Abstract
Cutaneous T-cell lymphoma (CTCL) is a lymphoproliferative skin disease with limited therapeutic options. Ten CTCL patients were treated with once-weekly intranodal injection of 1 × 106 mature monocyte-derived dendritic cells (DCs) pulsed with 100 μg/mL tumor lysate protein equivalent and keyhole limpet hemocyanin (50 μg/mL). Tumor-specific delayed-type hypersensitivity (DTH) reactions developed in 8 of 8 patients challenged with tumor-lysate-pulsed DCs and in 3 of 8 patients challenged with tumor lysate alone. Three of 5 patients showed significant tumor-lysate-specific increases of in vitro peripheral blood lymphocyte proliferation coinciding with increased interferon-α (IFN-α) production. Five of 10 (50%) patients had objective responses. Four patients had partial responses (PRs). Two are still in PR, and the other 2 patients had a mean PR duration of 10.5 months. One patient had a complete response (CR) for 19 months that is ongoing. The remaining 5 patients had progressive disease. In the 5 responder patients, 6.8 ± 1.4 vaccinations were necessary to induce an objective clinical response. Response was associated with low tumor burden. Continuation of vaccinations with new tumor lysate derived from progressive lesions reinduced treatment responses in 2 patients in PR. Selected patients had massive infiltration of CD8+ and TIA+ cytotoxic T cells at the site of regressing lesions and molecular remission after therapy. Intranodal injection of autologous tumor-lysate-pulsed DCs is well-tolerated and achieves immunologic and objective clinical responses in selected CTCL patients. (Blood. 2003;102:2338-2344)
Introduction
Cutaneous T-cell lymphomas (CTCLs) are characterized by the proliferation of clonally expanded T cells in skin with possible involvement of lymph nodes and visceral organs in advanced stages.1-3 The disease spectrum includes classical mycosis fungoides (MF), pleomorphic/anaplastic CTCL, and Sézary syndrome as leukemic variants.4-6 Therapeutic options are limited, especially in advanced stages.7 Nonspecific immunotherapy using interferon-γ (IFN-γ) in combination with psoralen plus ultraviolet light A (PUVA) therapy is effective in the early stages of CTCL.8 Recently, biologic agents such as interleukin-12 (IL-12) and denileukin diftitox (DAB389IL-2) have shown promising results in early clinical trials.9,10 One potential disadvantage of nonspecific immunotherapy using cytokines is the risk for considerable adverse effects with decreased quality of life. Specific immunotherapy aims to activate tumor-antigen-specific T cells with minimal concomitant systemic reactions. A study using mimotopes for tumor-specific T cells has demonstrated expansion of specific T cells in a patient with CTCL.11 There is accumulating evidence that the known therapeutic benefit of extracorporeal photopheresis (ECP), which induces complete remission in up to one quarter of patients with erythrodermic/leukemic CTCL, is a form of specific immunotherapy.12 Indeed, recent data support the evidence that DCs generated in the ECP chamber ingest apoptotic CTCL cells and stimulate a clinically relevant tumor-antigen-specific immune response after reinfusion to the patient.13,14 Tumor antigens that are targeted by that therapy include antigens derived from the clonotypic region of the CTCL T-cell-receptor (TCR)-β chain.
We have previously shown that antigen-specific vaccination using dendritic cells (DCs) induces immune responses and is of therapeutic value in selected patients with advanced melanoma.15,16 In the present pilot study, we directly addressed whether DC vaccination is of potential value in patients with CTCL refractory to standard treatment. We show that DC vaccination is feasible and safe and that it induces immunologic and clinical responses in selected CTCL patients.
Patients, materials, and methods
Patient selection and treatment
We included in the study 10 patients with CTCL refractory to standard treatment. The institutional ethical review board of the University Zurich Hospital approved the study, and informed consent was obtained from each patient before therapy. Diagnosis of CTCL was established based on clinical appearance, laboratory data, histomorphologic, routine immunohistology, and detection of clonality by TCR-γ chain polymerase chain reaction/denaturing gradient gel electrophoresis (PCR/DGGE).5 Patients with adequate renal and hepatic function and a Karnofsky index greater than 80% were included in the study (Table 1). Patients were treated with tumor-lysate-pulsed DCs from 1999 to 2002. Patients received a median of 9.5 weekly vaccinations. The vaccine preparation was administered intralymphatically into an inguinal lymph node under ultrasound control or was injected close to the regional lymph nodes. Booster vaccinations were performed depending on the clinical response. Before vaccination start, medical history was taken, and the following investigations were performed at baseline and after vaccination: full body physical examination, hematologic testing (hemoglobin level, leukocyte and platelet cell counts), blood chemistry analysis, ultrasound investigation of lymph nodes and abdomen, and chest x-ray examination.
Patient characteristics
No. . | Age, y/sex . | TNM stage . | Pretreatment therapy . | TBI before treatment . | TBI after treatment . | Clinical response . | DTH . |
|---|---|---|---|---|---|---|---|
| 1 | 47/F | IIb | PUVA | 3.3 | 1 | PR | Lysate− |
| IFN-α | Lysate/DC+++ | ||||||
| RT | KLH+++ | ||||||
| Retinoids | |||||||
| MTX | |||||||
| 2 | 52/F | Ib | PUVA | 3.0 | 1 | PR | Lysate− |
| Retinoids | Lysate/DC++ | ||||||
| IFN-α | KLH+++ | ||||||
| RT | |||||||
| 3 | 64/F | Ib | PUVA | 3.0 | 4.3 | PD | Lysate− |
| Retinoids | Lysate/DC+ | ||||||
| RT | KLH+++ | ||||||
| 4 | 62/M* | IIb | Steroids | 2.3 | 4.3 | PD | Lysate− |
| RT | Lysate/DC+ | ||||||
| KLH++ | |||||||
| 5 | 62/M | Ia | PUVA | 1 | 0 | CR | Lysate+ |
| Steroids | Lysate/DC+ | ||||||
| KLH+++ | |||||||
| 6 | 65/M* | IVa | PUVA | 4.3 | 6.3 | PD | ND |
| RT | |||||||
| IFN-α | |||||||
| Chemotherapy | |||||||
| 7 | 74/M* | IIb | PUVA | 5.3 | 6.3 | PD | ND |
| UVB | |||||||
| RT | |||||||
| 8 | 57/M | Ib | Steroids | 3 | 1 | PR | Lysate+ |
| Lysate/DC++ | |||||||
| KLH+++ | |||||||
| 9 | 47/M | IIb | UVB | 4.3 | 6.3 | PD | Lysate+ |
| RT, IFN-α | Lysate/DC+ | ||||||
| Retinoids | KLH++ | ||||||
| Chemotherapy | |||||||
| PUVA | |||||||
| 10 | 69/F | III | UVB | NA | NA | PR | Lysate− |
| RT | Lysate/DC+ | ||||||
| Photopheresis | KLH++ |
No. . | Age, y/sex . | TNM stage . | Pretreatment therapy . | TBI before treatment . | TBI after treatment . | Clinical response . | DTH . |
|---|---|---|---|---|---|---|---|
| 1 | 47/F | IIb | PUVA | 3.3 | 1 | PR | Lysate− |
| IFN-α | Lysate/DC+++ | ||||||
| RT | KLH+++ | ||||||
| Retinoids | |||||||
| MTX | |||||||
| 2 | 52/F | Ib | PUVA | 3.0 | 1 | PR | Lysate− |
| Retinoids | Lysate/DC++ | ||||||
| IFN-α | KLH+++ | ||||||
| RT | |||||||
| 3 | 64/F | Ib | PUVA | 3.0 | 4.3 | PD | Lysate− |
| Retinoids | Lysate/DC+ | ||||||
| RT | KLH+++ | ||||||
| 4 | 62/M* | IIb | Steroids | 2.3 | 4.3 | PD | Lysate− |
| RT | Lysate/DC+ | ||||||
| KLH++ | |||||||
| 5 | 62/M | Ia | PUVA | 1 | 0 | CR | Lysate+ |
| Steroids | Lysate/DC+ | ||||||
| KLH+++ | |||||||
| 6 | 65/M* | IVa | PUVA | 4.3 | 6.3 | PD | ND |
| RT | |||||||
| IFN-α | |||||||
| Chemotherapy | |||||||
| 7 | 74/M* | IIb | PUVA | 5.3 | 6.3 | PD | ND |
| UVB | |||||||
| RT | |||||||
| 8 | 57/M | Ib | Steroids | 3 | 1 | PR | Lysate+ |
| Lysate/DC++ | |||||||
| KLH+++ | |||||||
| 9 | 47/M | IIb | UVB | 4.3 | 6.3 | PD | Lysate+ |
| RT, IFN-α | Lysate/DC+ | ||||||
| Retinoids | KLH++ | ||||||
| Chemotherapy | |||||||
| PUVA | |||||||
| 10 | 69/F | III | UVB | NA | NA | PR | Lysate− |
| RT | Lysate/DC+ | ||||||
| Photopheresis | KLH++ |
Patient 1 continued subcutaneous IFN-α treatment during vaccination. Patient 8 used an intermediate potency topical steroid once a week. Patient 9 continued to use retinoids and intralesional IFN-α. Partial remission in the stage 3 patient 10 with Sézary syndrome was defined as greater than 50% reduction in erythroderma lasting for more than 1 month. Some additional tumors were treated with radiotherapy or topical imiquimod. Stabilization and regression occurred, even for tumors that received no additional therapy.
RT indicates radiotherapy; MTX, methotrexate; ND, not done; NA, not applicable; PD, progressive disease; −, less than 3 mm; +, 3-10 mm; ++, 11-20 mm; and +++, more than 20 mm.
indicates deceased.
Clinical response and toxicity criteria
Tumor sites were evaluated by physical examination using a quantitative score (tumor burden index [TBI]) as published.17 This score has proven to be a prognostic tool and to discriminate cumulative survival outcome in CTCL patients.17 The percentage of involved skin was documented using the rule of nines. Skin lesions were differentiated as patches (flat lesions, diameter larger than 1 cm), plaques (elevated, increased consistency), and tumors (nodular lesions). Tumor burden index was calculated as previously defined17 : TBI = 1 + 2 × (0 = patches < 30% or 1 = patches > 30%) + 2 × (0 = no plaques or 1 = plaques) + 1.3 × (0 = no tumor or 1 = tumor).
The range of TBI was 1 to 6.3. For complete remission, a TBI score of 0 was assigned. In patients with generalized erythroderma (patient 10), TBI was not evaluable. Standard clinical response criteria were applied.18 Complete response (CR) was defined as complete regression of all lesions lasting at least 1 month. Partial remission was defined as greater than 50% decrease of lesions (and greater than 50% decrease of TBI) lasting more than 1 month. Mixed response (MxR) was defined as a regression of CTCL lesions while other lesions remained stable or progressed. Stable disease (SD) was defined as less than a 25% change in size (and less than a 25% change in TBI) with no new lesions developing for 1 month. Adverse effects were recorded using common World Health Organization (WHO) toxicity criteria.
Preparation of tumor lysate
Tumor material was dispersed with a polytron (PT 3000; Kinematica AG, Littau, Switzerland) in 1 mL phosphate-buffered saline (PBS). Larger particles were removed by centrifugation. The supernatant underwent 4 freeze (liquid nitrogen) and thaw (room temperature) cycles. The lysate was irradiated with 12 000 rad (120 Gy) and thereafter was passed through a 0.2-μm filter. The protein concentration of the lysate was determined by the Bio-Rad protein assay (Bio-Rad Laboratories GmbH, Munich, Germany). One day before vaccination, immature DCs were incubated with the autologous tumor lysate (100 μg/mL) for 2 hours at 37°C.
Generation of dendritic cells
Peripheral blood mononuclear cells (PBMCs) were obtained by a leukapheresis procedure performed according to the standard operating procedures of the Department of Transfusion Medicine, University of Zurich. The leukapheresis product was diluted with PBS (Cantonal Pharmacy, Zurich, Switzerland) containing 10% ACD-A (Cantonal Pharmacy) for the isolation process with Ficoll-Hypaque (Amersham Pharmacia Biotech, Uppsala, Sweden). After isolation cells underwent 3 washing cycles with PBS containing 1 mM EDTA (ethylenediaminetetraacetic acid) (Cantonal Pharmacy). PBMCs (80 × 106/mL) were diluted with cold 20% human albumin solution (ZLB Bioplasma AG, Bern, Switzerland) containing 20% dimethyl sulfoxide (Fluka Chemie GmbH, Buchs, Switzerland) and were immediately stored at -80°C in 4.5 mL Nunc cryotubes (Nunc Nalge, Roskilde, Denmark). After 1 day they were transferred into liquid nitrogen until use.
Dendritic cells were generated from frozen PBMCs as previously described, with minor modifications.15,19 Briefly, 50 × 106 PBMCs were thawed and cultured in 10-cm diameter Petri dishes (Falcon-Becton Dickinson, Franklin Lakes, NJ) in complete RPMI containing 5 mM glutamine (Seromed, Berlin, Germany), 1 mM sodium pyruvate, and 0.2% gentamicin (both Gibco BRL, Carlsbad, CA) with 1% autologous plasma (AP) for 30 to 45 minutes. After removing the floating cells and washing with PBS, adherent cells were cultured overnight in complete RPMI. The following day RPMI was supplemented with 800 U/mL granulocyte macrophage-colony-stimulating factor (GM-CSF; Leucomax, Sandoz-Wander Pharma SA, Bern, Switzerland) and 1000 U/mL IL-4 (R&D Systems Europe, Oxford, United Kingdom). At day 6 the cells were stimulated with a maturation cytokine cocktail including TNF-α (10 ng/mL), IL-6 (1000 U/mL), and IL-1β (10 ng/mL) (R&D Systems Europe) for 24 hours.
Phenotypic DC changes were monitored by light microscopy and flow cytometric analysis. DCs used for the clinical study expressed high levels of HLA class 1, HLA class 2, and costimulatory molecules (CD80, CD86) as described.15 Mean value of DC surface molecule expression in selected samples was the following: CD83 = 56% ± 14.3%; CD86 = 91% ± 7.1%; HLADR = 95% ± 5.5%. DCs were pulsed with autologous tumor lysate (100 μg/mL) for 2 hours at room temperature at day 6 before the maturation cocktail was added. In all cases, the DC preparation was loaded with keyhole limpet hemocyanin (KLH) Megathura crenulata protein (50 μg/mL; Calbiochem, Bad Soden, Germany) for 2 hours at the time of tumor lysate pulsing. Before injection, DCs were washed 3 times in PBS and resuspended in a total volume of 0.5 mL PBS. Immediately afterward antigen-loaded DCs were administered to the patient. In some patients responding to treatment, booster DCs were prepared from freshly isolated peripheral blood leukocytes (PBLs) instead of frozen leukapheresis products.
DTH reactions
Delayed-type hypersensitivity (DTH) skin tests were performed with tumor lysate, tumor-lysate-pulsed DCs, KLH, and nonpulsed DCs before vaccination and after 8 vaccinations. Then 105 DCs, DCs pulsed with tumor lysate, KLH (5 μg), tumor lysate (10 μg), and PBS as negative control were injected intradermally into the forearm. A positive skin-test reaction was defined as erythema larger than 3-mm diameter and induration 48 hours after intradermal injection. DTH was read after 48 hours as follows: -, < 3 mm; +, 3-10 mm; ++, 11-20 mm; +++, > 20 mm.
Immunohistochemistry
Biopsy samples of CTCL skin were obtained if possible before, during, and after vaccination and were cryopreserved. Representative 5- to 7-μm serial cryostat sections were stained using the alkaline phosphatase anti-alkaline phosphatase (APAAP) technique as described.20 Unconjugated primary polyclonal antibodies or monoclonal antibodies (mAbs) used at 10 μg/mL were anti-CD3 (rabbit antihuman polyclonal; DAKO, Glostrup, Denmark), anti-CD4 (monoclonal mouse immunoglobulin G1 (IgG1); Novocastra Laboratories, Newcastle, United Kingdom), anti-CD8 (monoclonal mouse IgG1; DAKO), anti-CD45RO (monoclonal mouse IgG2a; DAKO), and anti-TIA (monoclonal mouse IgG1; Immunotech, Marseilles, France).
Measurement of peripheral blood lymphocyte proliferation
To measure the proliferation of peripheral blood lymphocytes (PBLs) before and after vaccination in response to tumor lysate and PBL lysate, [3H]thymidine incorporation was measured. PBLs from 5 patients (patients 1, 5, 8, 9, 10) were available for analysis. After the addition of 100 μg/mL tumor lysate or autologous PBL lysate, 105 cells per well in a 96-well plate were cultured for 7 days in RPMI containing 10% human AB serum. Autologous PBL lysate was prepared as described for tumor lysate from PBLs obtained through leukapheresis (“Preparation of tumor lysate”). Eighteen hours before culture termination, 1 μCi (0.037 MBq) [3H]thymidine (Amersham Biosciences, Uppsala, Sweden) was added to each well. Each sample was cultured in triplicate. Cell cultures were harvested using an automated multiwell harvester. Filter papers were then placed in a scintillation bag, and scintillation fluid was added and spread with a roller. Thereafter bags were heat-sealed, and radioactivity was measured in a scintillation counter (1450 Microbeta Trilux; Perkin Elmer, Norwalk, CT). Statistical analysis was performed using the unpaired t test.
Measurement of IFN-γ secretion
To measure IFN-γ secretion during the in vitro proliferation assay, cell culture supernatants were obtained at day 7 and were centrifuged and stored at -80°C until use. Supernatants from 3 wells were pooled, and secreted IFN-γ was measured using a sandwich enzyme-linked immunosorbent assay (ELISA) (R&D Systems, Minneapolis, MN) according to the manufacturer's guidelines.
TCR-gamma chain PCR/DGGE analysis
DNA was extracted from lesions on which biopsies were performed before and after treatment with tumor-lysate-pulsed DCs, as previously described.21 The concentration of DNA was determined in each sample and adjusted to 2 μg/μL for PCR amplification. Nested PCR for the TCR γ chain gene was performed with previously reported primers and PCR conditions.21 Positive control was the Myla cell line, and negative control was normal human lymphocyte DNA in the PCR step. For the DGGE, a D Gene System (Bio-Rad, Hercules, CA) was used with 6.5% polyacrylamide to obtain gradients of 30% to 60% urea/formamide. A 10-μL aliquot of the PCR product of each sample was loaded onto the gels. Electrophoresis was run at 150 V at 60°C for 6 hours. Ethidium bromide-stained gels were photographed under UV light.
Results
Feasibility and adverse effects
Dendritic cells were prepared and injected intranodally with minor modifications as described.15 One hundred fifty-one intranodal injections were performed in 10 patients. Injected DC solution accumulated in the paracortex of the lymph node and led to a visible swelling of the lymph node. Tumor lysate could be obtained in every patient with patch/plaque/tumor MF and the minimum amount of protein, necessary for pulsing the DCs (100 μg/mL). Adverse effects consisted of transient pain during injection, lymph node swelling, generalized exanthema in 2 patients after vaccination (25th vaccination in patient and 3rd vaccination in patient 4) that were not believed to be related to therapy. No severe constitutional symptoms or autoimmune-type clinical symptoms were observed.
Delayed-type hypersensitivity reactions
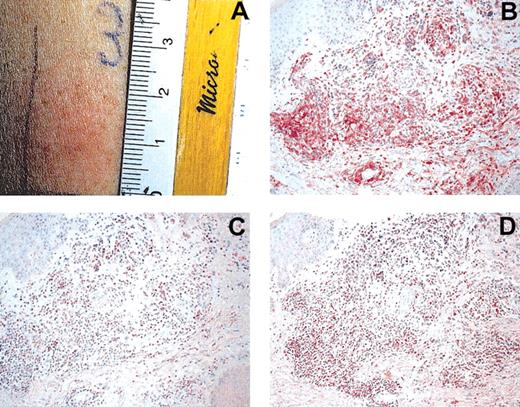
An important aim in vaccination trials is the assessment of a vaccine-specific immune response. With peptide vaccines, various immunomonitoring methods are available.22 A useful method to monitor immunoreactivity toward tumor lysate is DTH reactions. DTH preparations were injected before and after 8 cycles of DC vaccination. They consisted of: tumor lysate alone (10 μg), tumor-lysate-pulsed DCs, KLH alone (5 μg), KLH-pulsed DCs, DCs alone, and PBS as control in 0.25 mL PBS each. A reaction was assessed as positive if erythema and induration after 48 hours were greater than 3 mm. Eight of 10 patients were evaluable for DTH reactions. DTH reactions toward tumor lysate or tumor-lysate-pulsed DCs were negative before therapy. All 8 patients had positive KLH and tumor-lysate-pulsed DC DTH reactions (patients 1, 2, 3, 4, 5, 8, 9, 10; Figure 1A). Background reaction of DC alone was observed in patients 3, 4, and 5. Even after subtraction of this background reactivity, tumor-lysate-pulsed DTH reactions were positive, as shown in Table 1. Three patients had reactions to autologous tumor lysate alone (patients 5, 8, 9). Recruitment of effector lymphocytes to the DTH challenge site after vaccination was evaluated using immunohistochemistry. Infiltration of numerous CD4+ and CD8+ cells was observed in the dermis of DTH reaction biopsy samples. Infiltrating cells expressed the cytotoxic marker T-cell intracytoplasmic antigen (TIA; Figure 1B-D). In addition, the infiltrating cells were of the memory phenotype (CD45RO+; data not shown).
Induction of tumor-lysate-specific DTH after DC vaccination. (A) Positive DTH reaction (25 mm) in patient 1 after intradermal injection of tumor lysate. Immunohistochemical investigations were performed using mAbs for CD4 (B), CD8 (C), and TIA (D). Note heavy infiltration of DTH challenge site. Sections were stained using the APAAP technique and counterstained with hematoxylin. Original magnification, × 200 (B-D).
Induction of tumor-lysate-specific DTH after DC vaccination. (A) Positive DTH reaction (25 mm) in patient 1 after intradermal injection of tumor lysate. Immunohistochemical investigations were performed using mAbs for CD4 (B), CD8 (C), and TIA (D). Note heavy infiltration of DTH challenge site. Sections were stained using the APAAP technique and counterstained with hematoxylin. Original magnification, × 200 (B-D).
In vitro analysis of the tumor-lysate-specific immune response
To test whether injected tumor-lysate-pulsed DCs were able to induce a tumor-antigen-specific immune response, we compared cell proliferation and IFN-γ production of lymphocytes isolated before and after DC vaccination. PBLs were cultured in the presence of 100 μg/mL autologous tumor lysate, 100 μg/mL autologous PBL lysate, or medium alone. PBLs from 5 patients (patients 1, 5, 8, 9, 10) before and after vaccination were available for testing. Cell proliferation was measured using [3H]thymidine incorporation. Tumor-lysate-stimulated PBLs from all patients showed an increase in proliferation after vaccination; the increase was significant (P < .05) in 3 of 5 patients (patients 5, 8, 10). These patients showed objective tumor responses (see below). The addition of PBL lysate also induced statistically significant (P < .05) increased proliferation in patients 5, 8, and 10, which was less than the proliferation induced by tumor lysate (Figure 2A).
Induction of PBL proliferation and IFN-γ secretion after DC vaccination. PBLs from patients 1 (Ai,Bi), 5 (Aii,Bii), 8 (Aiii,Biii), 9 (Aiv,Biv), and 10 (Av,Bv) before (▪) and after (□) vaccination were cultured for 7 days in RPMI containing 10% human AB serum in the presence of 100 μg/mL tumor lysate or PBL lysate. No lysate was added to negative controls (-). (A) Cell proliferation was assessed by measuring [3H]thymidine incorporation using a scintillation counter. Data are shown as mean ± SD of triplicate wells. (B) Supernatants from 3 wells were pooled after 7 days of incubation, and IFN-γ secretion was measured by ELISA. *Significant difference (P < .05).
Induction of PBL proliferation and IFN-γ secretion after DC vaccination. PBLs from patients 1 (Ai,Bi), 5 (Aii,Bii), 8 (Aiii,Biii), 9 (Aiv,Biv), and 10 (Av,Bv) before (▪) and after (□) vaccination were cultured for 7 days in RPMI containing 10% human AB serum in the presence of 100 μg/mL tumor lysate or PBL lysate. No lysate was added to negative controls (-). (A) Cell proliferation was assessed by measuring [3H]thymidine incorporation using a scintillation counter. Data are shown as mean ± SD of triplicate wells. (B) Supernatants from 3 wells were pooled after 7 days of incubation, and IFN-γ secretion was measured by ELISA. *Significant difference (P < .05).
To assess IFN-γ production of PBLs stimulated with tumor lysate, supernatants from 3 wells were pooled at 7 days after stimulation and measured using IFN-γ-specific ELISA. In concordance with the data obtained with the proliferation assay, samples after vaccination from patients 5, 8, and 10 showed a strong increase in IFN-γ production after stimulation with tumor lysate compared with PBLs isolated before vaccination (increases of 94%, 166%, and 197%, respectively) (Figure 2B). Only minor increases in IFN-γ production were observed after stimulation with autologous PBL lysate or medium alone (Figure 2B).
Clinical response
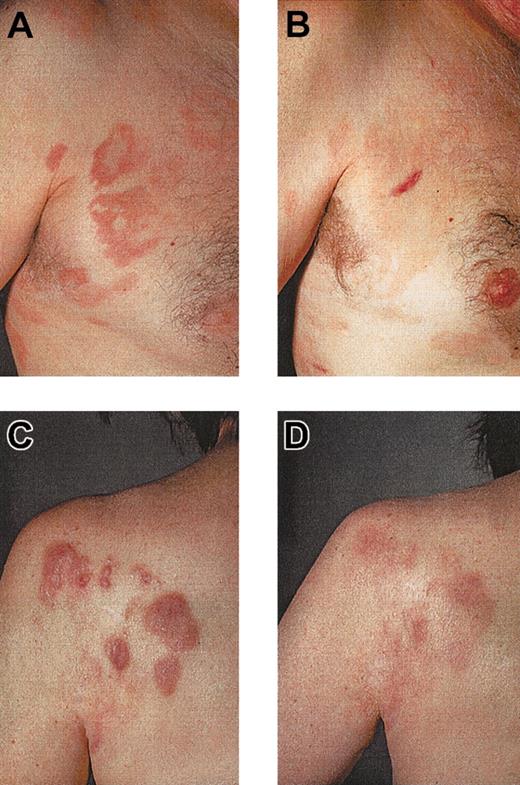
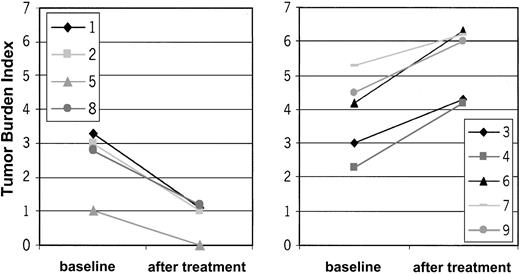
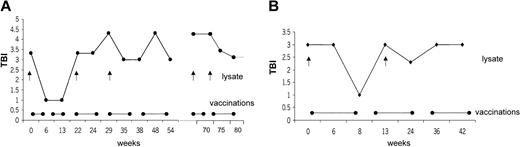
Ten patients with CTCL were included in the study. Mean age was 59.9 ± 9 years; 6 patients were men, and 4 were women. Stage of disease ranged from Ia to IVa (Table 1). Clinical response was assessed by standard criteria based on a previously established TBI.17,18 Four of 10 (40%) patients had PR (patients 1, 2, 8, 10), which has continued in 2 patients. The other 2 patients had PR for a mean duration of 10.5 months. Overall duration of the objective responses was 7.9 months. Regression was observed in patches, plaques, and tumors (patient 1; Figure 3C-D). In some lesions, regression started from the center of the lesion (patient 1; data not shown). There was regression of plaques to residual erythema without notable infiltration (patient 8; Figure 3A-B). One patient had CR for 19 months, and it continues (patient 5). This patient had also a DTH reaction to tumor lysate alone after 8 vaccinations. One patient with Sézary syndrome (patient 10) was not evaluable using TBI and experienced regression of more than 50% of her erythroderma, qualifying as PR. The remaining 5 patients had progressive disease (patients 3, 4, 6, 7, 9). In the 5 objective responder patients, 6.8 ± 1.4 vaccinations were necessary for the induction of a clinical response, which was associated with low tumor burden at baseline (median TBI of 3 in responder patients vs TBI of 4.3 in nonresponder patients) (Figure 4). Clinical relapse after cessation of treatment occurred in 2 of 5 responder patients after a mean of 10.5 ± 7.7 weeks. Continuation of vaccination with new tumor lysate derived from progressive lesions in these 2 patients again induced a treatment response (Figure 5A, patient 1; Figure 6B, patient 2). In patient 1, the interruption of vaccination led to disease progression (Figure 5A, week 13). Introduction of a new vaccine using DCs pulsed with tumor lysate of a progressing lesion (Figure 5A, week 29) again induced a treatment response. A similar observation was made in patient 2, in whom interruption of vaccination led to disease progression (Figure 5B, week 8). A change to new tumor lysate pulsed on DCs at week 13 led to a new clinical response (Figure 5B)
Regression of CTCL lesions after tumor-lysate-pulsed DC vaccination. Regression of infiltrated plaques of patient 8 (A, before therapy), leaving an area of residual erythema without palpable infiltration (B, after therapy). Regression of tumors, plaques, and patches in patient 1 (C, before therapy; D, after therapy).
Regression of CTCL lesions after tumor-lysate-pulsed DC vaccination. Regression of infiltrated plaques of patient 8 (A, before therapy), leaving an area of residual erythema without palpable infiltration (B, after therapy). Regression of tumors, plaques, and patches in patient 1 (C, before therapy; D, after therapy).
TBI in responding and nonresponding patients before and after DC vaccination. X-axis represents TBI. Y-axis differentiates baseline and posttreatment values. Please note the difference in the baseline TBI between the responder patients (left panel) and nonresponder patients (right panel). Low tumor burden at treatment start is associated with treatment response. Patient 10 with Sézary syndrome was not evaluable for TBI.
TBI in responding and nonresponding patients before and after DC vaccination. X-axis represents TBI. Y-axis differentiates baseline and posttreatment values. Please note the difference in the baseline TBI between the responder patients (left panel) and nonresponder patients (right panel). Low tumor burden at treatment start is associated with treatment response. Patient 10 with Sézary syndrome was not evaluable for TBI.
Correlation of vaccine application with clinical response. Correlation of disease development as assessed by TBI and vaccine application was followed over time. Interruption of vaccination led to disease progression with increased TBI. Repeated response was induced after revaccination (•——•) with new tumor lysate (↑) from progressive lesions. In patient 1, interruption of vaccination led to progression of disease (A, week 13). Introduction of a new vaccine using DCs pulsed with tumor lysate of a progressing lesion (A, week 29) induced a treatment response. A similar observation was made in patient 2, when interruption of the vaccination led to disease progression (B, week 8). A change to new tumor lysate led to a clinical response after week 13 (B).
Correlation of vaccine application with clinical response. Correlation of disease development as assessed by TBI and vaccine application was followed over time. Interruption of vaccination led to disease progression with increased TBI. Repeated response was induced after revaccination (•——•) with new tumor lysate (↑) from progressive lesions. In patient 1, interruption of vaccination led to progression of disease (A, week 13). Introduction of a new vaccine using DCs pulsed with tumor lysate of a progressing lesion (A, week 29) induced a treatment response. A similar observation was made in patient 2, when interruption of the vaccination led to disease progression (B, week 8). A change to new tumor lysate led to a clinical response after week 13 (B).
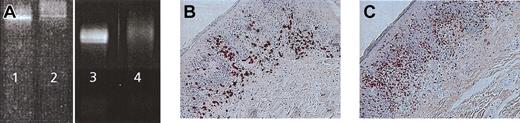
Induction of molecular remission in the presence of infiltration with CD8+ cytotoxic killer cells. (A) TCR-γ chain PCR DGGE of CTCL patient 1 demonstrating a Vγ 1-8 band before treatment (lane 2), positive control MyLa cell line (lanes 1 and 3). Disappearance of tumor-specific clone after vaccination in residual erythematous lesion (lane 4). Immunohistologic staining of regressive CTCL lesion demonstrates infiltration of CD8+ (B) and TIA+ (C) T cells at the dermo-epidermal junction. Sections were stained using the APAAP technique and counterstained with hematoxylin. Original magnifications, × 400 (B) and × 200 (C).
Induction of molecular remission in the presence of infiltration with CD8+ cytotoxic killer cells. (A) TCR-γ chain PCR DGGE of CTCL patient 1 demonstrating a Vγ 1-8 band before treatment (lane 2), positive control MyLa cell line (lanes 1 and 3). Disappearance of tumor-specific clone after vaccination in residual erythematous lesion (lane 4). Immunohistologic staining of regressive CTCL lesion demonstrates infiltration of CD8+ (B) and TIA+ (C) T cells at the dermo-epidermal junction. Sections were stained using the APAAP technique and counterstained with hematoxylin. Original magnifications, × 400 (B) and × 200 (C).
Immunohistology and T-cell-receptor clonality analysis of regressing lesions
In 2 patients (patients 1, 7), we obtained biopsy material from lesions before and after treatment and performed TCR-γ chain PCR DGGE. In both patients, a specific clone was found in the Vγ 1-8 band before vaccination. In addition, in both patients the tumor-specific clone disappeared on PCR at the end of treatment (Figure 6A, patient 1). One of these patients (patient 1) with molecular remission showed PR, whereas the other patient (patient 7) experienced disease progression during treatment. Immunohistologic stainings were performed on the lesion in responder patient 1. Heavy infiltration of CD8+ T cells along the dermo-epidermal junction was exactly observed where the CD4 tumor cells would have been expected (Figure 6B). Infiltrating cells were also TIA positive, indicating cytolytic potential (Figure 6C).
Discussion
We show that tumor-lysate-pulsed DC vaccination is feasible, well tolerated, and induces immunologic and clinical responses in selected CTCL patients. CTCL is a peculiar type of extranodal non-Hodgkin lymphoma (NHL) that progresses in its classical MF variant slowly, from patch to plaque to tumor stage, with possible involvement of lymph nodes and visceral organs in terminal stages. The rationale for immunotherapeutic intervention in CTCL is derived from several findings pointing to a role for the immune system in the surveillance of tumor cells: (1) presence of tumor-infiltrating T cells at the tumor site23 ; (2) therapeutic effectiveness of immunobiologic agents such as IFN-γ and IL-1224 ; (3) specific activation of the immune system mediated by dendritic cells as the rational mechanism of extracorporal photopheresis, a classic therapy of erythrodermic CTCL.12 To test directly whether dendritic cell vaccination induces immune responses or clinical responses in CTCL, we performed a pilot study in 10 patients with CTCL using intranodal injection of tumor-lysate-pulsed DC weekly for 8 treatment cycles. Tumor lysate was chosen as tumor antigen because the definition of CTCL-specific tumor antigens is still in its early development,11,25,26 and tumor lysate as antigen has demonstrated its usefulness in melanoma patients.15 In 10 of 10 CTCL patients, sufficient tumor material was available to prepare tumor lysates for 8 vaccination cycles. Adverse effects were minor, with some swelling and pain at the site of lymph node injections. No WHO grade III/IV toxicities were observed, nor were there clinical signs of autoimmunity induced by the vaccine.
Assessment of the immune response to tumor lysates is notoriously difficult. We performed DTH reactions before and after vaccination in combination with immunohistochemical analysis of infiltrating cells. In 8 of 8 patients, the induction of a positive DTH reaction toward tumor-lysate-pulsed DCs (after subtraction of the background values for DC injection alone) was observed after 8 vaccinations. In 3 patients, positive DTH reactions were induced after the injection of tumor lysate alone. Infiltrating cells consisted of numerous CD8 and CD4 cells. Infiltrating cells expressed the cytotoxic marker TIA, indicating that cells recruited to the DTH challenge site are indeed cytolytic effector cells. These findings point to an activation of tumor-lysate-specific immunity during DC vaccination. Immune monitoring methods to assess tumor-lysate-specific T-cell responses are difficult to perform. We chose proliferation assays and detection of IFN-γ secretion to analyze a tumor-lysate-specific immune response because they have proven in our hands to be the most reliable assays. Tumor-lysate-specific PBL proliferation was increased significantly in 3 of 5 patients tested. IFN-γ production was increased in 5 of 5 patients. Interestingly, there was also an increase of proliferation and IFN-γ production when autologous PBL lysate was used. This increase was less than that observed with tumor lysate. This indicates increased autoreactivity to PBLs induced by tumor-lysate-pulsed DC vaccination and might be attributed to the fact that, especially in early CTCL lesions, considerable numbers of nonmalignant cells are part of the “tumor lysate.”
An important end point in vaccination studies is clinical response. Few phase 3 studies have addressed clinical efficacy of vaccination. Even though our study was not specifically designed to evaluate clinical efficacy, we made several novel observations regarding this issue. CTCL is a potentially interesting therapeutic target for vaccination studies because tumor progression is slow (over years), patients often have low tumor burden at the beginning of their disease, and the impact of vaccination on the clinical course of the disease is clearly visible. This is in contrast to other tumor entities such as melanoma in which tumor progression is often rapid and correlation between tumor response and vaccination can only be assessed as a form of “snapshot” at the time of the 3 monthly staging investigations. A question in our study was, therefore, whether there is a correlation between vaccine application and development of the clinical response. In 2 responder patients, a clear correlation between vaccine application and clinical response was observed. Omitting the vaccine led to relapse of the tumor (mean time to relapse, 10.5 ± 7.7 weeks), whereas continuation with a new tumor lysate derived from progressing lesion again enabled the induction of a clinical response (Figure 5).
The overall objective response rate was 50% (5 of 10 CTCL patients), with 4 partial responders and 1 complete responder. Overall mean duration of objective responses was 7.9 months. CR is now ongoing for 19 months. In the 5 objective responder patients, a mean of 6.8 ± 1.4 vaccinations was necessary for the induction of a response. Response was associated with low tumor burden before vaccination, with a median TBI of 3 in responder patients versus 4.3 in nonresponders (Figure 4). Electron beam therapy is a possible way to reduce tumor burden in the absence of immunosuppression in future immunotherapy trials.27 In 2 patients, material from pretreatment and posttreatment lesions was obtained and analyzed by immunohistochemistry and TCR-γ PCR DGGE. Molecular remission in these patients was demonstrated by the disappearance of the tumor clone in posttreatment lesions in 2 of 2 patients after DC vaccination (Figure 6A). Disappearance of the malignant clone coincided with heavy infiltration of CD8+ and TIA+ cytolytic T cells at the site of tumor regression. These data suggest an involvement of CTCL-specific cytolytic T cells in eradication of the tumor and are similar to the findings of Rook et al9 after IL-12 therapy for CTCL patients. Classic therapeutic approaches for CTCL consist of, among others, ECP, nitrogen mustard, PUVA, and IFN-α.28-30 Recently, innovative therapeutic interventions have been reported that include chemotherapeutic agents such as nucleoside analogs,31 vitamin A derivatives such as bexaroten,32 cytokine fusion toxins such as DAB IL-2,10 and cytokine-based therapy such as IL-12.33 Our study compares with the pioneering DC vaccination study of Hsu et al,34 which demonstrated an idiotype (Id)-specific immune response and a clinical response in 2 of 4 patients with follicular lymphoma. A very recent follow-up confirmed Id-specific immune responses and durable clinical responses in a larger cohort.35 In myeloma patients, idiotypic T-cell responses were induced using Id-pulsed DCs.36-39 Our study is in line with recent interesting findings that the well-established therapeutic procedure of ECP is based on the loading of DCs with apoptotic CTCL cells and the induction of tumor-specific immunity.13
Taken together, our pilot study is one of the first to demonstrate that antigen-specific vaccination approaches using monocyte-derived DCs as adjuvant and tumor lysate as tumor antigen might have an impact on the antitumor immune response and clinical course of CTCL. Future strategies will most likely include a focus on patients with low tumor burden, repetitive vaccinations to keep clinical responses ongoing, and DC vaccination as first-line treatment in larger controlled studies.
Prepublished online as Blood First Edition Paper, April 24, 2003; DOI 10.1182/blood-2002-08-2455.
Supported by the Zürich Cancer League (F.O.N.) and the Swiss Cancer League (F.O.N.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr U. Schanz and his team from the Transfusion Medicine Unit for performing leukapheresis and Mrs Emy Ammann and Mrs Sylvia Sahner for excellent technical support.

![Figure 2. Induction of PBL proliferation and IFN-γ secretion after DC vaccination. PBLs from patients 1 (Ai,Bi), 5 (Aii,Bii), 8 (Aiii,Biii), 9 (Aiv,Biv), and 10 (Av,Bv) before (▪) and after (□) vaccination were cultured for 7 days in RPMI containing 10% human AB serum in the presence of 100 μg/mL tumor lysate or PBL lysate. No lysate was added to negative controls (-). (A) Cell proliferation was assessed by measuring [3H]thymidine incorporation using a scintillation counter. Data are shown as mean ± SD of triplicate wells. (B) Supernatants from 3 wells were pooled after 7 days of incubation, and IFN-γ secretion was measured by ELISA. *Significant difference (P < .05).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/7/10.1182_blood-2002-08-2455/6/m_h81935017002.jpeg?Expires=1767696788&Signature=kabefqxQyp3LyP8omhfaul7DLwy6ullSQr-wlRCqoD7g-5WRiFw8rpeSD1Ew08uFg-4Eyh-sWtdMuWkVGMU9UjlO3Op-0avhfkt~YiDFmVFWvg~x3F~K7pDUtnfwefjUkHeVKuqvxbitMdvi5voTS2Qn20QHLFNb8nO8BWSaNBM0aOKCccIv-g~2hjKN8zBSm124sM6PIF2EFzHl~wZgAkBNI3heJO5zNqBEwWL9vxF6JRnaYn4spnupQtcieWUfmxBJBe45oB8~el6dCvO5zQX4lTU8ZP8dpfJADWnk2WNvhJaJW2sswvha6IQeq5d2rZ588oOpOh2GXZvsi38Eug__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal