Atypical chronic myeloid leukemia (aCML) is a chronic myeloproliferative disorder with a clinical and hematologic picture similar to chronic myelocytic leukemia (CML) but lacking Philadelphia chromosome and BCR-ABL rearrangement. Cytogenetic studies have shown either a normal karyotype or numeric chromosomal changes.1 Recently the molecular cloning of t(5;10)(q33;q22) has been reported in 2 patients with aCML.2,3 This translocation creates a H4(D10S170)/platelet-derived growth factor receptor beta (PDGFβR) fusion transcript and suggests an association between deregulated tyrosine kinases and aCML. We report on a patient with an aCML and a t(5;10) who achieved a clinical and cytogenetic response after imatinib mesylate therapy.
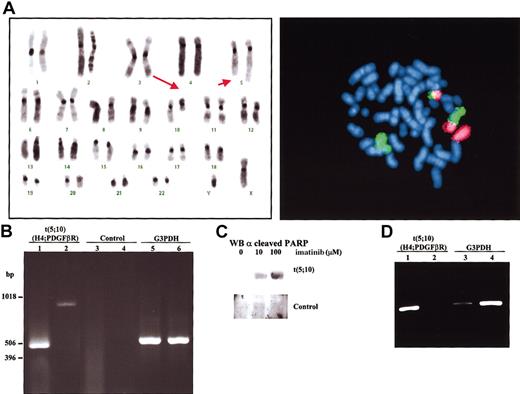
The patient, a 44-year-old man, presented with leukocytosis and splenomegaly. The white blood cell count was 158 × 109/L (3% myelocytes; 6% metamyelocytes; 4% bands; 68% neutrophils; 8% eosinophils; 10% lymphocytes; 1% monocytes), hemoglobin level was 91 g/L, and platelet count was 352 × 109/L. Analysis of peripheral blood smear revealed a remarkable dysplasia in myeloid cells. Cytogenetic analysis showed the following: 46,XY,t(5;10)(q33;q22)[24]/46,XY[1] after G-banding and fluorescence in situ hybridization (FISH) studies (Figure 1A). Both FISH and polymerase chain reaction (PCR) studies failed to demonstrate the presence of BCR-ABL fusion. Therefore, nested reverse transcriptase (RT)-PCR analysis using specific primers flanking the predicted breakpoints was performed. Using 2 different sets of primers the region implicated in the translocation was amplified (Figure 1B). These results demonstrated that t(5;10)(q33;q22) involved the genes H4 and PDGFβR. Sequencing of the amplified bands confirmed that there was a fusion H4-PDGFβR occurring at exactly the same breakpoint as found in the previous t(5;10) reports.2-4 Based on the presence of PDGFβR rearrangement, the patient began treatment with imatinib, at a daily dose of 400 mg. The therapy was well tolerated, without obvious side effects. Clinical and cytogenetic complete response to imatinib was achieved after 3 weeks of therapy. At 8 weeks after initiation of imatinib therapy, semiquantitative RT-PCR analysis showed a 99% reduction in H4/PDGFβR expression in peripheral blood compared with blood samples taken prior to treatment (Figure 1D). The patient remains in complete response after one year of therapy.
Identification of t(5;10) translocation with H4/PDGFβR fusion genes and induction of apoptosis by imatinib treatment. (A) Left panel: FISH using chromosome-specific painting probes to chromosome 5 (green) and chromosome 10 (red). Right panel: representative karyotype of a metaphase from bone marrow aspirate at diagnosis of disease. Arrows point to derivative chromosomes resulting from t(5;10)(q33;q22). (B) Amplification of the flanking region implicated in the translocation t(5;10) by nested RT-PCR with 2 sets of primers, set I (lanes 1,3) and set II (lanes 2,4). Total RNA from bone marrow collected from the t(5;10) patient (lanes 1-2) as well as from a Philadelphia-positive patient (lanes 3-4) was used as negative control. A450-bp region from the housekeeping gene G3PDH was also amplified from t(5;10) (lane 5) and control (lane 6) RNAs. (C) Bone marrow aspirate from patient and a control donor was cultured for 24 hours in the presence of the indicated amounts of imatinib. Cells were then lysed and submitted to Western blot analysis using anticleaved PARP (poly-adenosine diphosphate ribose polymerase) antibodies to determine the ratio of apoptosis. (D) Recession of H4/PDGFβR expression after therapeutic treatment with imatinib. Isolated RNAfrom peripheral blood before (lanes 1,3) and after (lanes 2,4) imatinib treatment was used in a nested RT-PCR analysis. The G3PDH gene (lanes 3-4) was used as an internal control to normalize the expression of H4/PDGFβR gene product (lanes 1-2). Intensity for each of the bands was quantified with a BioRad GelDoc2000 analyzer (Hercules, CA) and the ratio between them determined.
Identification of t(5;10) translocation with H4/PDGFβR fusion genes and induction of apoptosis by imatinib treatment. (A) Left panel: FISH using chromosome-specific painting probes to chromosome 5 (green) and chromosome 10 (red). Right panel: representative karyotype of a metaphase from bone marrow aspirate at diagnosis of disease. Arrows point to derivative chromosomes resulting from t(5;10)(q33;q22). (B) Amplification of the flanking region implicated in the translocation t(5;10) by nested RT-PCR with 2 sets of primers, set I (lanes 1,3) and set II (lanes 2,4). Total RNA from bone marrow collected from the t(5;10) patient (lanes 1-2) as well as from a Philadelphia-positive patient (lanes 3-4) was used as negative control. A450-bp region from the housekeeping gene G3PDH was also amplified from t(5;10) (lane 5) and control (lane 6) RNAs. (C) Bone marrow aspirate from patient and a control donor was cultured for 24 hours in the presence of the indicated amounts of imatinib. Cells were then lysed and submitted to Western blot analysis using anticleaved PARP (poly-adenosine diphosphate ribose polymerase) antibodies to determine the ratio of apoptosis. (D) Recession of H4/PDGFβR expression after therapeutic treatment with imatinib. Isolated RNAfrom peripheral blood before (lanes 1,3) and after (lanes 2,4) imatinib treatment was used in a nested RT-PCR analysis. The G3PDH gene (lanes 3-4) was used as an internal control to normalize the expression of H4/PDGFβR gene product (lanes 1-2). Intensity for each of the bands was quantified with a BioRad GelDoc2000 analyzer (Hercules, CA) and the ratio between them determined.
Imatinib mesylate has been shown to efficiently inhibit the activity of certain tyrosine kinases, including BCR-ABL, c-Kit, PDGFβR, PDGFαR, and ARG kinase.5,6 Thus to further investigate the role of chimeric protein H4/PDGFβR, imatinib mesylate was tested in bone marrow primary cultures. Interestingly, bone marrow primary cultures from the t(5;10) patient displayed marked apoptosis when treated with imatinib (Figure 1C).
Our results demonstrate the efficiency of imatinib in the treatment of patients displaying the translocation involving H4 and PDGFβR genes. Imatinib binds tightly into the adenosine triphosphate (ATP)-binding pocket within the tyrosine kinase domain of BCR-ABL,7 and we predict a similar mode of action with PDGFβR. Hence, the observed positive response strongly suggests that inhibition of PDGFβR activity may also be effective in other myeloproliferative diseases involving this tyrosine kinase receptor.
Partially supported by Grants of Spanish Fondo de Investigaciones Sanitarias (01/3153 and G03/136) and by the Council of Castilla-León, Spain (Sa 113/01).
We are thankful to Witte-Maria Weber and Novartis for providing imatinib (CGP 57148B) used for the in vitro culture studies.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal