12 and lipid metabolism13 or on well-known genes whose relationship to atherosclerosis had not been reported before. However, the incidence of novel highly up-regulated genes in the outcome of these investigations indicated that a full understanding of the atherosclerotic process is far from established and studies elucidating presently unknown genes/proteins are indispensable to solve the “missing links.” Nevertheless, studies concentrating on these novel atherosclerosisrelated genes are sparse. De Vries et al6 revealed 30 cytokine-responsive human genes of unknown function in cultured vascular smooth muscle cells (SMCs). Moreover, Horrevoets et al7 and Yoshisue et al8 identified novel genes in human umbilical vein endothelial cells, whose expression was modulated by cytokines or shear stress, respectively.
The low abundance of reports describing novel genes can, at least partially, be explained by the lack of antigen-specific immunologic tools. Indeed, the availability of antibodies or antibody fragments is a prerequisite to study genes at the protein level, and although these tools are ample for well-established proteins, it is a laborious task to obtain high-quality antibodies for novel proteins. In order to meet the need for antigen-specific tools, in the present study we selected single-chain variable fragments (scFv's) from a phage-displayed library.14 These scFv's were demonstrated to serve as useful instruments to evaluate protein expression in Western blot and immunohistochemical analyses.
Recently, suppressive subtractive hybridization (SSH) analysis has been performed on whole-mount human atherosclerotic plaques to establish a library of cDNA fragments preferentially expressed in plaques with a thrombus versus stable atherosclerotic plaques.15 Sequence analysis of clone SSH6 revealed that its cDNA shares partial overlap with a public database cDNA template coding for a putative protein with unknown function, but in addition it contains a 120-bp insertion absent in the homologous sequence.
In this report, we describe the cloning and initial characterization of this novel mRNA/protein, which was named tentatively vasculin as an acronym for vascular wall–linked protein. We isolated full-length cDNA from a vascular SMC library and characterized its genomic organization. Furthermore, we developed immunologic tools to characterize the protein and to evaluate its presence in human tissues. Our data indicate a regulated gene expression of vasculin in the vascular wall, suggesting an in vivo function for vasculin in normal vascular biology as well as in atherosclerosis. Moreover, the presence of vasculin in plasma opens perspectives for the development of vasculin as a marker for atherosclerosis.
Patients, materials, and methods
Tissue sampling
Atherosclerotic plaques were collected from patients undergoing vascular surgery or at autopsy (Departments of Surgery and Pathology, Academic Hospital Maastricht). Approval was obtained according to the protocols of the Medical Ethical Committee of the Academic Hospital Maastricht. Informed consent was provided according to the Declaration of Helsinki. Vascular specimens were processed as described15 and classified according to Virmani et al.16 Nondiseased samples originated from the abdominal aorta or from the subclavian, mammary, or carotid artery; venous specimens were from the saphenous or iliac veins; samples from early diseased arteries were from the abdominal or thoracic aorta or the subclavian or carotid artery; advanced plaques were from the abdominal aorta, common iliac artery, or the (common) femoral or carotid artery; and plaques containing a thrombus originated from abdominal aorta or the femoral, iliac, or carotid artery.
Cell culture
Human vascular SMCs were obtained from abdominal aortic lesions as described previously with minor modifications.17 Cultures were maintained in Dulbecco modified Eagle medium (DMEM; Life Technologies, Bethesda, MD) complemented with 20% fetal calf serum, l-alanyl-l-glutamine (1 × Glutamax I; Life Technologies), penicillin (100 IU/mL), and streptomycin (50 μg/mL) and were used for further experiments between passage 2 and 5.
Screening of vascular SMC cDNA library
A 1490-bp probe including the unique 120-bp insertion of clone SSH6 was polymerase chain reaction (PCR)–generated from first-strand cDNA using the appropriate primers (Table 1), 32P-labeled (High Prime; Roche Applied Science, Indianapolis, IN), and was used to screen a human arterial (activated) SMC-derived cDNA library.6 Positive clones were identified, tested using PCR, and inserts were sequenced with the Thermo Sequenase cycle-sequencing kit (Amersham Pharmacia Biotech, Piscataway, NJ). Bioinformatics were performed using the nucleotide Blast programs (National Center for Biotechnology [NCBI], http://www.ncbi.nlm.nih.gov) and EXPASy Molecular Biology Services (http://www.expasy.ch).
Oligonucleotide sequences of primers used in PCR
Application . | Amplified fragment, bp* . | DNA strand . | Oligonucleotides . |
|---|---|---|---|
| Screening SMC library | 1083-2572 | Forward | 5′TGAGTTGGAGAGACTGGACC 3′ |
| Screening SMC library | 1083-2572 | Reverse | 5′GAGAGATGTATCACACTAAG 3′ |
| MTE, complete cDNA clone SSH6 | 743-1292 | Forward | 5′GGCTCGTAAATTGGATA 3′ |
| MTE, complete cDNA clone SSH6 | 743-1292 | Reverse | 5′TTACCTCCATTAGGACGTCC 3′ |
| MTE, unique insert in clone SSH6 | 1048-1168 | Forward | 5′GGACTTGCCATGAGGTGTTGAAG 3′ |
| MTE, unique insert in clone SSH6 | 1048-1168 | Reverse | 5′CTTTGTTGATGATGGTGGAGTAG 3′ |
| Cloning GST-vasculin | 1597-2545 | Forward | 5′CCTAAATCTAGAGCGTCGACGATGCTGG 3′ |
| Cloning GST-vasculin | 1597-2545 | Reverse | 5′AAGCTGTTAGTCGACCCTTCACA 3′ |
Application . | Amplified fragment, bp* . | DNA strand . | Oligonucleotides . |
|---|---|---|---|
| Screening SMC library | 1083-2572 | Forward | 5′TGAGTTGGAGAGACTGGACC 3′ |
| Screening SMC library | 1083-2572 | Reverse | 5′GAGAGATGTATCACACTAAG 3′ |
| MTE, complete cDNA clone SSH6 | 743-1292 | Forward | 5′GGCTCGTAAATTGGATA 3′ |
| MTE, complete cDNA clone SSH6 | 743-1292 | Reverse | 5′TTACCTCCATTAGGACGTCC 3′ |
| MTE, unique insert in clone SSH6 | 1048-1168 | Forward | 5′GGACTTGCCATGAGGTGTTGAAG 3′ |
| MTE, unique insert in clone SSH6 | 1048-1168 | Reverse | 5′CTTTGTTGATGATGGTGGAGTAG 3′ |
| Cloning GST-vasculin | 1597-2545 | Forward | 5′CCTAAATCTAGAGCGTCGACGATGCTGG 3′ |
| Cloning GST-vasculin | 1597-2545 | Reverse | 5′AAGCTGTTAGTCGACCCTTCACA 3′ |
Underlined sequence indicates SalI restriction site; mutated residues are indicated in bold.
Amplified vasculin cDNA fragment, according to position in clone A6.3 (Figure 1).
Multiple-tissue Northern blot analysis
Multiple tissue Northern blot was performed using the multiple-tissue expression array (MTE; Clontech, Palo Alto, CA), essentially according to the protocol of the manufacturer. In brief, probes matching the complete cDNA fragment present in clone SSH6 or the unique 120-bp insert only were PCR amplified using the appropriate primers indicated in Table 1. Subsequently, the MTE was hybridized with the denatured 32P-labeled probe at 65°C overnight and exposed to x-ray film at –70°C for 48 hours. mRNA from a primary vascular SMC culture derived from an atherosclerotic plaque with a thrombus, as well as plasmid DNA containing exon 3, were used as positive controls.
Expression plasmid for Schistosoma japonicum glutathione S–transferase–vasculin (GST-vasculin) fusion protein
A cDNA fragment corresponding to the 302 C-terminal amino acids of the open reading frame (ORF) of vasculin was PCR amplified under standard conditions using the primers mentioned in Table 1 in order to introduce SalI restriction sites for the construction of the expression plasmid. As a result of the introduction of the desired restriction sites, a proline (CCA) and arginine (AGG) codon in the open reading frame of vasculin were mutated into a serine (TCG) and threonine (ACG) codon, respectively. Subsequently, the PCR product was SalI digested and the 938-bp gel-purified fragment was ligated into plasmid pGEX-4T-2 (Amersham Pharmacia Biotech). BL21 strain Escherichia coli cells were transformed with the ligation product and the construct used for the expression of GST-vasculin (pGEX-GST-vasculin) was sequenced entirely in the GST-vasculin coding region.
Expression and purification of GST-vasculin and GST
GST-vasculin was expressed basically as described before.18 Wild-type GST protein, produced in BL21 E coli cells transformed with a pGEX-4T-2 without insert, was used as a control. For the purification of GST and GST-vasculin, cells were lysed by sonication in buffer 20 mM Tris–HCl (tris(hydroxymethyl)aminomethane-HCL) (pH 8.0), containing 1% Triton X-100, 100 mM NaCl, 1 mM EDTA (ethylenediaminetetraacetic acid), 1 mM dithiothreitol (DTT), 2 mM phenylmethylsulfonyl fluoride (PMSF), and 0.1 mM benzamidine. The soluble fraction was purified with glutathione agarose by batch absorption (Amersham Pharmacia Biotech), essentially as described by the manufacturer. After dialysis against phosphate-buffered saline (PBS), purity was checked using sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and Coomassie Blue staining. Protein concentration was determined using a colorimetric assay (Bio-Rad Protein Assay; Bio-Rad Laboratories, Hercules, CA).
Selection of vasculin-specific scFv's from a phage-display library
The Griffin.1 library, a human synthetic VH+VL scFv library in the phagemid vector pHEN2,19 was kindly provided by Dr Heather Griffin (Medical Research Council [MRC] Centre for Protein Engineering, Cambridge, United Kingdom).
Three rounds of selection of the library were performed by panning in immunotubes coated with 200 μg GST-vasculin, essentially as described previously.20 Prior to the final round of selection, the enriched library was depleted for GST binders by panning on 200 μg GST. After each round, single clones were characterized by size and BstNI fingerprinting of PCR-amplified inserts. To identify vasculin-specific scFv's resulting from the third round of selection, an enzyme-linked immunosorbent assay (ELISA) was performed on purified vasculin-GST and GST, essentially as described before.21 In short, individual bacterial clones were grown in 96-well microtiter tubes, soluble scFv's were expressed as described,20 and the bacterial supernatants were screened for antigen binding. Bound scFv's were detected with horseradish peroxidase (HRP)–conjugated monoclonal antibody 9E10 (9E10-HRP) directed against the human c-myc amino acid tag within the scFv and visualized using trimethylbenzidine (TMB) and hydrogen peroxidase. The scFv-encoding region of phagemids coding for vasculin-specific scFv's were sequenced.
Expression and purification of scFv-2A4 and scFv-1E5
E coli HB2151 cells were transformed with the phagemids pHEN2-scFv-2A4 or pHEN2-scFv-1E5. ScFv's were expressed as described20 and purified from the whole-cell extract (B-per; Pierce, Rockford, IL) using Ni-NTA (Qiagen, Hilden, Germany) according to the manufacturers instructions. Prior to further applications, scFv's were dialyzed overnight at 4°C against PBS and protein purity and concentration was determined as described in “Expression and purification of GST-vasculin and GST.”
Affinity of scFv's
Affinity constants for the binding of scFv's with GST-vasculin was determined using the BIAcore 2000 analytical system equipped with the CM5 sensor chip (BIAcore Amersham Pharmacia Biotech, Maarssen, The Netherlands) as described previously.22 In brief, purified GST-vasculin was coupled covalently up to 1100 resonance units (RUs) to the sensor chip in 10 mM acetate buffer, pH 4.0. ScFv's diluted to a final concentration ranging from 50 to 800 nM in 0.01 M HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), pH 7.4, 0.15 mM NaCl, 3 mM EDTA, and 0.005% Tween 20, were injected at a flow rate 10 μL/minute (injection volume 65 μL). After each cycle the chip was regenerated using 15 μLof a 100 mM H3PO4 solution. The analyses of the association and dissociation phases were performed with the software of the BIAcore 2000 (Langmuir binding, local fit).
Western blot and 2-dimensional (2D) analysis
Proteins were extracted essentially as described.23 In brief, cultured cells scraped in PBS or vascular tissue, respectively, were homogenized in ice-cold lysis buffer (10 mM Tris-HCl, pH 8.0, 2.5 mM KCl, 150 mM NaCl, 0.5% Nonidet P40, 0.5% Triton X-100, 20 mM β-glycerolphosphate, 50 mM NaF, 1 mM dithiothreitol, 10 μg/mL soytrypsin inhibitor, 10 μg/mL leupeptin, 2 mM NaVO3, 200 mM benzamidin) and incubated for 30 minutes on ice. After centrifugation (20 minutes, 4500g), the protein concentration of the soluble fraction was determined as described in “Expression and purification of GST-vasculin and GST.” Human aorta, muscle, testis, ovary, and SMC protein medleys were obtained from Clontech.
For Western blot analysis, samples equivalent to 25 μg of total protein were separated by SDS-PAGE (9%) under reducing conditions, whereas for 2D gel electrophoresis, samples containing 50 μg protein were separated using a 7-cm Immobiline pH gradient strip (pH 4-7; Amersham Pharmacia Biotech) prior to separation by 12% SDS-PAGE. After transfer onto nitrocellulose (Protran; Schleicher & Schuell, Dassel, Germany) and blocking (MPBS; PBS containing 2% [wt/vol] skimmed milk powder), blots were incubated with scFv-2A4 (5 μg/mL). Bound scFv-2A4 was detected with anti-myc tag antibody 9E10 (1:1000 dilution of the hybridoma supernatant), followed by incubation with a horseradish-coupled rabbit antimouse antibody (RAM-HRP; 1:1000; Dako, Glostrup, Denmark). Specific antibody binding was visualized using enhanced chemiluminiscence (Amersham Pharmacia Biotech). All antibodies were diluted in MPBS containing 0.5% gelatin. Between each incubation step the membrane was washed with PBS containing 0.1% (vol/vol) Tween 20.
Immunohistochemical analysis
After deparafination and rehydration, 4-μm tissue sections were pretreated for 30 minutes with pepsin (1 mg/mL in 0.1 N HCl) and blocked for 60 minutes with Tris-buffered saline (TBS; Tris-HCl 0.1 M, NaCl, 2.7 M) containing 0.1% (vol/vol) Tween 20 and 1% bovine serum albumin (BSA). Sections were incubated subsequently with scFv-2A4 (120 μg/mL), anti-myc tag antibody 9E10 (1:750 dilution of the hybridoma supernatant), biotinylated sheep antimouse antibody (1:250; Dako), and alkaline phosphatase–coupled streptavidin biotin complex (ABC) reagent (1:200; Dako). Each incubation step was performed for 30 minutes at room temperature. Alkaline phosphatase activity was visualized using the Alkaline Phosphatase Kit I (Vector, Burlingame, CA) containing 1 mM levamisole (Sigma, St Louis, MO), resulting in a red precipitate. Sections were counterstained with hematoxylin. To determine cell types expressing vasculin and/or SSH6-β, double immunohistochemical stainings were performed. Sections labeled for vasculin and/or SSH6-β were labeled subsequently for either α-smooth muscle actin (ASMA) (1:500; Dako; staining SMC), CD31 (1:100; Dako; staining endothelial cells), or CD68 (1:100; Dako; staining macrophages), as described previously.
Statistical analysis
All data are expressed as mean ± SD. Means between groups were compared by the use of the Mann-Whitney U test. A P value of less than .05 was considered statistically significant.
Results
SSH6gene, vasculin mRNA, and protein
Bioinformatic analysis revealed that the cDNA of clone SSH6 differs from the homologous cDNA template AL161991 due to the insertion of a unique 120-bp fragment.15 Further sequence analysis demonstrated that this insert includes the putative start codon of a previously unidentified ORF, suggesting that this ORF corresponds to a novel mRNA and protein.
In order to obtain the full-length ORF, a human artery SMC cDNA library6 was screened with a probe that includes the unique 120-bp insert of clone SSH6. Among more than 10 cDNA clones, all containing an insert more than 2000-bp long, we isolated and characterized clone A6.3, which contained the longest cDNA fragment (2858 bp, AY226828; Figure 1). Clone A6.3 comprises an 1105-bp 5′ untranslated region, an ORF of 1419 bp, and a 334-bp 3′ untranslated region.
Nucleotide sequence of clone A6.3 and the deduced amino acid sequence for vasculin. The deduced amino acid sequence is shown below the DNA sequence. The nucleotide sequence is numbered at the left and the amino acid sequence at the right. The putative vasculin start codon (ATG) and the termination codon (TGA) are boxed. Vertical bars indicate the exon limits and exons are numbered as indicated in Table 2. Exon 3 is indicated in bold.
Nucleotide sequence of clone A6.3 and the deduced amino acid sequence for vasculin. The deduced amino acid sequence is shown below the DNA sequence. The nucleotide sequence is numbered at the left and the amino acid sequence at the right. The putative vasculin start codon (ATG) and the termination codon (TGA) are boxed. Vertical bars indicate the exon limits and exons are numbered as indicated in Table 2. Exon 3 is indicated in bold.
Comparison of the sequence of clone A6.3 with the human genome demonstrated that the gene was located on chromosome 5 (5q11.2). The gene comprises at least 12 exons and spans a region of more than 90 000 bp. Exon-intron boundaries and sizes of exons and introns are summarized in Table 2. Remarkably, exon 3, containing the start codon of the ORF identified in clone SSH6, exactly matches the unique 120-bp fragment absent in most of clone SSH6 cDNA variants in the databases. These data strongly suggest that alternative splicing of exon 3 underlies the existence of various variants of the SSH6 gene and that the ORF cloned in the current study corresponds to a unique splice variant containing exon 3. Because this ORF was identified originally in a library of vascular wall–derived cDNAs and subsequently isolated from a vascular smooth muscle cDNA library, we entitled the corresponding mRNA and protein as vasculin. The gene was named SSH6, referring to the original SSH6 clone.
Exon-intron junctions of the human SSH6 gene
Exon . | Exon-intron junction . | Exon size, bp . | Intron size, bp . |
|---|---|---|---|
| 1 | ACT GTG... CAG AAG gta aca | > 95 | 1236 |
| 2 | tta cag GTG ATT... GAA TTT gta agt | 953 | 37 657 |
| 3 | tga cag GGA CTT... ACA AAG gta ctc | 120 | > 16 000 |
| 4 | ttt cag TCG TCA... ATG GAG gta aaa | 124 | 128 |
| 5 | ata aag GTA ACT... GAT TTT gta agt | 224 | 4644 |
| 6 | ttc cag CCG TCT... TGT GGG gta agt | 67 | 10 267 |
| 7 | atg cag AAT ATC... ACA AAA gta agt | 185 | 590 |
| 8 | ctt tag CCT ACA... AAA GAG gta tga | 141 | 2193 |
| 9 | tct tag TGT AAT... GAG AAG gta att | 168 | > 1600 |
| 10 | aat cag GAT GAC... ACA CAG gct agt | 188 | 10 038 |
| 11 | ttt cag ATT GTT... GAA CAG gta aga | 103 | 1311 |
| 12 | ttt tag TTA CAG... ATT TTC | > 490 | NA |
Exon . | Exon-intron junction . | Exon size, bp . | Intron size, bp . |
|---|---|---|---|
| 1 | ACT GTG... CAG AAG gta aca | > 95 | 1236 |
| 2 | tta cag GTG ATT... GAA TTT gta agt | 953 | 37 657 |
| 3 | tga cag GGA CTT... ACA AAG gta ctc | 120 | > 16 000 |
| 4 | ttt cag TCG TCA... ATG GAG gta aaa | 124 | 128 |
| 5 | ata aag GTA ACT... GAT TTT gta agt | 224 | 4644 |
| 6 | ttc cag CCG TCT... TGT GGG gta agt | 67 | 10 267 |
| 7 | atg cag AAT ATC... ACA AAA gta agt | 185 | 590 |
| 8 | ctt tag CCT ACA... AAA GAG gta tga | 141 | 2193 |
| 9 | tct tag TGT AAT... GAG AAG gta att | 168 | > 1600 |
| 10 | aat cag GAT GAC... ACA CAG gct agt | 188 | 10 038 |
| 11 | ttt cag ATT GTT... GAA CAG gta aga | 103 | 1311 |
| 12 | ttt tag TTA CAG... ATT TTC | > 490 | NA |
Exon/intron boundaries were determined by comparing the vasculin cDNA sequence (Figure 1) to human genomic contigs (accession numbers AC008435 and hCG40617, from public and CELERA databases, respectively). The nucleotide sequence of each exon (uppercase letters) and intron (lowercase letters) at the exon/intron boundaries is shown. NA indicates not applicable.
Analysis of the ORF of vasculin, spanning from the putative start codon in exon 3 to the stop codon in exon 12, revealed that it encodes a 473–amino acid (aa) protein (Figure 1). The presence of a TAA stop codon at position –3 to –1 from the putative start codon ascertains that the ORF is identified completely at the 5′ end and codes for a full-length protein. The calculated mass of vasculin is 53 kDa and its iso-electric point is 6.56, ignoring the potential contribution of posttranslational modifications. Extensive bioinformatic analysis did not result in the identification of any typical hydrophobic signal nor mitochondrial, peroxisomal, or vacuolar targeting peptide. Moreover, neither DNA/RNA binding motifs nor a model predicting nuclear or transmembrane localization of vasculin were detected. Furthermore, we were not able to identify a functional protein domain. Therefore, at present we are unable to assign a function to this protein.
Vascular SMC–specific expression pattern of vasculin
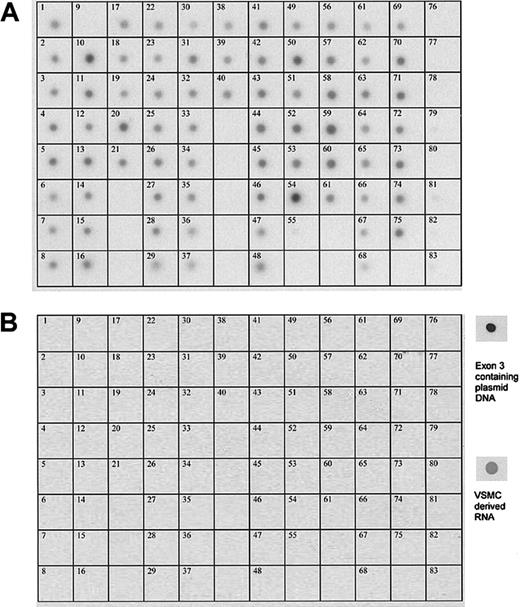
To examine the tissue distribution of mRNA transcripts from the SSH6 gene, a multiple-tissue Northern blot analysis was performed on 61 adult human tissues, 8 human cell lines, 7 fetal tissues, and 6 controls. With the original insert of clone SSH6 as a probe, which covers 305-bp length of exon 2, the entire exons 3 and 4 (120 bp and 124 bp, respectively), and 4 bp of exon 5, mRNA was detected in nearly all tested samples (Figure 2A). In contrast, multiple-tissue Northern blot analysis using an exon 3–specific probe (120 bp) resulted in the absence of any hybridization signal in any tested tissue or cell line, except for RNA derived from human vascular SMCs and our positive control, exon 3 containing plasmid DNA (Figure 2B). These data strongly suggest a differential tissue distribution of various splice variants of the SSH6 gene (ie, vasculin cDNA), containing a unique 120-bp fragment and detected exclusively in primary vascular SMCs, can be discriminated from other ubiquitously expressed variant(s) lacking this 120-bp insert.
Expression of the SSH6 gene in a variety of human tissues and cell lines. Northern blot analysis of a commercially available human multiple-tissue expression array (MTE; Clontech). Hybridization with a probe corresponding to (A) exon 2-3-4 and (B) exon 3. Samples 1 to 61 correspond to poly A+ RNA from human tissues (1, whole brain; 2, cerebral cortex; 3, frontal lobe; 4, parietal lobe; 5, occipital lobe; 6, temporal lobe; 7, paracentral gyrus of cerebral cortex; 8, pons; 9, left cerebellum; 10, right cerebellum; 11, corpus callosum; 12, amygdala; 13, caudate nucleus; 14, hippocampus; 15, medulla oblongata; 16, putamen; 17, substantia nigra, 18, accumbens nucleus; 19, thalamus; 20, pituitary gland; 21, spinal cord; 22, heart; 23, aorta; 24, left atrium; 25, right atrium; 26, left ventricle; 27, right ventricle; 28, interventricular septum; 29, apex of the heart; 30, esophagus; 31, stomach; 32, duodenum; 33, jejunum; 34, ileum; 35 ilocecum; 36, appendix; 37, ascending colon; 38 transverse colon; 39, descending colon; 40, rectum; 41 kidney; 42, skeletal muscle; 43, spleen; 44, thymus; 45, peripheral blood leukocyte; 46, lymph node; 47, bone morrow; 48, trachea; 49, lung; 50, placenta; 51, bladder; 52, uterus; 53, prostate; 54, testis; 55, ovary; 56, liver; 57 pancreas; 58, adrenal gland; 59, thyroid gland; 60, salivary gland; 61, mammary gland); from human cell lines (62, leukemia HL-60; 63, HeLa S3; 64, leukemia, K-562; 65, leukemia MOLT-4; 66, Burkitt lymphoma Raji; 67, Burkitt lymphoma Daudi; 68, colorectal adenocarcinoma, A549); from fetal tissues (69, fetal brain; 70, fetal heart; 71, fetal kidney; 72, fetal liver; 73, fetal spleen; 74, fetal thymus; 75, fetal lung); and from controls (76, yeast total RNA; 77, yeast tRNA; 78, E coli rRNA; 79, E coli DNA; 80, Poly r(A); 81, human C0t-1 DNA; 82, 100 ng human DNA; 83, 500 ng human DNA). Panels at the right show the hybridization signal of the exon 3 probe with vascular SMC–derived RNA and exon 3 containing plasmid DNA, respectively.
Expression of the SSH6 gene in a variety of human tissues and cell lines. Northern blot analysis of a commercially available human multiple-tissue expression array (MTE; Clontech). Hybridization with a probe corresponding to (A) exon 2-3-4 and (B) exon 3. Samples 1 to 61 correspond to poly A+ RNA from human tissues (1, whole brain; 2, cerebral cortex; 3, frontal lobe; 4, parietal lobe; 5, occipital lobe; 6, temporal lobe; 7, paracentral gyrus of cerebral cortex; 8, pons; 9, left cerebellum; 10, right cerebellum; 11, corpus callosum; 12, amygdala; 13, caudate nucleus; 14, hippocampus; 15, medulla oblongata; 16, putamen; 17, substantia nigra, 18, accumbens nucleus; 19, thalamus; 20, pituitary gland; 21, spinal cord; 22, heart; 23, aorta; 24, left atrium; 25, right atrium; 26, left ventricle; 27, right ventricle; 28, interventricular septum; 29, apex of the heart; 30, esophagus; 31, stomach; 32, duodenum; 33, jejunum; 34, ileum; 35 ilocecum; 36, appendix; 37, ascending colon; 38 transverse colon; 39, descending colon; 40, rectum; 41 kidney; 42, skeletal muscle; 43, spleen; 44, thymus; 45, peripheral blood leukocyte; 46, lymph node; 47, bone morrow; 48, trachea; 49, lung; 50, placenta; 51, bladder; 52, uterus; 53, prostate; 54, testis; 55, ovary; 56, liver; 57 pancreas; 58, adrenal gland; 59, thyroid gland; 60, salivary gland; 61, mammary gland); from human cell lines (62, leukemia HL-60; 63, HeLa S3; 64, leukemia, K-562; 65, leukemia MOLT-4; 66, Burkitt lymphoma Raji; 67, Burkitt lymphoma Daudi; 68, colorectal adenocarcinoma, A549); from fetal tissues (69, fetal brain; 70, fetal heart; 71, fetal kidney; 72, fetal liver; 73, fetal spleen; 74, fetal thymus; 75, fetal lung); and from controls (76, yeast total RNA; 77, yeast tRNA; 78, E coli rRNA; 79, E coli DNA; 80, Poly r(A); 81, human C0t-1 DNA; 82, 100 ng human DNA; 83, 500 ng human DNA). Panels at the right show the hybridization signal of the exon 3 probe with vascular SMC–derived RNA and exon 3 containing plasmid DNA, respectively.
Production of recombinant GST-vasculin protein and the selection and characterization of scFv's
In order to study vasculin at the protein level, high-affinity antibodies were selected to serve as immunologic tools. Therefore, a fusion protein (GST-vasculin, 535 aa's) consisting of GST and the 302 C-terminal aa's of vasculin was expressed in E. coli. For control experiments, recombinant wild-type GST protein was prepared. SDS-PAGE analysis and Coomassie Blue staining of purified proteins revealed a major band of the expected size for GST-vasculin (61 kDa) and GST (26 kDa).
Vasculin-specific scFv's were selected from a combinatorial VH+VL phage displayed library (MRC, Cambridge, United Kingdom) by panning on purified GST-vasculin and GST. After the final round of selection, soluble scFv's were screened for antigen binding by ELISA on plates coated either with GST-vasculin or GST. Thus it was demonstrated that all selected scFv's were directed against the vasculin segment of the fusion protein (n = 80). Fingerprinting and subsequent sequence analysis of the phagemids of positive clones demonstrated that at least 2 different scFv's could be discriminated. Clone 2A4 and 1E5, each coding for a different scFv, were used in further experiments because the corresponding scFv's (named scFv-2A4 and scFv-1E5, respectively) generated highest signals in ELISA.
Dot blot and ELISA analysis indicated that both scFv's reacted as well with denatured as nondenatured GST-vasculin but lacked reactivity with nonrelated proteins (data not shown). BIAcore analysis demonstrated that scFv-2A4 exhibits an affinity of 71 ± 55 nM (KD) for GST-vasculin, whereas the affinity of scFv-1E5 was more than 2-fold lower (KD, 163 ± 118 nM). Therefore, scFv-2A4 was used in further experiments.
Analysis of vasculin protein in human tissues and cell lines
To investigate the ability of scFv-2A4 to bind to native, full-length vasculin, we used scFv-2A4 to probe a Western blot of human atherosclerotic plaque lysates, in parallel with GST-vasculin, which was used as a positive control. As shown in Figure 3, the predicted 61-kDa band was detected for GST-vasculin. In plaque lysates, strongly immunoreactive bands were identified with an apparent molecular weights of 45 kDa and 35 kDa. As discussed in “Discussion,” the detected 45-kDa protein probably corresponds to vasculin, whereas the 35-kDa protein might be a transcriptional or translational variant of the SSH6 gene. From now on this low–molecular weight variant will be referred to as SSH6-β. Interestingly, these data are the first to indicate the existence of multiple variants of the SSH6 gene at the protein level. In this context, we have to emphasize that scFv-2A4 does not distinguish SSH6-β from vasculin.
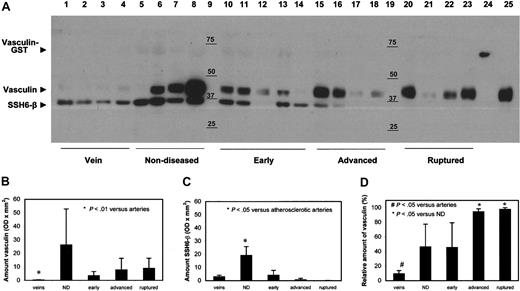
Vasculin and SSH6-β in the vascular wall. (A) Representative Western blot analysis of lysates of complete segments of veins, nondiseased arteries, and arteries displaying different stages of atherosclerosis (n = 2). Lanes 1 to 4, veins; lanes 5 to 8, nondiseased arteries; lanes 10 to 14, early atherosclerotic plaques; lanes 15 to 18, advanced atherosclerotic plaques; lanes 20 to 23, atherosclerotic plaques with a thrombus; lane 24 GST-vasculin; and lane 9 and 19, marker. For lysates and GST-vasculin, samples equivalent to 25 μg and 1 μg protein, respectively, were applied. (B-C) Quantitative data of vasculin and SSH6-β content, respectively, as judged by densitometric scanning of 2 independent Western blot analyses (mean ± SD, n = 4-5). ND indicates nondiseased. (D) Relative amount of vasculin versus the total amount of vasculin + SSH6-β, as determined in panels B-C, in veins and arteries displaying various stages of atherosclerosis (mean ± SD, n = 4-5). ND indicates nondiseased.
Vasculin and SSH6-β in the vascular wall. (A) Representative Western blot analysis of lysates of complete segments of veins, nondiseased arteries, and arteries displaying different stages of atherosclerosis (n = 2). Lanes 1 to 4, veins; lanes 5 to 8, nondiseased arteries; lanes 10 to 14, early atherosclerotic plaques; lanes 15 to 18, advanced atherosclerotic plaques; lanes 20 to 23, atherosclerotic plaques with a thrombus; lane 24 GST-vasculin; and lane 9 and 19, marker. For lysates and GST-vasculin, samples equivalent to 25 μg and 1 μg protein, respectively, were applied. (B-C) Quantitative data of vasculin and SSH6-β content, respectively, as judged by densitometric scanning of 2 independent Western blot analyses (mean ± SD, n = 4-5). ND indicates nondiseased. (D) Relative amount of vasculin versus the total amount of vasculin + SSH6-β, as determined in panels B-C, in veins and arteries displaying various stages of atherosclerosis (mean ± SD, n = 4-5). ND indicates nondiseased.
To evaluate the presence of vasculin and SSH6-β protein in veins and arteries displaying various stages of atherosclerosis, Western blot analysis was performed on lysates from individual samples of veins, nondiseased arteries, arteries containing early or advanced plaques, or arteries containing plaques with a thrombus (n = 4-5 for each group; Figure 3). Expression of vasculin and SSH6-β was detected in all samples, although to different extents and in different ratios. Most strikingly were the minimal amounts of vasculin in veins and the minimal amounts of SSH6-β in plaques with a thrombus. Quantification of the intensity of the vasculin and SSH6-β bands by densitometric scanning of the blots demonstrated that vasculin content was significantly higher in arteries than in veins (P < .01; Figure 3B). However, no correlation was observed between the amount of vasculin and the stage of atherosclerosis. Analysis of the SSH6-β content (Figure 3C) revealed no significant difference between veins and arteries. Remarkably, the amount of SSH6-β was significantly higher in nondiseased arteries than in early and advanced plaques and plaques with a thrombus (P < .05). Comparison of the vasculin/SSH6-β ratio demonstrates large differences between various vessels. Figure 3D illustrates the ratio of vasculin versus SSH6-β and shows that the relative amount of vasculin differs significantly between veins and arteries, between nondiseased arteries and advanced atherosclerotic plaques, and between nondiseased arteries and plaques with a thrombus (P < .05).
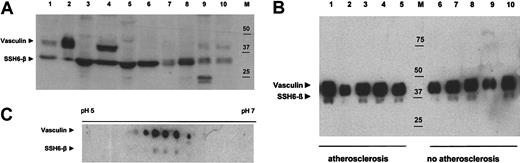
In addition to the tissue-specific expression of vasculin mRNA, evidence for a differential expression pattern of vasculin and SSH6-β in various human tissues and cell lines was deduced from Western blot analysis on the respective lysates (Figure 4A). Vasculin was highly expressed in plaques and human umbilical vein endothelial cells and was present to a lower extent in ovary and aorta. Most interestingly, vasculin was also detected at high levels in human plasma. In contrast, vasculin was not observed in a vascular SMC line, a colon or lung carcinoma–derived cell line, or in skeletal muscle and brain lysates. On the other hand, SSH6-β was identified in all samples.
Western blot analysis of vasculin and SSH6-β. For each sample, amounts equivalent to 25 μg protein were applied. Vasculin and SSH6-β were detected by scFv-2A4 (5 μg/mL) and visualized using enhanced chemiluminescence. (A) Analysis of a variety of human cell lines and tissues. Lane 1 shows atherosclerotic plaque; lane 2, human plasma; lanes 3 to 6, human cell lines (3, colon carcinoma; 4, human umbilical vein endothelial cells; 5, Louis lung carcinoma; 6, vascular SMC line); lanes 7 to 11, human tissues (7, muscle; 8, brain; 9, ovary; 10, aorta); and M, marker. (B) Plasma samples from patients with (lane 1 to 5) or without (lane 6 to 10) clinical manifestations of renal atherosclerotic disease. M indicates marker. (C) A 2D gel electrophoresis analysis of a human atherosclerotic plaque lysate. Advanced plaque lysate (50 μg) was separated using a 7-cm pH gradient strip (pH 4-7) and a 12% SDS-PAGE and transferred onto nitrocellulose.
Western blot analysis of vasculin and SSH6-β. For each sample, amounts equivalent to 25 μg protein were applied. Vasculin and SSH6-β were detected by scFv-2A4 (5 μg/mL) and visualized using enhanced chemiluminescence. (A) Analysis of a variety of human cell lines and tissues. Lane 1 shows atherosclerotic plaque; lane 2, human plasma; lanes 3 to 6, human cell lines (3, colon carcinoma; 4, human umbilical vein endothelial cells; 5, Louis lung carcinoma; 6, vascular SMC line); lanes 7 to 11, human tissues (7, muscle; 8, brain; 9, ovary; 10, aorta); and M, marker. (B) Plasma samples from patients with (lane 1 to 5) or without (lane 6 to 10) clinical manifestations of renal atherosclerotic disease. M indicates marker. (C) A 2D gel electrophoresis analysis of a human atherosclerotic plaque lysate. Advanced plaque lysate (50 μg) was separated using a 7-cm pH gradient strip (pH 4-7) and a 12% SDS-PAGE and transferred onto nitrocellulose.
Analysis using Western blot analysis of vasculin and SSH6-β plasma levels of 5 patients with proven renal atherosclerosis and of 5 individuals with no symptoms of atherosclerotic disease (Figure 4B) does not reveal significant differences between vasculin and SSH6-β plasma levels of individuals with and without clinical manifestations of atherosclerosis, respectively. Therefore, more elaborated studies are required to investigate the possible correlation between vasculin, SSH6-β levels, or the vasculin/SSH6-β ratio and atherosclerotic load.
A 2D gel electrophoresis and subsequent Western blot analysis (Figure 4C) of lysates from atherosclerotic plaques containing whole-artery segments demonstrated the presence of at least 5 vasculin variants with an isoelectric point (pI) ranging between 5.5 and 6.5, indicating the occurrence of various posttranslational modifications. In addition, we demonstrated at least 3 posttranslational variants of SSH6-β. Analysis of 3 stable plaques and 3 plaques with a thrombus suggested no difference in posttranslational modification between the respective plaque types (data not shown).
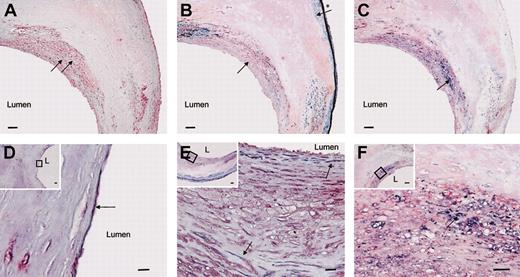
To localize vasculin and SSH6-β protein in the vascular wall, immunohistochemical analysis was performed with scFv-2A4 (Figure 5A). Immunohistochemical analysis of vascular samples revealed immunoreactivity in the cytoplasm of SMCs, macrophages, and vascular endothelial cells, as confirmed by a combined staining of scFv-2A4 and anti-SMC actin (SMC), anti-CD68 (macrophages/monocytes), or anti-CD31 (endothelial cells) antibodies, respectively (Figure 5B-F). In nondiseased arteries vasculin and/or SSH6-β were localized essentially in (a subpopulation of) medial SMCs and in vascular endothelial cells (data not shown). In atherosclerotic arteries, immunohistochemical staining was observed also in neointimal SMCs and in macrophages. Strikingly, signals were more intense in neointimal SMCs than in medial SMCs (Figure 5B,E inset). In addition, pronounced immunohistochemical signals were observed in the endothelium and the SMCs of small vessels inside the adventitia (data not shown). In negative controls, omitting incubation with scFv-2A4, no signals were detected.
Localization of vasculin and SSH6-β protein in the vascular wall. Cross-sections of an atherosclerotic plaque stained by immunohistochemistry. (A) Vasculin and/or SSH6-β is visualized in red (black arrows). (B,E) Combined immunohistochemical staining for vasculin and/or SSH6-β (red) and anti–smooth muscle actin demonstrating SMC (blue). Pronounced vasculin/SSH6-β signals are observed in neointimal SMC (black arrow), whereas vasculin/SSH6-β signals are minimal in medial SMCs (arrow with *). (C,F) Combined immunohistochemical staining of vasculin and/or SSH6-β with anti-CD68 (blue) demonstrates the presence of SSH6 gene products in monocytes/macrophages, as indicated by a black arrow. (D) Combined immunohistochemical staining of vasculin and/or SSH6-β with anti-CD31 (blue) shows vasculin/SSH6-β in endothelial cells. The black arrow indicates an endothelial cell expressing vasculin/SSH6-β. (D-F) Insets represent overview pictures with regions in D-F outlined by a ⋄. L indicates the lumen. Bars in panels A-C and the insets in D-F represent 100 μm; bars in D-F represent 10 μm.
Localization of vasculin and SSH6-β protein in the vascular wall. Cross-sections of an atherosclerotic plaque stained by immunohistochemistry. (A) Vasculin and/or SSH6-β is visualized in red (black arrows). (B,E) Combined immunohistochemical staining for vasculin and/or SSH6-β (red) and anti–smooth muscle actin demonstrating SMC (blue). Pronounced vasculin/SSH6-β signals are observed in neointimal SMC (black arrow), whereas vasculin/SSH6-β signals are minimal in medial SMCs (arrow with *). (C,F) Combined immunohistochemical staining of vasculin and/or SSH6-β with anti-CD68 (blue) demonstrates the presence of SSH6 gene products in monocytes/macrophages, as indicated by a black arrow. (D) Combined immunohistochemical staining of vasculin and/or SSH6-β with anti-CD31 (blue) shows vasculin/SSH6-β in endothelial cells. The black arrow indicates an endothelial cell expressing vasculin/SSH6-β. (D-F) Insets represent overview pictures with regions in D-F outlined by a ⋄. L indicates the lumen. Bars in panels A-C and the insets in D-F represent 100 μm; bars in D-F represent 10 μm.
Immunohistochemical analysis of vasculin and/or SSH6-β in a wide range of human tissues, including ovary, testis, colon, salivary gland, and small intestine, demonstrated the presence of (one or both of) these proteins in other types of smooth muscle and endothelial cells, whereas a positive signal was also obtained in secretory cells (for example, mucus-producing cells and goblet cells; data not shown).
Discussion
In a previous study,15 a first inventory was made of genes differentially expressed in whole-mount human plaques with a thrombus versus stable atherosclerotic lesions. One of the identified cDNAs, coding for part of a previously unidentified ORF, was chosen for further investigations because of its pronounced upregulation in plaques with a thrombus versus stable plaques, as validated by macroarray and reverse transcriptase–PCR analysis on individual samples. The current study concentrates on this novel RNA/protein, which was entitled vasculin, as an acronym for vascular wall linked protein.
Multiple tissue Northern blot analysis, in combination with genomic analysis, revealed that vasculin differs from other splice variants of the SSH6 gene due to alternative splicing of exon 3, which is demonstrated to be expressed exclusively in the vascular wall. This is not surprising since expression of alternatively spliced mRNA variants in specific cells and tissues, or even at specific stages of development, are reported to contribute frequently to the functional diversity of human genes.24 Extensive bioinformatic analysis confirmed the existence of various splice variants of the SSH6 gene (Figure 6). As mentioned before,15 vasculin is homologous to clone AL161991,25 except for the unique 120-bp fragment corresponding to exon 3. Recent analyses of 2 other cDNA clones that are also homologous to vasculin (BC000267.1 and AK024807) revealed a putative ORF of 181 aa's, corresponding to aa294-aa473 of vasculin. In addition, a vasculin cDNA homologue originating from a testis cDNA library (clone AL13684425 ) has been identified to be nearly identical to vasculin, with the exception of a 21-bp insertion located at the connection between exon 3 and 4 of vasculin mRNA. Genomic analysis demonstrated that this insert may correspond to an additional exon of the SSH6 gene (exon 3A; Figure 6). Interestingly, Northern blot analysis performed in the current study did not reveal the presence of an exon 3 containing SSH6 mRNA variant in testis. Therefore, it is not unlikely that AL136844 originates from vascular SMCs, ubiquitously present in the strongly vascularized testicular tissue. Very recently, computational gene prediction analysis of genomic NCBI contig NT_006431 resulted in the model reference sequence XM_042059. The aa sequence of the resulting hypothetical protein is identical to the sequence of vasculin.
Schematic presentation of public domain mRNA transcripts homologous to vasculin mRNA (clone A6.3). (A) Schematic overview of the exons of the SSH6 gene (Table 2). (B) Exon use of known splice variants. ORFs corresponding to the hypothetical proteins represented in the public database are indicated in gray. mRNA and hypothetical protein sizes are indicated.
Schematic presentation of public domain mRNA transcripts homologous to vasculin mRNA (clone A6.3). (A) Schematic overview of the exons of the SSH6 gene (Table 2). (B) Exon use of known splice variants. ORFs corresponding to the hypothetical proteins represented in the public database are indicated in gray. mRNA and hypothetical protein sizes are indicated.
The presence of various SSH6 splice variants in the mRNA databases correlates to our Western blot data revealing various SSH6 variants at the protein level (Figure 4A), including vasculin (45 kDa) and SSH6-β (34 kDa). Strikingly, the size of SSH6-β exactly matches a translation product of 302 aa's using the internal methionine at position 172 of vasculin as an alternative start. However, further analysis is required to identify unambiguously the aa sequence of vasculin and SSH6-β and to clarify whether SSH6-β expression is regulated at the transcriptional, translational, or posttranslational level.
Western blot analysis validated the vascular wall–specific expression pattern for vasculin, as it is detected mainly in arteries, and demonstrated a ubiquitous expression profile for SSH6-β. Interestingly, analysis of arteries displaying various stages of atherosclerosis indicated an increase of the vasculin versus SSH6-β ratio throughout the various stages of atherogenesis (Figure 3D), suggesting a switch from SSH6-β into vasculin during the development of an atherosclerotic plaque. In this respect it should be noted that the amount of vasculin is negligible in veins, which are not susceptible for atherosclerosis. In contrast, Western blot analysis of surgically removed occluded venous grafts (n = 3, data not shown) indicated that vasculin is the predominant variant in venous bypass grafts, suggesting that a switch from the expression of SSH6-β into vasculin is concomitant with the development of an atherosclerotic plaque in the venous graft.
The SSH6-β→vasculin switch may be the result of an alternative exon use of the SSH6 gene, whereby vasculin is the major transcriptional variant in advanced atherosclerotic plaques and plaques with a thrombus. Interestingly, exon switching in the scavenger receptor CD36 gene is also suggested to be involved in the activation of SMCs during the formation of atherosclerotic lesions.26 Alternatively, SSH6-β may be considered as a cleavage product from vasculin. In this view, the SSH6-β→vasculin switch during atherogenesis may reflect a decreased processing of vasculin, resulting in a proatherogenic stimulus of vasculin or in the inhibition of an antiatherogenic effect of SSH6-β.
Apart from the presence of vasculin in plaques, vasculin is also observed in human plasma. The lack of a well-defined signal peptide, as predicted by computational analysis, complicates the query for the origin of plasma vasculin. One of the hypotheses is that circulating vasculin originates from vascular endothelial cells or vascular SMCs via transcytosis or as a consequence of cell damage, leading to the release of intracellular vasculin into the circulation. However, future quantitative analysis to evaluate vasculin plasma levels in patients and healthy individuals will shed more light on the (patho)physiologic importance of vasculin in blood and on the potential use of vasculin plasma levels (or the ratio between vasculin and SSH6-β levels) as a marker for the atherosclerotic load.
Despite these observations suggesting a significant role for vasculin in atherogenesis, the key question remains: What is the function of vasculin? At this moment, we cannot assign vasculin to any established gene or protein family. Therefore, we can only speculate about its function. Firstly, the ubiquitous expression profile of SSH6 splice variants hints at a function in the basal cell machinery. The presence of SSH6-related expressed-sequence tags in the 2-cellular stage of development, as demonstrated by electronic Northern analysis, supports this hypothesis. In this context, the vascular wall–specific, N-terminal segment of vasculin might have a regulatory function that is specific for the development of vascular SMCs. It may, for example, interact with other SMC-particular agents or make vascular SMCs susceptible for atherogenic stimuli. Second, bioinformatic analysis revealed the presence of SSH6-related genes (homology more than 85%) in the murine and rat genome, indicating that this protein (family) has vastly expanded in vertebrates. On the other hand, we were not able to identify SSH6 homologues in “model systems” like Caenorhabditis elegans and Saccharomyces cerevisiae, suggesting that SSH6 has no direct counterpart in lower life forms. A final clue comes from its cytoplasmic localization, as indicated by immunohistochemical staining and corresponding to our bioinformatic data, showing no obvious nuclear (transmembrane) signal sequence. These findings suggest that vasculin is not involved in intranuclear processes. Together, our data insinuate that the SSH6 gene is involved in an essential but unexplored pathway in cell biology of vertebrates in general and that vasculin might play an important role in vascular biology in particular.
Prepublished online as Blood First Edition Paper, July 3, 2003; DOI 10.1182/blood-2003-01-0306.
Supported by the Netherlands Organisation for Scientific Research (NWO, grant 902-26-223; K.B.J.M.C.) and by the Academic Hospital Maastricht (grant PF1197; A.P.J.J.B.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors want to thank Marjo Donners for performing 2-dimensional electrophoresis, Natasja Kisters for excellent technical assistance and Dr Heather Griffin (MRC Centre for Protein Engineering, Cambridge, United Kingdom) for the Griffin.1 library.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal