Abstract
Antiphospholipid antibodies (aPLs) are associated with thrombosis and recurrent abortions during autoimmune pathologies, but they are also produced in healthy individuals and during infectious diseases. To analyze the possible links between physiologic and pathologic aPLs, it is of importance to characterize normal aPL production. We took advantage of the known tropism of Epstein-Barr virus (EBV) for B cells in general, and memory B cells in particular, during primary infectious mononucleosis (IMN) in 3 patients to get access to anticardiolipin (aCL)-producing B cells. Flow cytometry analysis of these cells showed that, depending on the patient, 10% to 60% of immunoglobulin M (IgM) aCL-producing B cells express the CD27 marker of memory B cells. Single cell sorting of aCL B cells, followed by single cell reverse transcription-polymerase chain reaction (RT-PCR) amplification of their immunoglobulin variable region genes, showed that some of these cells produce mutated forms of aCL antibodies, confirming their memory B-cell origin. Considering that, during primary IMN, EBV infects and expands already pre-existing memory B cells, we conclude that healthy individuals have a discrete pool of aCL memory cells able to produce mutated forms of antibodies. The implications of this new information are discussed in light of different hypotheses regarding the origin of aCL. (Blood. 2003;102:2459-2465)
Introduction
Antiphospholipid antibodies (aPLs) constitute a complex group of autoantibodies that bind to anionic phospholipids in the presence or absence of protein cofactors.1-5 Some of these antibodies can be detected under a nonpathogenic form in healthy individuals,6,7 or during infectious diseases,8-14 but pathogenic aPLs are strongly associated with thrombosis and recurrent abortions during autoimmune pathologies like the primary antiphospholipid syndrome (PAPS) and systemic lupus erythematosus (SLE).15-18 Despite intensive efforts trying to understand the aPL-thrombosis association, it is somewhat surprising that we remain so ignorant about the origin of these antibodies in healthy individuals and in autoimmune patients, as well as about the mechanisms of their pathogenicity in patients. Even though it is likely that most of the difficulties arise from the complex pattern of aPL reactivity,19 it is also possible that the lack of information on the precise molecular and cellular aspects of aPL-producing B cells in healthy individuals precludes any further insight into the origin of aPL.
We recently described the clonal analysis of anticardiolipin (aCL) antibodies in a patient with PAPS using a combination of PL-specific selection of single cells sorted by flow cytometry and cloning of immunoglobulin variable region genes by the single cell polymerase chain reaction (PCR) technique.19 We were surprised by the extreme molecular heterogeneity of the antibodies: indeed, not only the fine specificity of these antibodies was diverse but also their variable regions were either in a germ line configuration or mutated in a way that suggested an antigen-driven process. Considering that aCLs can be detected in healthy individuals and are, therefore, components of the so-called natural antibodies (Abs), it is of importance to know if the mutations of some aCL Abs represented recent events directly linked to the autoimmune pathology (APS) or constituted the molecular imprint of the patient's past immunologic history. In the last-mentioned scenario, the producing B cells should be memory B cells and would have just been reactivated by the autoimmune state, but not induced by it.
To gain new information on the molecular aspects and the cellular origin of aPL in healthy individuals, we analyzed the aPL-producing cells in 3 nonautoimmune patients suffering from acute Epstein-Barr virus (EBV)-induced infectious mononucleosis (IMN). This approach combines 2 major advantages: first, EBV-induced IMN is a known clinical situation in which nonpathogenic aPLs are produced,20 and second, it was shown that EBV directly infects already pre-existing memory B cells during the acute phase of IMN.21 By analyzing the aCL isotypes, the phenotype of aCL-producing B cells, the molecular structures of aCLs derived from the previously described aPL-specific cell sorting, and the reactivity of selected in vitro produced monoclonal aCLs, we conclude that healthy individuals have memory B cells producing mutated forms of aPL.
Patients, materials, and methods
Patients
Patient 1, a 20-year-old man, presented with fever and weary state for 5 days. Peripheral blood cell analysis revealed high lymphocytosis and the presence of numerous lymphocytes with large hyperbasophilic cytoplasm. The EBV serology showed positive for IgM antiviral capsid antigen (anti-VCA), but negative for IgG anti-Epstein-Barr nuclear antigen (EBNA), confirming the diagnosis of EBV-induced primary IMN. During this acute phase, the patient's activated cephalin time was slightly prolonged (ratio 1.3), and both IgM and IgG aCLs were detected (33 U IgG aPL [GPL] and 70 U IgM aPL [MPL] according to internal controls, enzyme-linked immunosorbent assay [ELISA] assay; Diagnostica Stago, Asnières, France). No thrombotic event was observed, and later controls, after the patient recovered from IMN, indicated negative tests for aCL.
Patient 2, an 18-year-old woman, presented with a very similar medical status with a 6-day history of EBV-induced primary IMN (IgM anti-VCA, but no IgG anti-EBNA). aCLs were also transiently detected in her serum (25 U GPL and 50 U MPL), without any thrombotic manifestation.
Patient 3, a 23-year-old man, also presented with an acute status related to an EBV-induced primary IMN (IgM anti-VCA, but no IgG anti-EBNA) with transient IgM aCL (25 U MPL) but no thrombotic event was observed.
Approval was obtained from the INSERM institutional review board for these studies. After informed consents were obtained from these patients, 20 mL peripheral blood was withdrawn during the acute phase of IMN for cell labeling and sorting.
Cell labeling and sorting
Sorting of aPL-producing B cells was performed by staining of Ficoll-Isopaque-purified peripheral blood mononuclear cells as previously described.19 Briefly, cells were stained with either R-phycoerythrin-conjugated F(ab′)2 antihuman IgG Fcγ or antihuman IgM Fcμ (Jackson Immunoresearch, West Grove, PA) and cardiolipin (CL) vesicles containing 29% CL, 58.3% phosphatidylcholine (PC), 11.6% cholesterol, and 0.9% labeled PC in the presence of 2% fetal calf serum (FCS).
Single doubled-stained cells were sorted into thin-wall polycarbonate 96-well plates (Costar, Corning, NY) using an Epics Altra flow cytometer (Beckman Coulter, Brea, CA) outfitted with an automatic cell deposit unit. Each well contained 6 μL water and 3 μL reverse transcriptase buffer, 5 × concentrated (Gibco, Life Technologies, Gaithersburg, MD). Plates were immediately frozen on dry ice and stored at -80°C for further DNA amplification.
The flow cytometric analysis of IgM+CL+CD27+ B cells was performed by 4-color fluorescence staining on the patient's peripheral blood mononuclear cells (PBMCs) according to standard protocols. The following reagents were used: CL vesicles as described earlier, antihuman CD27-phycoerythrin (PE; Immunotech, Luminy, France), biotinylated antihuman IgM (Rockland, Gilbertsville, PA) plus Streptavidin-Cy-5 (Jackson Immunoresearch). Only live cells were included in the analysis by excluding propidium iodide-stained cells. Samples were analyzed on a FACScalibur (Becton Dickinson, Mountain View, CA) flow cytometer using CellQuest software (Becton Dickinson).
Amplification of rearranged VH and VK chain gene segments
Preparation of complementary DNA and PCR amplification was performed according to the single cell reverse transcription (RT)-PCR technique described elsewhere.19 Briefly, the RT reaction was performed by adding to each well 6 μL RT reaction mix (1 mM dithiothreitol; 0.1 mM each deoxyadenosine triphosphate [dATP], deoxythymidine triphosphate [dTTP], deoxycytidine triphosphate [dCTP], and deoxyguanosine triphosphate [dGTP]; 40 U RNase inhibitor, pd(N)6; and 25 U Superscript RTase) followed by an incubation of 1.5 hours at 37°C.
VH and VK gene rearrangements were amplified by a seminested PCR approach using the High Fidelity Taq DNA polymerase (Roche Molecular Biochemicals, Mannheim, Germany) and family-specific V gene primers together with 2 sets of the respective J gene primers. The sequences of the sets of oligonucleotides used as primers were published previously.19
To detect EBV-infected cells, transcribed fragments of EBV-encoded small nuclear RNAs (EBER) were amplified in parallel. Therefore, 2 EBER-specific primers (EBER F1, 5′-CTT TAA AAC TCT AAA AAT CAA AAC TTT AGA-3′, and EBER R1, 5′-ACC AGA AAT AGC TGC AGG ACC ACT TTA TAC-3′) were added to the primer mix of the first round of PCR. The second round was carried out in a separate reaction tube using primer EBER F2, 5′-AAT GGG CGC CAT TTT G-3′, and EBER R2, 5′-TCC CTA GAA CTG ACA ATT-3′. The PCR program was the same as the second round of the VH PCR with the following modifications: the annealing temperature was 50°C and the number of cycles was increased to 35. The analysis of the expression of the EBV-encoded EBNA2 gene was also performed by PCR, using for the first amplification the primers EBNA 2 F1, 5′-CAT AGA AGA AGA AGA GGA TGA AGA-3′, and EBNA 2 R1, 5′-GTA GGG ATT CGA GGG AAT TAC TGA-3′, which were added to the primer mix. The second round of amplification was carried out in separate reaction with the primer EBNA 2 F2, 5′-ATG CCT ACA TAC TAT CTT GCG TTA CA -3′, and EBNA 2 R1. The PCR program differed from the second round of the VH PCR by the annealing temperature (65°C) and the number of cycles (40 cycles).
Sequence analysis
The PCR products were gel purified and sequenced with the primers used in the second round of PCR amplification and the Big Dye Terminator cycle DNA sequencing kit (Applied Biosystems, Foster City, CA), on an automated ABI 310 sequencer (Applied Biosystems).
The V region genes of immunoglobulin were analyzed using the international ImmunoGeneTics (IMGT) database (http://imgt.cines.fr).22
Cloning of PCR products and antibody expression
Cloning strategy was detailed in Lieby et al.19 VH and VK gene segments originating from a single cell were cloned in a forced orientation in the transfer vectors p119Cγ1 (containing the γ1 exons) and pBHuCK, respectively, for in vitro antibody production in a baculovirus expression system. Cloning of VH and VK fragments needed extension amplifications using overlapping oligonucleotides (primer sequences are accessible online at http://aloes.u-strasbg.fr/Publications/Sequences_PRIMERS_RALLONGEMENTS.htm). Integrity of the sequences of the cloned fragments was verified by sequencing the plasmids.
Double recombinant viruses were obtained by cotransfection of the plasmidic DNA containing the VH and VK gene segments with the baculovirus DNA. Human monoclonal antibodies were obtained using plaque assays and virus propagation according to published methods.23 Secreted Abs produced by Sf9 insect cells in culture medium without FCS were purified with a protein A column (Bio-Rad Laboratories, Hercules, CA) according to the manufacturer's instructions. Purified IgG was dialyzed against phosphate-buffered saline (PBS) 1 × and quantified by a direct ELISA.
Antiphospholipid activity assay
Polystyrene microtiter plates (Polysorp; Nunc, Roskile, Denmark) were coated with 50 μL/well CL, PS, or PC 50 μg/mL (Sigma Chemical, St Louis, MO) in absolute alcohol and evaporated at room temperature overnight. Plates were saturated with PBS 10% FCS for 2 hours at 4°C. After washing, purified mAbs were incubated for 3 hours at room temperature. After 3 washing steps with PBS 0.01% Tween 20, 50 μL/well peroxidase-conjugated goat antihuman IgG (Jackson Immunoresearch) was added and revealed with O-phenylenediamine dihydrochloride peroxidase substrate (Sigma). After 30 minutes of incubation, plates were read at 492 nm using a Titertek Multiskan (Labsystem, Helsinki, Finland).
Purified mAbs were tested for their cofactor dependency. The reactivity of each mAb with CL was tested in the presence of purified human cofactors β2GP1, prothrombin (PT), protein S (prot S), protein C (prot C), and annexin V (An V). Plates were coated with CL and blocked with PBS containing 0.25% gelatin. Samples were diluted in PBS 0.25% gelatin, 2 mM CaCl2 containing either 10 μg/mL PT, 5 μg/mL prot S, 5 μg/mL prot C, 5 μg/mL An V, or with 10 μg/mL β2GP1 (in PBS 0.25% gelatin). Samples were added in duplicate and incubated 3 hours at room temperature.
Human β2GP1 and prot C are from Kordia (Leiden, the Netherlands). Human PT and prot S were purified from human plasma by chromatography, and human An V was purified from human placenta.24
Results
The 3 patients, without any previous history of autoimmune disease or thrombotic manifestation, presented with typical symptomatic acute EBV-induced IMN. They had detectable and transient serum aCL during the acute phase of the infectious disease and no thrombotic complication.
Presence of newly infected aCL-producing B cells in peripheral blood
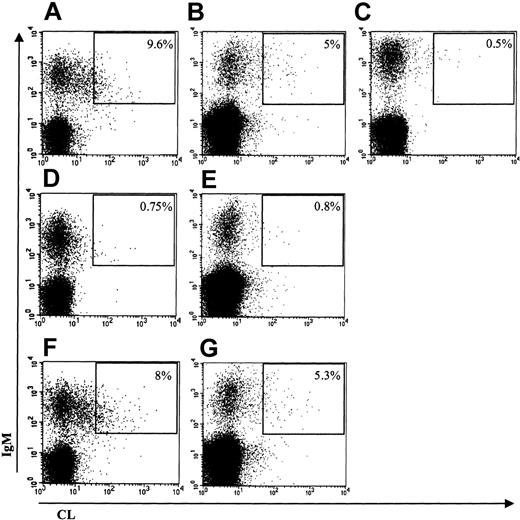
To understand the origin of aCL in nonautoimmune conditions, we first looked for the presence of APL-producing B cells in the patient's peripheral blood. In these 3 patients, double staining with anti-IgM and CL-coated labeled vesicles showed the presence of circulating IgM aCL-producing B cells: 9.6% of IgM+ B cells in the first patient, 5% in the second, and 4% in the third (Figure 1 and data not shown). This labeling analysis was previously extensively validated in patients with PAPS by showing that labeled cells were indeed producing aCL antibodies.19
CL binding of IgM-expressing B cells. The percentage of double-positive cells (upper right gate) is expressed as the percentage of IgM-positive B cells. Binding of labeled CL vesicles and anti-IgM: (A) patient 1; (B) patient 3; and (C) healthy subject. Inhibition of labeled CL vesicles binding with nonlabeled CL vesicles: (D) patient 1 and (E) patient 3. Inhibition of labeled CL vesicles binding with nonlabeled PC vesicles: (F) patient 1 and (G) patient 3.
CL binding of IgM-expressing B cells. The percentage of double-positive cells (upper right gate) is expressed as the percentage of IgM-positive B cells. Binding of labeled CL vesicles and anti-IgM: (A) patient 1; (B) patient 3; and (C) healthy subject. Inhibition of labeled CL vesicles binding with nonlabeled CL vesicles: (D) patient 1 and (E) patient 3. Inhibition of labeled CL vesicles binding with nonlabeled PC vesicles: (F) patient 1 and (G) patient 3.
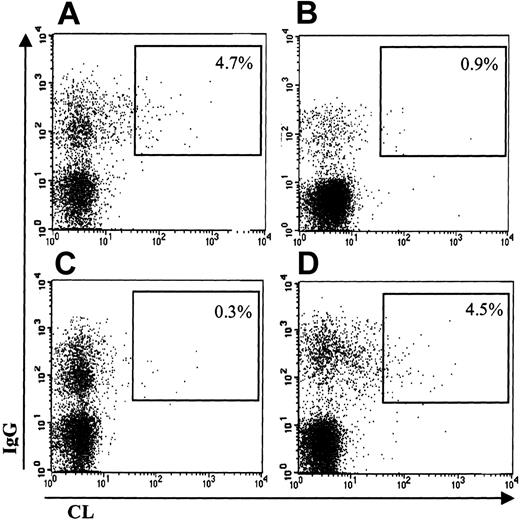
As an example, Figure 1 shows the results obtained in the first and second patients: IgM-positive B cells bind CL-coated vesicles (Figure 1A-B), this binding being specific for CL because (1) an excess of unlabeled CL-coated vesicles clearly inhibits the binding of labeled vesicles (Figure 1D-E), and (2) an excess of unlabeled neutral PL vesicles (PC only) does not inhibit the binding of labeled CL vesicles (Figure 1F-G). For comparison, it is also shown that such aCL-producing B cells are hardly detectable in a healthy control subject (Figure 1C). Double staining with anti-IgG revealed the presence of approximately 4% IgG aCL-producing B cells in the peripheral blood of patients 1 and 2. As shown in Figure 2, the patient 1 expresses IgG aCL B cells (Figure 2A), the binding of these cells to CL-labeled vesicles being also inhibitable by an excess of unlabeled CL-coated vesicles (Figure 2C). Again, these cells are not present in a healthy control subject (Figure 2B).
CL binding of IgG-expressing B cells. The percentage of double-positive cells (upper right gate) is expressed as the percentage of IgG-positive B cells. Binding of labeled CL vesicles and anti-IgG: (A) patient 1 and (B) healthy subject. Inhibition of labeled CL vesicles binding with nonlabeled CL vesicles (C) or with nonlabeled PC vesicles (D).
CL binding of IgG-expressing B cells. The percentage of double-positive cells (upper right gate) is expressed as the percentage of IgG-positive B cells. Binding of labeled CL vesicles and anti-IgG: (A) patient 1 and (B) healthy subject. Inhibition of labeled CL vesicles binding with nonlabeled CL vesicles (C) or with nonlabeled PC vesicles (D).
To make sure that the B-cell receptors were indeed engaged in the specific binding of CL-coated labeled vesicles, we tested the ability of anti-F(ab′)2 antibodies to inhibit this binding, as previously described.19 The results of such an inhibition experiment confirmed that the B-cell antigen receptors (BCRs) are responsible for the binding to CL-coated vesicles (data not shown).
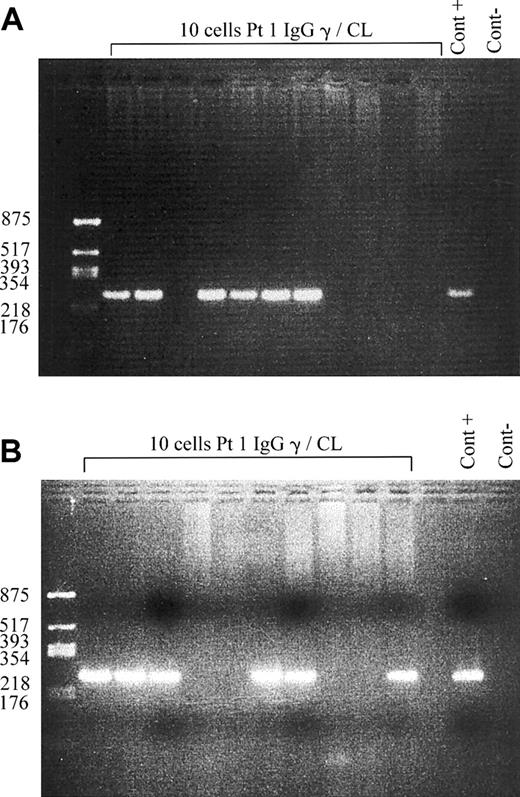
Despite the known tropism of EBV for CD21+ B cells, the presence of aCL-producing cells in the peripheral blood in these 3 patients could be related to either polyclonal activation of uninfected cells or to EBV per se-induced B-cell expansion and activation. To clarify this question, we sorted individual aCL B cells and looked by single cell PCR for the presence of EBER transcripts in both IgM and IgG aCL-producing B cells. Among these 2 cell types, approximately 60% were EBER positive (Figure 3) in patient 1, indicating that, at least, this proportion of aCL B cells corresponded to recently EBV-infected cells. We confirmed these results by amplifying EBNA2 transcripts by single cell RT-PCR in approximately 60% of the aCL-sorted B cells (not shown). Thus, during EBV-induced IMN, healthy individuals have circulating EBV-infected IgM and IgG aCL-producing B cells.
PCR amplification of EBER transcripts. EBV-specific transcripts are amplified by single cell PCR of sorted IgG anti-CL B cells (A) and IgM anti-CL B cells (B) and analyzed on ethidium bromide-stained agarose gels. Products are visible as bands of 253 bp. Negative control of PCR (Cont -) and positive control (Cont +) are shown: cells infected with the B95-8 strain of EBV are used for the positive control.
PCR amplification of EBER transcripts. EBV-specific transcripts are amplified by single cell PCR of sorted IgG anti-CL B cells (A) and IgM anti-CL B cells (B) and analyzed on ethidium bromide-stained agarose gels. Products are visible as bands of 253 bp. Negative control of PCR (Cont -) and positive control (Cont +) are shown: cells infected with the B95-8 strain of EBV are used for the positive control.
Some of the aCL-producing B cells are memory B cells
The presence of aCL with the IgG isotype in serum in patients 1 and 2, as well as the detection of IgG aCL B cells in their peripheral blood, already suggest that aCL memory B cells were activated during EBV infection. To test this hypothesis, we analyzed both the phenotype of aCL B cells and the molecular structure of the aCL variable regions.
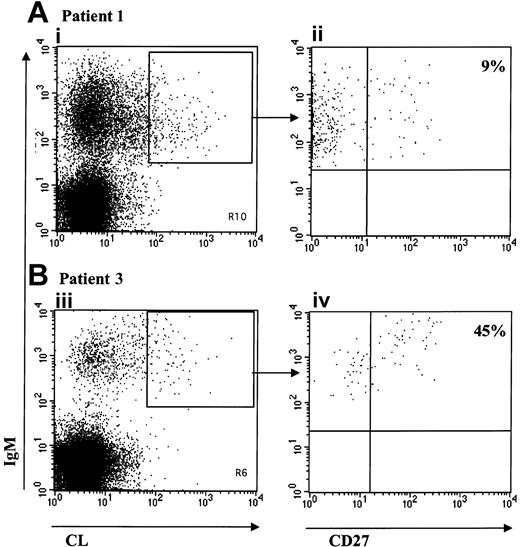
The CD27 molecule, which belongs to the tumor necrosis factor receptor family, was shown to be a reliable phenotypic marker of memory B cells.25,26 The peripheral blood (PB) CD27+ B-cell pool in humans represents about 40% of B cells and can be subdivided on the basis of the expression of the membrane immunoglobulin heavy chain isotype.25 Most of these CD27+ cells are not classical IgG or IgA class-switched memory B cells but are IgM only or IgM- and IgD-expressing cells. Thus, we used the CD27 marker to look for the presence of memory cells among the aCL-producing B cells by 3 color cytofluorometry. The results, presented in Figure 4 for patients 1 and 2, show, respectively, that about 10% (Figure 4Aii) and 45% (Figure 4Bii) of IgM aCL-gated B cells are CD27+. This proportion was higher in the third patient, reaching 60% (not shown).
Phenotype of aCL IgM-bearing B cells. Representation of the 3 color FACS analysis on viable (PI-) lymphocytes after staining with labeled CL vesicles, anti-IgM and anti-CD27 of PBMCs from patient 1 (A) and patient 3 (B). IgM+/CL+ double-stained population is shown for patient 1 (Ai) and patient 3 (Biii). Panels Aii and Biv are gated on the double-stained population IgM+/CL+. The percentage of CD27+ cells is given.
Phenotype of aCL IgM-bearing B cells. Representation of the 3 color FACS analysis on viable (PI-) lymphocytes after staining with labeled CL vesicles, anti-IgM and anti-CD27 of PBMCs from patient 1 (A) and patient 3 (B). IgM+/CL+ double-stained population is shown for patient 1 (Ai) and patient 3 (Biii). Panels Aii and Biv are gated on the double-stained population IgM+/CL+. The percentage of CD27+ cells is given.
The other important marker of memory B cells is the presence of somatically mutated antigen receptors. Indeed, on antigen activation, naive B cells undergo proliferation, isotype switching, and somatic mutations of rearranged immunoglobulin V region genes within the germinal centers and eventually become memory B cells if they survive or do not differentiate into plasmocytes. Thus, we analyzed the heavy chain variable region sequences of aCL-bearing B cells in the first patient. These cells were sorted on the basis of their IgG or IgM expression as well as on their ability to bind CL-coated labeled vesicles. The heavy chain variable regions of IgM and IgG aCL, and the light chain variable regions of IgM aCL, were sequenced after single cell sorting and amplification as described.19 The results are presented in Tables 1 (IgM aCL-sorted B cells) and 2 (IgG aCL-sorted B cells).
VH and VL sequence analysis of IgM aCL (MVH and MVL) originating from single cell sorting (patient 1)
. | Vhfamily . | Vkfamily . | Germline gene . | Homology (%)* . | R/S CDR . | R/S FR . | D gene . | Jhgene . | Jkgene . |
|---|---|---|---|---|---|---|---|---|---|
| MVH1 | 1 | — | V1-2 | 100 | — | — | D2-2 | 4 | — |
| D3-3 | |||||||||
| MVH2 | 3 | — | V3-48 | 96.3 | 1/1 | 6/1 | D3-16 | 4 | — |
| MVH3 | 1 | — | V1-2 | 99.7 | — | 0/1 | D3-22/in† | 4 | — |
| MVH4 | 4 | — | V4-b | 100 | — | — | D5-5 | 4 | — |
| MVH5 | 3 | — | V3-13 | 98 | 1/0 | 2/0 | D3-10 | 6 | — |
| P = .329‡ | |||||||||
| MVH6 | 3 | — | V3-21 | 98.7 | 1/0 | 1/1 | D5-5 | 4 | — |
| P = .335 | |||||||||
| MVH7 | 2 | — | V2-5 | 100 | — | — | D3-3 | 5 | — |
| MVH8 | 1 | — | V1-3 | 90.7 | 7/1 | 7/8 | D3-10 | 4 | — |
| P = .0375 | |||||||||
| MVH9 | 3 | — | V3-48 | 96.8 | 4/1 | 0/2 | D1-26 | 4 | — |
| P = .0116 | |||||||||
| MVL1 | — | 2 | V2-28 | 100 | — | — | — | — | 1 |
| MVL2 | — | 1 | V1-39 | 99.7 | — | 1/0 | — | — | 3 |
| MVL3 | — | 4 | V4-1 | 100 | — | — | — | — | 2 |
| MVL4 | — | 1 | V1-13 | 100 | — | — | — | — | 5 |
| MVL5 | — | 1 | V1-39 | 100 | — | — | — | — | 3 |
| MVL6 | — | 4 | V4-1 | 99.3 | — | 1/0 | — | — | 1 |
| MVL7 | — | 3 | V3-20 | 99.6 | — | 0/1 | — | — | 1 |
| MVL8 | — | 1 | V1-5 | 97 | 3/1 | 2/2 | — | — | 2 |
| P = .0195 | |||||||||
| MVL9 | — | 1 | V1-8 | 100 | — | — | — | — | 4 |
. | Vhfamily . | Vkfamily . | Germline gene . | Homology (%)* . | R/S CDR . | R/S FR . | D gene . | Jhgene . | Jkgene . |
|---|---|---|---|---|---|---|---|---|---|
| MVH1 | 1 | — | V1-2 | 100 | — | — | D2-2 | 4 | — |
| D3-3 | |||||||||
| MVH2 | 3 | — | V3-48 | 96.3 | 1/1 | 6/1 | D3-16 | 4 | — |
| MVH3 | 1 | — | V1-2 | 99.7 | — | 0/1 | D3-22/in† | 4 | — |
| MVH4 | 4 | — | V4-b | 100 | — | — | D5-5 | 4 | — |
| MVH5 | 3 | — | V3-13 | 98 | 1/0 | 2/0 | D3-10 | 6 | — |
| P = .329‡ | |||||||||
| MVH6 | 3 | — | V3-21 | 98.7 | 1/0 | 1/1 | D5-5 | 4 | — |
| P = .335 | |||||||||
| MVH7 | 2 | — | V2-5 | 100 | — | — | D3-3 | 5 | — |
| MVH8 | 1 | — | V1-3 | 90.7 | 7/1 | 7/8 | D3-10 | 4 | — |
| P = .0375 | |||||||||
| MVH9 | 3 | — | V3-48 | 96.8 | 4/1 | 0/2 | D1-26 | 4 | — |
| P = .0116 | |||||||||
| MVL1 | — | 2 | V2-28 | 100 | — | — | — | — | 1 |
| MVL2 | — | 1 | V1-39 | 99.7 | — | 1/0 | — | — | 3 |
| MVL3 | — | 4 | V4-1 | 100 | — | — | — | — | 2 |
| MVL4 | — | 1 | V1-13 | 100 | — | — | — | — | 5 |
| MVL5 | — | 1 | V1-39 | 100 | — | — | — | — | 3 |
| MVL6 | — | 4 | V4-1 | 99.3 | — | 1/0 | — | — | 1 |
| MVL7 | — | 3 | V3-20 | 99.6 | — | 0/1 | — | — | 1 |
| MVL8 | — | 1 | V1-5 | 97 | 3/1 | 2/2 | — | — | 2 |
| P = .0195 | |||||||||
| MVL9 | — | 1 | V1-8 | 100 | — | — | — | — | 4 |
R/S indicates the ratio of replacement mutations (R) and silent mutations (S) in the complementary-determining regions (CDR1 and CDR2) and framework (FR1, FR2, and FR3).—indicates not applicable.
Homology % provides the percentage of nucleotide sequence homology between the Ig V gene and its putative germ line.
In indicates sequence inserted in inverted orientation.
P is the probability that an excess of R mutations in the CDRs resulted by chance.
VH and sequence analysis of IgG aCL (GVH and GVL) originating from single cell sorting (patient 1)
. | Vhfamily . | Vkfamily . | Germline gene . | Homology (%)* . | R/S CDR . | R/S FR . | D gene . | Jhgene . | Jkgene . |
|---|---|---|---|---|---|---|---|---|---|
| GVH1 | 1 | — | V1-69 | 95.6 | 3/0 | 3/5 | D1-20/in† | 4 | — |
| P = .09‡ | |||||||||
| GVH2 | 1 | — | V1-69 | 99.2 | — | 0/2 | D3-16 | 3 | — |
| GVH3 | 2 | — | V2-5 | 100 | — | — | D3-3 | 5 | — |
| GVH4 | 3 | — | V3-21 | 97.6 | 1/0 | 4/1 | D5-5 | 4 | — |
| P = .339 | |||||||||
| GVH5 | 3 | — | V3-23 | 93.9 | 7/5 | ½ | D1-14 | 3 | — |
| P = .0027 | |||||||||
| GVH6 | 3 | — | V3-30 | 100 | — | ND | 3 | — | |
| GVH7 | 4 | — | V4-4 | 92.2 | 5/3 | 6/5 | D4-41 | 4 | — |
| GVH8 | 3 | — | V3-7 | 95.5 | 5/1 | 4/1 | D6-13 | 4 | — |
| P = .015 | |||||||||
| GVH9 | 3 | — | V3-73 | 92.5 | 5/3 | 6/5 | D3-22 | 6 | — |
| GVL5 | 1 | V1-9 | 98.8 | 2/0 | 1/0 | — | 3 | ||
| GVL6 | — | 3 | V3-11 | 96.7 | 4/0 | 2/1 | — | 2 | |
| P = .0025 | |||||||||
| GVL7 | — | 1 | V1-9 | 99.7 | — | 1/0 | — | — | 3 |
| GVL8 | — | 1 | V1-33 | 99.6 | — | 1/0 | — | — | 1 |
| GVL9 | — | 1 | V1-39 | 97.2 | 3/1 | 2/1 | — | — | 2 |
| P = .013 |
. | Vhfamily . | Vkfamily . | Germline gene . | Homology (%)* . | R/S CDR . | R/S FR . | D gene . | Jhgene . | Jkgene . |
|---|---|---|---|---|---|---|---|---|---|
| GVH1 | 1 | — | V1-69 | 95.6 | 3/0 | 3/5 | D1-20/in† | 4 | — |
| P = .09‡ | |||||||||
| GVH2 | 1 | — | V1-69 | 99.2 | — | 0/2 | D3-16 | 3 | — |
| GVH3 | 2 | — | V2-5 | 100 | — | — | D3-3 | 5 | — |
| GVH4 | 3 | — | V3-21 | 97.6 | 1/0 | 4/1 | D5-5 | 4 | — |
| P = .339 | |||||||||
| GVH5 | 3 | — | V3-23 | 93.9 | 7/5 | ½ | D1-14 | 3 | — |
| P = .0027 | |||||||||
| GVH6 | 3 | — | V3-30 | 100 | — | ND | 3 | — | |
| GVH7 | 4 | — | V4-4 | 92.2 | 5/3 | 6/5 | D4-41 | 4 | — |
| GVH8 | 3 | — | V3-7 | 95.5 | 5/1 | 4/1 | D6-13 | 4 | — |
| P = .015 | |||||||||
| GVH9 | 3 | — | V3-73 | 92.5 | 5/3 | 6/5 | D3-22 | 6 | — |
| GVL5 | 1 | V1-9 | 98.8 | 2/0 | 1/0 | — | 3 | ||
| GVL6 | — | 3 | V3-11 | 96.7 | 4/0 | 2/1 | — | 2 | |
| P = .0025 | |||||||||
| GVL7 | — | 1 | V1-9 | 99.7 | — | 1/0 | — | — | 3 |
| GVL8 | — | 1 | V1-33 | 99.6 | — | 1/0 | — | — | 1 |
| GVL9 | — | 1 | V1-39 | 97.2 | 3/1 | 2/1 | — | — | 2 |
| P = .013 |
R/S indicates the ratio of replacement mutations (R) and silent mutations (S) in the complementary-determining regions (CDR1 and CDR2) and framework (FR1, FR2, and FR3); and—, not applicable.
Homology % provides the percentage of nucleotide sequence homology between the Ig V gene and its putative germ line.
In indicates sequence inserted in inverted orientation.
P is the probability that an excess of R mutations in the CDRs resulted by chance.
The analysis of these sequences should take into account 2 phenomena that occur during the germinal center B-cell maturation:27 (1) replacement mutations are usually counterselected within the framework (FR) regions of the V genes to preserve the overall structure of the binding domains, resulting in low values of replacement-to-silent (R/S) ratios in the FRs; and (2) high values of R/S ratios within the CDRs are usually indicative of an antigen-driven selection.
First of all, such an analysis of the sequences of aCL VH and VK regions indicates that these antibodies are heterogeneous, using different VH and VK families, and different V gene segments without any apparent bias. Second, although most of the IgM aCL V regions are poorly mutated or unmutated, 2 of 9 sequenced antibodies (MVH8 and MVH9) are mutated with a pattern suggesting an antigen-driven process: M8 contains mutations on both the heavy (MVH8) and the light (MVL8) chain V regions, and M9 is mutated on the VH region only (MVH9). Third, the same analysis of 9 VH regions and 5 VL regions originating from IgG aCL B cells shows 10 mutated V regions. The pattern of the mutations affecting GVH5, GVH8, GVL6, and GVL9 suggests that, at least, these 4 antibodies have been selected by an antigen. Taken together, this information confirms that some of IgM and IgG aCLs originate from memory B cells.
Reactivities of 3 IgM aCL mAbs originating from single B cells
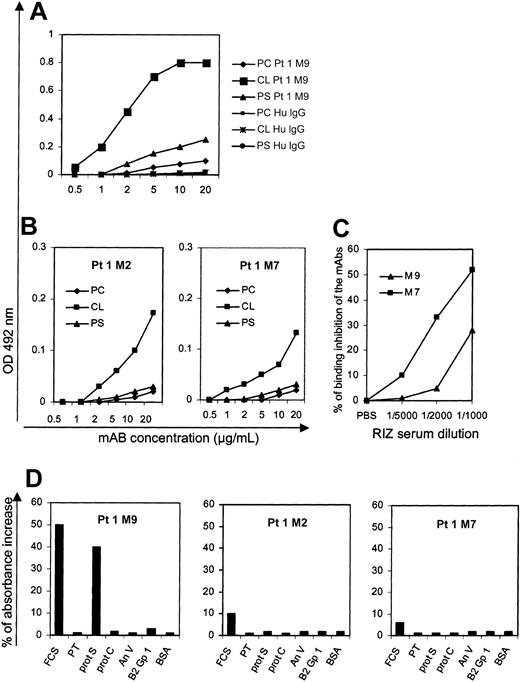
We have previously shown that the sorting procedure allows us to obtain authentic antigen-specific B cells.19 In order both to confirm this point and to precisely determine some characteristics of “normal” anti-CL antibodies, we cloned the heavy and light chain rearranged V region sequences originating from 3 different aCL B cells (patient 1) into recombinant baculovirus to produce the corresponding antibodies in vitro, as previously described.19 We have deliberately chosen informative pairs of V regions to produce these mAbs: one pair probably comes from a single IgM memory B cell making an antibody whose VH region is mutated in a way suggesting an antigen-driven process (the mutated MVH9 and the germ line MVL9), one pair of germ line V regions originating from a naive B cell (MVH7 and MVL7), and one pair of V regions whose mutations are not indicative of an antigen-driven affinity maturation (MVH2 and MVL2). The anti-CL reactivity of these mAbs (respectively, M9, M7, and M2), as well as their cofactor dependency, are shown in Figure 5. The 3 mAbs do react with CL, albeit with different affinities: the mutated M9 (probably selected by an antigen) being more efficient than M7 and M2. To be more precise, M9 and M7 were biotin labeled, and their respective binding to cardiolipin was tested in the presence of varying dilutions of the patient 1 serum containing aPL antibodies. As shown in Figure 5C, the ability of the patient's serum to inhibit the binding of M7 was higher than it was for M9. The CL reactivity of the mAbs was dependent on a serum cofactor only for M9, but not for M7 and M2; as shown in Figure 5D, it appears that M9 is precisely dependent on Protein S.
PL-binding and cofactor dependency of 3 selected monoclonal antibodies originated from patient 1. (A-B) The mAbs are tested with 2 anionic PLs (CL and PS) and with a neutral PL (PC) in the presence of FCS. (A) Results with the somatically mutated M9; (B) results with the germline M7 and the mutated (but apparently not antigen driven) M2. Protein A-purified human IgG (PC, CL, or PS Hu IgG) are used as negative controls. The results are expressed as OD at 492 nm minus background (mean ± SE of 2 experiments). (C) Competition experiments using biotin-labeled M9 and M7 mAbs and varying dilutions of the patient 1 serum to test the ability of the serum to inhibit the binding of the mAbs on solid phase cardiolipin. (D) The mAbs are tested for their reactivity with CL in the absence or the presence of FCS or purified human cofactors (β2GP1, PT, prot S, prot S, and AnV). The results are expressed as the percentage of OD increase in the presence of FCS or purified cofactors.
PL-binding and cofactor dependency of 3 selected monoclonal antibodies originated from patient 1. (A-B) The mAbs are tested with 2 anionic PLs (CL and PS) and with a neutral PL (PC) in the presence of FCS. (A) Results with the somatically mutated M9; (B) results with the germline M7 and the mutated (but apparently not antigen driven) M2. Protein A-purified human IgG (PC, CL, or PS Hu IgG) are used as negative controls. The results are expressed as OD at 492 nm minus background (mean ± SE of 2 experiments). (C) Competition experiments using biotin-labeled M9 and M7 mAbs and varying dilutions of the patient 1 serum to test the ability of the serum to inhibit the binding of the mAbs on solid phase cardiolipin. (D) The mAbs are tested for their reactivity with CL in the absence or the presence of FCS or purified human cofactors (β2GP1, PT, prot S, prot S, and AnV). The results are expressed as the percentage of OD increase in the presence of FCS or purified cofactors.
Discussion
In this paper, we show that, during acute EBV-induced IMN, healthy individuals have circulating anti-CL-producing B cells with the phenotypic and molecular characteristics of memory B cells. Depending on both the marker that was used and on the patients, 10% to 60% of IgM aCL cells were memory B cells. We believe that these B cells existed before the acute primary EBV infection in these subjects for the following reasons: (1) in each case, the sampling of the patient's cells was performed during the symptomatic phase of the infectious disease, which probably did not leave sufficient time to allow de novo production of aCL memory B cells from the naive B-cell pool; (2) in these patients, we observed variable amounts of both serum IgM and IgG aCLs at a period of time when the specific B-cell response against EBV-encoded proteins did not yet switch the heavy chain from IgM to IgG (no IgG anti-VCA and no IgG anti-EBNA); and, finally, (3) during IMN, it is known that EBV is able to infect all the B cells bearing CD21, induces them to proliferate, but does not drive naive B cells to undergo somatic hypermutations in the germinal centers.21 We also looked for the presence of aCL-expressing B cells in uninfected healthy individuals, but we were unable to sort a reasonable number of cells and to amplify both Ig heavy and light chain V regions from single cells (data not shown). The reasons can be multiple: the frequency of aPL B cells is very low in the healthy situation, not far from the background of the assay (the percentage of positive cells after inhibition of binding with unlabeled CL vesicles in Figure 1D-E), and maybe more importantly, the total amounts of immunoglobulin mRNAs of quiescent B cells are certainly less important than in activated EBV-infected B cells.
During the autoimmune PAPS, we showed that the aCL antibodies were extremely diverse both in terms of PL and cofactor specificity, and in terms of the primary sequences of their variable regions. Although we have not yet produced a collection of monoclonal aCLs originating from the IMN patient, their molecular structures also appear extremely diverse in this situation. We were surprised by the frequencies (4%-9%) of peripheral B cells reactive with CL-coated vesicles in these patients. These high frequencies could be related mainly to the proliferative capacity of EBV which made it possible to have experimentally access to these cells (60% of CL B cells were EBV infected) and also partly to the polyclonal activation of uninfected cells. To what extent some antigens exposed at the membrane of activated lymphocytes also may stimulate aCL B-cell expansion remains to be determined.28 In any case, during IMN, naive and germinal center-infected B cells rapidly become the target of EBV-specific cytotoxic T cells which will eliminate most of them. But this is not the case for memory B cells which were shown to be the specific cellular site of EBV latency, enabling these cells to escape the immune surveillance.21,29-31 Thus, it is highly likely that at least some EBV-infected aCL-producing memory B cells will survive the massive T-cell-induced B-cell destruction after IMN recovery.
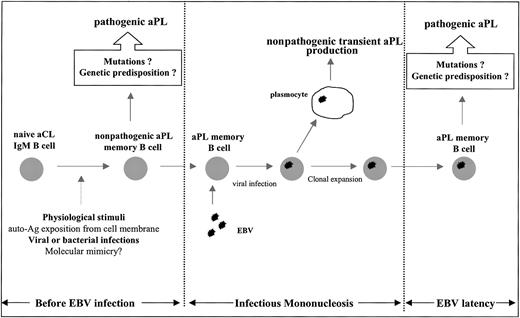
The important information that healthy individuals do have memory B cells able to produce aCL antibodies raises 2 crucial questions: what could be the physiologic significance of these aCL B cells, and are they related to those which are activated during autoimmune PAPS? The physiologic existence of mutated aCL antibodies indicates that the B cells crossed the germinal centers where some of them were selected by an antigen-driven process. This could have occurred during, for instance, past infectious diseases, because it is known that aCL antibodies are frequently produced during a variety of infections of viral and bacterial origins.8-11 However, the precise stimulus for aCL production is not completely understood: it was suggested that physiologic apoptosis, by allowing phospholipid extracellular exposure on apoptotic cell membranes, can induce aPL antibodies without the requirement for any infection.32-34 For instance, it was recently shown that human natural IgM antibodies are able to bind to apoptotic cells and activate complement after exposure of lysophosphatidylcholine.35 The link between physiologic aPL and autoimmune aPL is hypothesized in Figure 6 and could be related to the repetition of the antigenic stimuli which can result in modifying the aPL affinity and/or reactivity by introducing, for instance, a new cofactor dependency in a favorable genetic context.
Schematic hypothetical representation of aPL memory B-cell generation before acute EBV infection and of aPL memory B-cell expansion during acute EBV infection. APL memory B cells may appear in healthy individuals by means of physiologic stimuli or infectious diseases. These memory B cells are directly infected by EBV during IMN, leading to transient production of nonpathogenic aPL antibodies and clonal expansion. Genetic predisposition, environmental factors, or acquired additional somatic mutations may lead to the production of pathogenic aPL antibodies (“Discussion,” third paragraph).
Schematic hypothetical representation of aPL memory B-cell generation before acute EBV infection and of aPL memory B-cell expansion during acute EBV infection. APL memory B cells may appear in healthy individuals by means of physiologic stimuli or infectious diseases. These memory B cells are directly infected by EBV during IMN, leading to transient production of nonpathogenic aPL antibodies and clonal expansion. Genetic predisposition, environmental factors, or acquired additional somatic mutations may lead to the production of pathogenic aPL antibodies (“Discussion,” third paragraph).
It is interesting to note that, among the mAbs that were produced, the only one (M9) that is dependent on a protein cofactor is somatically mutated in a way that suggests an antigen-driven process. The risk of thrombosis could follow a prolonged production of mutated aCL because of a failure of the B-cell tolerance mechanisms (which appear to be tightly regulated in the mouse36 ) or the introduction of another unknown factor that would link aCL to thrombosis, or both. In any case, the detailed comparative analysis of the structures and functions of the different monoclonal aCL observed in the healthy situation and in pathologic autoimmune conditions should help in understanding the mechanisms of the PAPS.
Prepublished online as Blood First Edition Paper, June 5, 2003; DOI 10.1182/blood-2003-01-0180.
Supported by grants from INSERM (CRI 9702, EMI 0222). P.L. was supported by fellowships from the Fondation pour la Recherche Médicale (France).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal