Abstract
Identical infant twins with concordant leukemia were first described in 1882, and since that time many such pairs of infants and older children have been described. It has long been recognized that this situation offers a unique opportunity to identify aspects of the developmental timing, natural history, and molecular genetics of pediatric leukemia in general. We reviewed both the older literature and more recent molecular biologic studies that have uncovered the basis of concordance of leukemia. Molecular markers of clonality, including unique, genomic fusion gene sequences, have provided unequivocal evidence that twin pairs of leukemia have a common clonal origin. The only plausible basis for this, first suggested more than 40 years ago, is that following initiation of leukemia in one twin fetus, clonal progeny spread to the co-twin via vascular anastomoses within a single, monochorionic placenta. This explanation has been endorsed by the identification of clonotypic gene fusion sequences in archived neonatal blood spots of individuals who subsequently developed leukemia. These analyses of twin leukemias have thrown considerable light on the natural history of disease. They reveal a frequent prenatal origin and an early or initiating role for chromosome translocations. Further, they provide evidence for a variable and often protracted latency and the need, in childhood acute lymphoblastic leukemia (ALL)/acute myeloblastic leukemia (AML), for further postnatal exposures and/or genetic events to produce clinical disease. We argue that these insights provide a very useful framework for attempts to understand etiologic mechanisms. (Blood. 2003;102:2321-2333)
Introduction
Around 3 to 4 per 1000 live births produce a clone of 2 genetically identical twins. These twins are monozygotic, being derived from a single fertilized ovum followed by splitting of the early embryo. In contrast, fraternal or nonidentical twins are simultaneously derived from independent, fertilized ova and have the same genetic diversity as non-twinned siblings. The common genetic heritage of monozygotic twins is instantly recognizable in their striking similarity of appearance, and, principally for this reason, they have been singled out in almost all human cultures, historical and contemporary, as deserving of special attention. This unique status is reflected in a rich mythology, literature, and art.1,2 In some respects, this interest has been relatively trivial, focusing, as did Shakespeare, on the comic potential of mistaken identity. But twins also raise more fundamental philosophical and ethical issues. They challenge our precepts of identity, individuality, and fate. Recently, their “natural” origins have been used, in our view erroneously, to legitimize the prospect of human reproductive cloning. Their common inheritance also raises questions of importance to medicine and, in particular, provides an opportunity to explore vulnerability to disease. Not surprisingly, therefore, twins have been much used, and occasionally abused, in biomedical research.
In 1875, Francis Galton, cousin of Charles Darwin, introduced the idea of exploiting the shared genetic identity of monozygotic twins to assess the relative effect of heredity and environment—or nature versus nurture—in the development of unique characteristics, both normal and pathologic.3 Galton's evidence was anecdotal, but it persuaded him of the omnipotence of genetic predetermination. The effect of Galton is difficult to exaggerate. Not only did he initiate more than 100 years of productive medical research on twins, but he also spawned and advocated the eugenics movement that led inexorably to the pseudo-scientific justification for Nazi fascism and Mengele's sadistic experimentation on twins in Auschwitz.1 Contrary to what is often quoted, Galton did not introduce the “classical” twin research method of comparing concordance rates of disease or phenotype in identical versus nonidentical twins. This method came about 50 years later when the zygosity and genetic status of these 2 different twin types was recognized.4
In the modern era of human genetics, very many human traits and medical conditions have been subject to twin pair analysis.5,6 Some human disorders have close to 100% concordance in identical twins; this concordance applies to co-inheritance of mutant genes that are dominant and highly penetrant, for example, in Huntington chorea.7 Most diseases or traits (but not all) show a concordance rate in identical twins in the broad range of 5% to 75% and some 2 to 5 times higher than in fraternal twins. Examples include the following: tuberculosis,8 other infectious diseases,9 autoimmune diseases,10 autism,11 obesity,12 some adult cancers,13 cardiovascular diseases,5 cognitive abilities,14 self-esteem,15 and appreciation of musical pitch.16 In the cancers, concordance rates for monozygotic (versus dizygotic) twins vary markedly for subtypes, being high for prostate and breast cancers, low for lung cancer.13 These data have been generally taken to indicate that most of our ailments (as well as many of our talents) are a consequence of complex genotype-environment interactions. In some cases, such as type 1 diabetes,17 alleles involved in inherited susceptibility have been uncovered, and many more relevant gene polymorphisms can be anticipated with the advent of the human genome completion and new high-throughput single nucleotide polymorphism (SNP) assays. Although all of these studies enforce the notion that inherited genetics contributes to disease or trait susceptibility, interpretation is not without difficulty.5,18 Aside from ascertainment bias and the possibility that monozygotic twins may be more likely to share the same postnatal environmental exposures than fraternal twins, the in utero placental environment is usually different in the 2 twin types, and this difference could influence subsequent risk of disease. This situation turns out to be of some considerable relevance to leukemia in twins.
Leukemia in twins
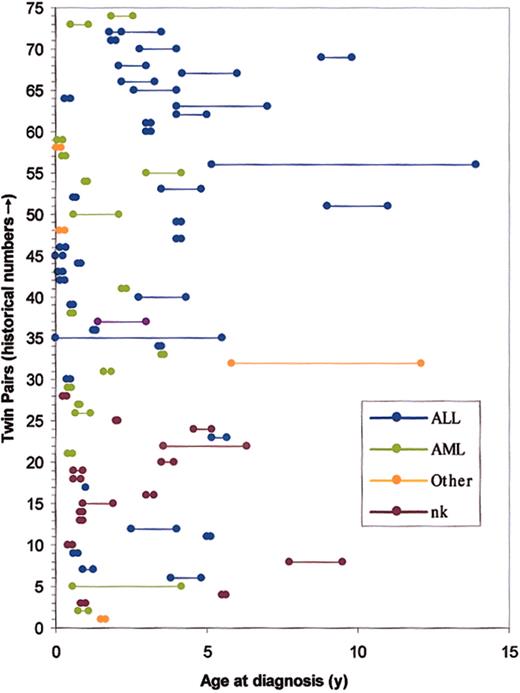
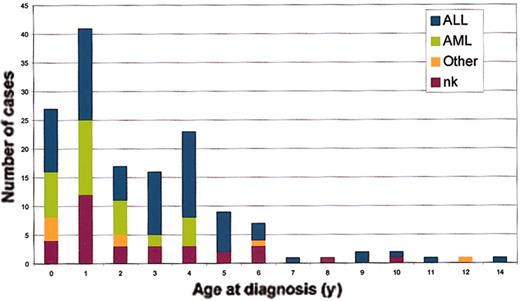
The first case report of concordant leukemia in twin children appeared in the German literature in 1882.19 This anecdote was followed considerably later by a few case reports beginning in the 1930s and subsequent reviews on the topic in the 1960s and 1970s.20-25 All told, more than 70 same-sex or known monozygotic twin pairs with concordant disease have been recorded in variable detail. These cases are listed in their historical sequence in Figures 1 and 2, which illustrates the ages at diagnosis in each twin pair and the leukemia subtypes. Concordant leukemia in unlike sex or known dizygotic twin pairs is exceedingly rare.25 Because of the rarity of the condition, the limited availability of suitable databases, possible ascertainment biases, and uncertain zygosity status in the early case pairs, there is no very accurate calculation of the concordance rates in monozygotic twins. In an article published in 1964, MacMahon and Levy22 provided the first estimates of concordance rates for childhood leukemia and also drew the important inference that their data strongly suggested a prenatal origin of the disease (or, as they conceded, a prezygotic, genetic influence). All told, a number of surveys (Table 1), albeit involving only small numbers of twin pairs with leukemia, suggest that the concordance rate for acute leukemia (including both acute lymphoblastic leukemia [ALL] and acute myeloblastic leukemia [AML]) in children aged birth to 15 years is between 5% and 25%. Infant ALL is a biologically and clinically distinct disease from childhood ALL.26 It would be more appropriate, therefore, to consider concordance rates separately for infant versus childhood ALL. If infant pairs are removed from the series reported (Table 1), the concordance rate for ALL remains at around 10%. Note, however, that in very few pairs of twins concordant for ALL does presentation occur after the age of 6 years (Figure 2; see also Figure 7). This information suggests that the concordance rate for the common variant of B-cell precursor ALL in the 2- to 5-year age incidence peak is higher than 10%, perhaps around 15%. For infants, there is no firm estimate of concordance, but it is clearly considerably higher than that in older children. Note that infant pairs (< 1 year at diagnosis) constitute around half of the reported concordant pairs (Figure 2); this finding contrasts with their proportional representation among singleton cases diagnosed with leukemia of approximately 5%. In reviewing the published data prior to 1970, both Zuelzer and Cox25 and Keith et al27 concluded that concordance rate for infant leukemia (< 1 year) was close to 100%. We discuss this issue further in “Concordance rates in twins, latency, and the need for a second, postnatal `hit'.”
Cumulative historical record of concordant leukemia in twin children. Nk indicates subtype not known; single or 2 fused dots, twins diagnosed within 1 month of each other. Note: some reported cases with inadequate details have been omitted. First reported pair in 1882 are no. 1 in this sequence. References for this historical data set can be found at http://www.icr.ac.uk (mgreaves).
Cumulative historical record of concordant leukemia in twin children. Nk indicates subtype not known; single or 2 fused dots, twins diagnosed within 1 month of each other. Note: some reported cases with inadequate details have been omitted. First reported pair in 1882 are no. 1 in this sequence. References for this historical data set can be found at http://www.icr.ac.uk (mgreaves).
Age distribution of twins with leukemia of different subtypes. Color coding as in Figure 1.
Age distribution of twins with leukemia of different subtypes. Color coding as in Figure 1.
Calculated concordance rates for leukemia in identical twin children
Method of ascertainment . | No. pairs (infants)* . | Concordance rate, %† . | Reference . |
|---|---|---|---|
| Regional vital statistics (death certificates); 1947-1960 plus 3 clinical case series, 1954-1962 (US) | 5 (2) | 25 | MacMahon and Levy22 |
| Nationwide vital statistics survey, 1960-1967 (US) | 7 (3) | 17 | Miller24 |
| Twin database plus clinical trial databases (US/United Kingdom) | 4 (1) | 5-10‡ | Buckley et al34 |
| Cancer registry, 1958-1998 (Sweden) | 3 (0) | 15 | Hemminki and Jiang138 |
| Nationwide clinical database, 1972-1998 (The Netherlands) | 2 (0) | 26 | § |
Method of ascertainment . | No. pairs (infants)* . | Concordance rate, %† . | Reference . |
|---|---|---|---|
| Regional vital statistics (death certificates); 1947-1960 plus 3 clinical case series, 1954-1962 (US) | 5 (2) | 25 | MacMahon and Levy22 |
| Nationwide vital statistics survey, 1960-1967 (US) | 7 (3) | 17 | Miller24 |
| Twin database plus clinical trial databases (US/United Kingdom) | 4 (1) | 5-10‡ | Buckley et al34 |
| Cancer registry, 1958-1998 (Sweden) | 3 (0) | 15 | Hemminki and Jiang138 |
| Nationwide clinical database, 1972-1998 (The Netherlands) | 2 (0) | 26 | § |
Number of concordant pairs documented and used to calculate rate. In parentheses, the number of concordant pairs that were diagnosed as infants (<12 months). The differential diagnosis in these cases was ALL (in 10), AML (in 3), and acute leukemia of uncertain subtype in the rest.
The calculation of concordance rate depends on how cases are ascertained. If twins in a pair are identified independently, for example, via a cancer registry database, then the rate is considered “casewise” (as opposed to “pairwise” if detection of one case leads to identification of the other). A casewise rate in essence computes the risk of a second twin having leukemia if one twin is known to be affected.
Concordance rate varied according to whether all cases incompletely followed up were included and in relation to age cut-off point.
Unpublished data (E. van Wering and M. G., June 1999). We have calculated this casewise rate. Two pairs of twins with concordant ALL were referred to the LRF Twin Survey (see “Triplets with ALL”); these were the only concordant twins documented in the period 1972 to 1998 in the comprehensive Dutch Childhood Leukemia Study Group database in which there were 11 identical twins with discordant ALL (ie, only one twin affected).
Concordant acute leukemia in monozygotic twins: LRF Series 1984-2002.*Samples and data are referred via St Jude Children's Research Hospital, Memphis, TN (courtesy of Dr W. Crist). These 19 pairs are also included in Figures 1-2.
Only rare cases of concordant leukemia have been recorded in adults,28 and the rate is probably less than 1%. An exception to this may be in chronic lymphocytic leukemia (CLL) for which several twin pairs are recorded.29,30 If the concordance rate is indeed higher than is expected by chance, then this rate is likely to be linked to the recognized familial or inherited genetic factors in CLL.31 The adult leukemia data are, however, entirely anecdotal, and it would be worthwhile to analyze concordance more systematically as has been done for childhood acute leukemia.
The concordance rate for other hematologic malignancies is also unknown, except for Hodgkin disease in young adults in which it is approximately 5%.32 Four pairs of identical twins concordant for Langerhans cell histiocytosis (or histiocytosis X), a very rare clonal neoplastic disorder, have been reported.33
Basis of concordance: the intraplacental metastasis hypothesis
Historically, most researchers favored the conventional view that concordance in twins reflected a shared, inherited, or genetic susceptibility. There were, however, several facts that sat uneasily with this interpretation. First, the concordance rate, at least for infants, appeared to be extraordinarily high. Nothing like this rate was seen with other pediatric cancers in which, aside from the well-recognized familial, inherited component in retinoblastoma, the concordance rate is only around 2%.34 Diagnosis of leukemia in infant twin pairs was also remarkably synchronous in most cases (Figure 1). Additionally, although there were no reliable data on the frequency of concordant leukemia in dizygotic infant twins or non-twinned siblings, it appeared to be very low; not what one would expect if familial and highly penetrant leukemia-susceptible genes were involved. Several researchers speculated that a common placental environment of monozygotic twins might contribute in some way toward concordance of leukemia. In 1962, Wolman35 suggested that “the disease may have originated in one twin within the uterus and have been transmitted to the other through the conjoined circulation?” This suggestion, as it turns out, was correct, but the idea was largely ignored until it was resurrected and developed more fully in a letter to the Lancet in 1971 from Bayard Clarkson and Ed Boyse36 at the Memorial Sloan-Kettering Institute.
Clarkson, a leukemia physician, was aware of the rare but striking instances of leukemia in twins. Boyse, an immunologist, was familiar with the observations of Ray Owen37 on blood chimerism in twin cattle in which dizygotic twins share a single placenta. This situation had been exploited by Peter Medawar (Billingham et al38 ) to discover neonatal immunologic tolerance and subsequent allograft acceptance, a finding that led to Medawar, with MacFarlane Burnet, being awarded the Nobel Prize for Medicine in 1960. Clarkson and Boyse pointed out that monozygotic human twins were likely in most cases to be blood chimeras also. They, literally, have shared blood. This reasoning, in turn, suggested a very plausible, nongenetic (inheritance-based) explanation for concordance of leukemia—that it arises as a single leukemia in one twin in utero and then spreads, via intraplacental anastomoses, to the other twin.
Twin embryogenesis, placental anastomoses, and blood cell chimerism
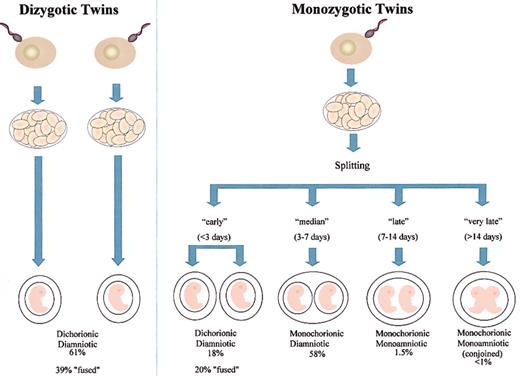
Placental status and blood chimerism in monozygotic twins depends on the timing of embryo splitting, and not all monozygotic twins share a single or monochorionic placenta39 (Figures 3-4). Embryos that split within the first 3 days after ovum fertilization develop separate, dichorionic placentas (Figure 3). Embryo splitting after day 3 but before day 7 results in separate diamniotic embryos, developing in a single monochorionic placenta (Figure 4A), and 60% of monozygotic twins are in this category. The occasional very late (14-day plus) splitting of an embryo results in conjoined (or “Siamese”) twins. Dizygotic twins, in contrast, are always dichorionic. Occasionally, however, the 2 placentae can fuse with some exchange of cells, and around 8% of dichorionic twins have blood group chimerism.40
Placental status in twin embryos. Frequency data taken from Strong and Corney.39
Placental status in twin embryos. Frequency data taken from Strong and Corney.39
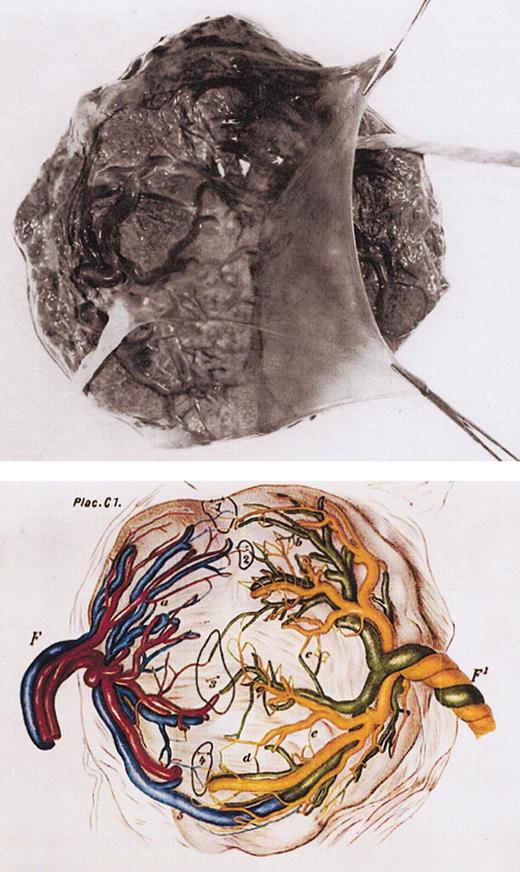
Monochorionic placenta in monozygotic twinning. Top panel: photograph of single, monochorionic placenta with dividing amnion tissue and 2 umbilical cords. Bottom panel: diagrammatic regeneration of monochorionic twin placenta with vascular anastomoses (labeled 1-5). F and F1 indicate umbilical cord of 2 twins. Taken from Strong and Corney.39
Monochorionic placenta in monozygotic twinning. Top panel: photograph of single, monochorionic placenta with dividing amnion tissue and 2 umbilical cords. Bottom panel: diagrammatic regeneration of monochorionic twin placenta with vascular anastomoses (labeled 1-5). F and F1 indicate umbilical cord of 2 twins. Taken from Strong and Corney.39
German pathologists in the 19th century discovered that a variety of vascular anastomoses exist within monochorionic placentas39 (Figure 4B). These anastomoses provide the anatomic traffic route for blood cell passage. They are also the cause of pathology as recognized in the twin-twin transfusion syndrome.41 This problem arises in some 20% of monozygotic, monochorionic twin pairs and accounts for around 15% to 20% of perinatal mortality in twins. Unequal blood distribution resulting from asymmetry of vascular anastomoses produces reciprocal polycythemia and anemia and, in some cases, cardiac, neurologic, and renal complications. The syndrome was first recorded by Herlitz42 in 1941 but is presumably inherent to twinning, a possible case being recorded in much earlier artistic representation (Figure 5).43
Artistic representation of newborn infant twins with probable twin-twin transfusion syndrome. Painting from 1617 titled De Wikkelkinderen (the swaddled children) from the Muiderslot castle near Amsterdam. Artist is unknown, but the infants are believed to be the infant male offspring of Jacob Dirkszoon de Graeff, mayor of Amsterdam. It is thought that the twins died shortly after birth. Taken from Berger et al.43
Artistic representation of newborn infant twins with probable twin-twin transfusion syndrome. Painting from 1617 titled De Wikkelkinderen (the swaddled children) from the Muiderslot castle near Amsterdam. Artist is unknown, but the infants are believed to be the infant male offspring of Jacob Dirkszoon de Graeff, mayor of Amsterdam. It is thought that the twins died shortly after birth. Taken from Berger et al.43
The intraplacental transfer hypothesis, therefore, had a sound anatomical basis, but it was nevertheless radical, implicating as it did a prenatal clonal origin in one twin followed by what was in effect a metastasis to another individual. Clarkson and Boyse36 suggested that this prenatal, monoclonal explanation might be proven by a demonstration of shared, nonconstitutive, cytogenetic abnormalities in leukemic cells from pairs of twins.
Molecular evidence for a monoclonal, prenatal origin of leukemia in twins
Both before and following the Clarkson-Boyse letter, several papers appeared reporting karyotyping investigations on single pairs of identical twins with acute leukemia.44-49 Some of these investigations recorded a sharing of a chromosome marker, compatible with a single cell or clonal origin. These data were, however, equivocal; the quality of cytogenetic studies at the time was less than ideal, and it was not generally appreciated that consistent chromosome markers are shared by entirely independent leukemias of the same subtype. Clearly, cytogenetic evidence for the single-clone idea would only be strongly supportive if the markers were very uncommon and/or if they were complex. One more recent cytogenetic report on concordant AML in monozygotic twins (diagnosed at age 3 and 4 years) found that the leukemic cells did share the karyotype +8, inv16, +21.50 In this case, it could well be that this pattern of chromosome abnormalities developed rapidly in one twin, in utero. Further in this section, we describe a recent twin pair in whom the leukemic cells shared a remarkable pattern of chromosomal instability indicative of a prenatal clonal origin.
Unambiguous evidence for a common clonality can be derived from molecular markers of clonal uniqueness. There are several candidate markers in this respect, although each has limitations (Table 2). A significant finding in this direction was provided by a short report that a pair of infant twin's leukemic cells appeared to share the same or similar IGH gene rearrangement, that is, same-sized restriction fragment in a Southern blot.51 This evidence was limited, however, by the fact that only one restriction enzyme was used, and, furthermore, the twins were conjoined with shared vascular connections after birth as well as before. IGH and TCR can provide supportive evidence for a common clonal origin of twin leukemias (below), but unequivocal evidence comes from the use, as clonal markers, of leukemia fusion genes generated by chromosome translocation.
Possible markers of monoclonality in twin leukemia
Marker . | Limitations . |
|---|---|
| Chromosome markers | Some markers common in independent leukemias |
| Marker may be secondary or late event | |
| IGH/TCR VD(N)J clonal rearrangements | Initial leukemic cell transformation may precede rearrangements |
| Rearrangements may not be stable | |
| X-linked allele expression in female pairs | Can be concordant for allele expression by chance (50%), but discordance of allele expression is strong evidence against monoclonality |
| “Oncogene”-specific sequence (eg, fusion gene) | May not be initiating or early event |
Marker . | Limitations . |
|---|---|
| Chromosome markers | Some markers common in independent leukemias |
| Marker may be secondary or late event | |
| IGH/TCR VD(N)J clonal rearrangements | Initial leukemic cell transformation may precede rearrangements |
| Rearrangements may not be stable | |
| X-linked allele expression in female pairs | Can be concordant for allele expression by chance (50%), but discordance of allele expression is strong evidence against monoclonality |
| “Oncogene”-specific sequence (eg, fusion gene) | May not be initiating or early event |
Chimeric fusion genes are formed by normal, error-prone repair of DNA double-strand breaks.52,53 The critical breaks occur in clustered intronic regions. The boundaries of the breakpoint regions are circumscribed by functional requirements of the resultant hybrid protein, and the breakpoint cluster region itself can vary from a few base pairs to 100 kilobase (kb) or more in length, depending on the gene. Within these breakpoint regions of the paired genes, the breaks can be widely scattered, although not entirely randomly and with significant microclustering.54 Critically, each patient's leukemic cells have, in each partner gene and in the resultant fusion gene junction, a unique or clonotypic genomic breakpoint and fusion sequence (Figure 6).52-54 This sequence then provides a highly specific and stable marker of the leukemic clone and a stringent test for the clonal relationship of leukemic populations in a pair of twins. If the leukemic cells of twins were found to share the same acquired clonotypic breakpoint, then this would indicate a monoclonal origin of their leukemia. An important caveat is, however, that clonotypic fusion genes are only ideal markers in this context if they are very early or initiating events in leukemogenesis. If they were postnatal, secondary events, then they would have different breakpoints regardless of the clonal origins of the leukemias.
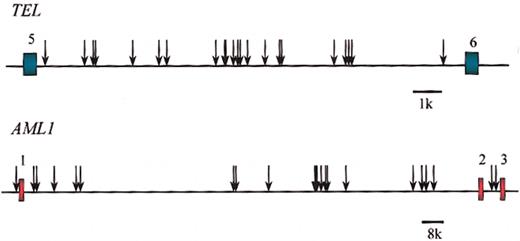
Clonotypic genomic breakpoints in TEL andAML1. Breakpoints in unrelated pediatric patients with ALL. Each arrow is a sequenced breakpoint of individual leukemia. Exons of TEL (in green) and AML1 (in red) are shown. Data are from Wiemels et al.54
Clonotypic genomic breakpoints in TEL andAML1. Breakpoints in unrelated pediatric patients with ALL. Each arrow is a sequenced breakpoint of individual leukemia. Exons of TEL (in green) and AML1 (in red) are shown. Data are from Wiemels et al.54
The most common chromosome translocation in infant leukemia results in the fusion of the MLL gene at 11q23 with a variety of partner genes, but principally AF4 in ALL.55 Following the cloning of the MLL gene, it was shown that MLL gene breakpoints, as indicated by restriction site mapping, were scattered throughout an 8.3-kb breakpoint region (introns between exons 5 to 11) and individualistic.56,57 Taking advantage of these data, Ford et al58 showed that, in 3 pairs of identical twin infants with concordant ALL, each pair shared the same MLL gene rearrangements as indicated by identical-sized restriction fragments (in Southern blots) of MLL with multiple enzyme digests. The rearrangements were, as anticipated, absent from nonblood cells and were also absent from remission samples and were, therefore, acquired and not constitutive. Subsequently, 2 additional infant twin pairs with shared, identical MLL fusions were recorded.59,60
Clarkson and Boyse36 regarded the very short latency and near synchronous diagnosis of leukemia in identical twin infants as the plausible outcome of in utero leukemogenesis. However, they speculated that leukemia in noninfant children would most likely be postnatal in origin. This idea appears to have embraced the (false) premise that leukemogenesis is a single hit phenomenon with short latency but also begs the question of what might underlie cases of concordant ALL in older children.
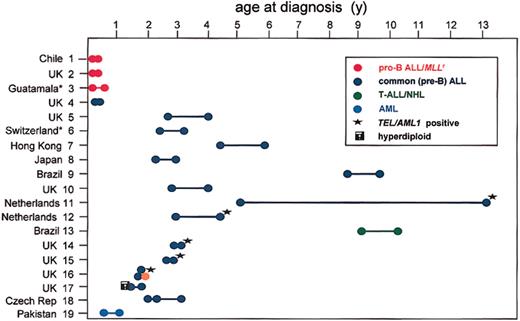
The studies of Ford et al58 of infant twins were part of a concerted attempt to identify twin pairs via a worldwide collaborative survey to resolve this issue of clonal origins. The prior hypothesis here was that the common (c) form of childhood ALL might involve a minimum of 2 independent genetic hits, one before and one after birth.61 Initiated in the 1980s, the Leukaemia Research Fund (LRF) twin survey (“Appendix”) has, to date, collected 19 pairs of concordant ALL (18 pairs) or AML (1 pair), and we have informative molecular data for clonality status on 11 of these. For all these pairs for which we have molecular data on clonality, monozygosity was confirmed using microsatellite markers.62 The subtypes and ages of these cases are represented in Figure 7. Of these 19 pairs, 3 had MLL gene fusions (the pro-B ALL infant cases nos. 1-3, in red in Figure 7). Fourteen pairs had a common B-cell precursor (CD10+) ALL, and, of these, 5 (nos. 11, 12, 14, 15, and 16; Figure 7) had TEL-AML1 fusion. Only one had a hyperdiploid karyotype (no. 17; Figure 7). In the remaining 8 cALL pairs, the chromosome karyotype was nonascertained or “normal” except for pair no. 5. In this pair, there was a discordant karyotype: 46XY, 2p- in one twin; 46XY, t(3;16)(p13;p13), 12p+, ?del (14q) in the other.
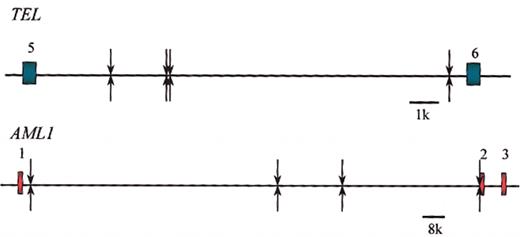
The most common chromosome translocation in ALL is the t(12;21), resulting in TEL(ETV6)-AML1(RUNX1) fusion.63,64 Some 25% of ALL have this marker,65 and the age distribution of positive cases at diagnosis mirrors that of the major 2- to 5-year incidence peak of ALL.66 Of those twin pairs in the LRF series, 5 had a TEL-AML1 fusion (marked * in Figure 7). Four of these have been molecularly characterized by cloning67 or long-distance polymerase chain reaction (PCR) methods and sequencing.68-70 The key observation was that within each of the 4 pairs of twins the same, identical, or clonotypic TEL and AML1 breakpoints were present (Figure 8). Pair no. 11 had a very asynchronous diagnosis, age 5 and 14 years. In the latter twin, it was possible to demonstrate the presence of the clonotypic TEL-AML1 fusion sequence in an archived bone marrow slide taken 9 years before diagnosis (and considered at the time to be hematologically normal) when her twin sister was diagnosed.69 This observation was important as it suggested that during a protracted period of postnatal latency, the leukemia or preleukemia was widely disseminated. The first of these TEL-AML1-positive twin pairs to be reported (no. 12; Figure 7) also shared a clonotypic IGH DN-J sequence.67
Shared clonotypic TEL and AML1 breakpoints in leukemias from identical twin pairs. Arrows above and below line for TEL and AML1 intron are leukemic pairs. Data from Ford et al,67 Maia et al,68 and Wiemels et al.69,70
A common clonal origin was also documented for a pair of twins with T-cell malignancy at age 9 and 11 years (pair no. 13 in Figure 7). In this case, the malignant blood cells shared a common, clonal T-cell receptor-β (TCRβ) DJ sequence, including an 11-base pair N region.71 Interestingly, this pair had divergent clinical diagnoses, one as T-ALL, the other as T-cell non-Hodgkin lymphoma (T-NHL). This case perhaps then illuminates the longstanding suspicion that these 2 disorders in children are variable presentations of the same underlying thymic transformation.
Somewhat surprisingly, only one pair of twins in the LRF series (pair no. 17 in Figure 7) was known to have the most common genetic abnormality in childhood ALL—hyperdiploidy. Triploidy of the same chromosomes, as in this pair, could not be taken, by itself, as firm evidence for a common clonality, but this pair shared unique TCRδ DN-J and IGH DN-J sequences, indicating that, in this case also, the leukemia was probably initiated prenatally.72 Interestingly, their IGH gene rearrangements were clonally divergent at the VDJ level, indicative of continued postnatal rearrangement involving distinctive V region additions or replacements.72
In 7 of the “early” pairs of cALL (Figure 7), we had no informative molecular data on clonality and limited availability of DNA. The IGH rearrangements all appeared to be different within pairs in Southern blot and PCR analysis, but, in the absence of sequencing, this cannot be considered as evidence against a common clonal origin.
The children in pair no. 19 were infants with AML. Their leukemic cells had an extraordinarily diverse clonal and intraclonal karyotype.73 Detailed analysis by fluorescence in situ hybridization (FISH) revealed that a stem line with alterations in 6 chromosomes (with deletions, duplications, and an insertion) was common to the twins' leukemic blasts, but the further and extensive subclonal chromosomal abnormalities were distinct. In this case, chromosome instability generated a rapid evolution of intraclonal diversity leading to leukemogenesis, and the twin comparison provided a distinction between the initiating, prenatal events and subsequent postnatal genetic divergence of the clones.
Triplets with ALL
In this LRF twin series, there were 2 sets of triplets with ALL (pairs no. 16 and no. 18 in Figure 7), and these triplets were particularly informative. In pair no. 16, 2 monozygotic twins that had shared a single placenta developed ALL, with a shared TEL-AML1 fusion but distinctive secondary genetic changes (see “Concordance rates in twins, latency, and the need for a second postnatal `hit'”).68 The third co-twin was dizygotic, developed in a separate placenta, and remains leukemia free by molecular screening. In set no. 18, all 3 twins were monozygotic (we assume a fourth twin was lost in utero), and all 3 developed ALL. No TEL-AML gene was present in these patients, but their leukemic cells shared clonotypic IGH sequences.74
These data suggest that both monozygosity and placental status may be critical for risk of concordant leukemia. All the patients in the LRF series (Figure 7) with known placental status (17 of 19) had a single placenta. One concordant pair of monozygotic infant twins with ALL was reported to have had independent (dichorionic) placentae in utero.59 As around 8% of dizygotic, dichorionic twins have blood cell chimerism,40 possibly based on occasional placental fusion, and something similar may have occurred in this pair.
Overall, these data have provided compelling evidence that concordant leukemia at any age in identical twin children is due to a clonal origin in utero. An important corollary is that postnatal latency following prenatal initiation can be very variable and occasionally protracted (ie, up to 14 years).
Twins versus singletons with leukemia
Concordant infant ALL or concordant childhood common, B-cell precursor ALL in identical twins is no different biologically, clinically, or in its age incidence (Figure 2) from ALL in singletons, and there is no basis for considering that leukemia might be uniquely initiated in utero only in the context of twinning. Therefore, the conclusion from the twin studies that leukemia can originate prenatally must apply more generally to pediatric patients. The crucial question, however, is how often is this likely to be the case? A prenatal origin of infant ALL is not surprising, given the very young age of patients (average = about 6 months); indeed some cases of 11q23/MLL fusion gene-positive cases are diagnosed neonatally75,76 or, in one case, in a stillborn fetus.77 Moreover, because monozygotic infant twins discordant for leukemia appear to be extremely rare, this finding suggests that, when an infant who happens to be a twin has ALL, it will inevitably have been of prenatal origin. This suggestion should then apply also to leukemia in singleton infants.
The same logic does not apply to leukemia in older children. If we accept that the concordance rate for common B-cell precursor ALL in noninfant children in the 2- to 5-year age incidence range is, say, 10%, then it follows that there is 90% discordance. This discordance could arise via 2 different mechanisms. One possibility, favored by Clarkson and Boyse,36 is that most of leukemias in twin children (> 1 year), as well as in singleton children, are initiated postnatally, setting a lower limit of approximately 10% on the cases in twins that can be concordant. Alternatively, and as some earlier commentators on twin leukemia suggested,22,78 leukemia in non-twin children might be initiated in utero but requires an essential postnatal exposure and/or genetic (or stochastic) event for which discordance was the norm in twins. The latter would imply that childhood cALL has a somewhat different biology and natural history than infant ALL, but this is already evident from molecular genetics and from the twin data with the highly variable postnatal latency and asynchronous diagnostic dates.
One test that would distinguish these alternatives would be to demonstrate that, in cases of discordant identical twin children with ALL, the healthy co-twin usually does have the clonotypic TEL-AML1 fusion gene in his or her blood, despite the absence of overt disease. This idea, to our knowledge, has not been examined; our attempts to do so, to date, have been thwarted by logistical and ethical difficulties. The significance of the modest concordance rate of ALL in twin children has, however, been resolved by a different tactic.
Backtracking leukemia fusion gene sequences to birth
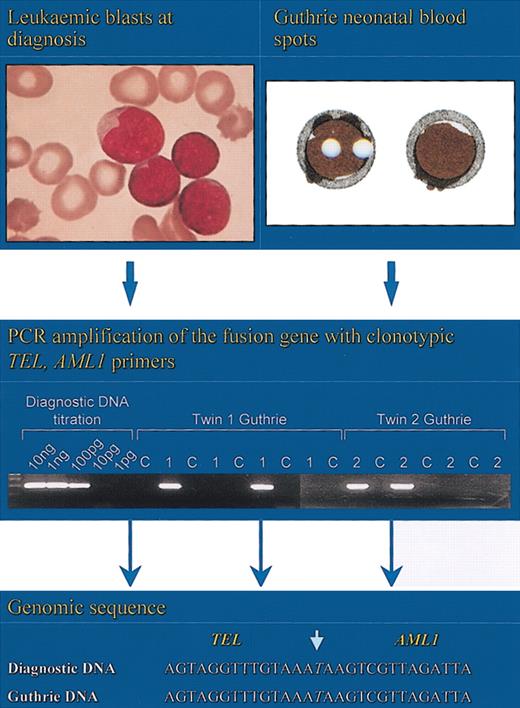
For the twin leukemia interpretation to be correct, then the clonal progeny of the transformed fetal “preleukemic” cells, have to be present in circulating blood before birth. If this is the case, then it should be possible to identify them directly. This identification has now been achieved by retrospective scrutiny of archived neonatal blood spots or Guthrie cards. These cards have been used for many years for genetic screening for inborn errors of metabolism such as phenylketonuria (PKU).79 They are also a source of reasonably intact constitutive DNA that can be amplified by PCR to reveal inherited mutations80 or exogenous viral sequences.81 Neonatal blood is usually taken during the first week of life by heel prick, and each spot is approximately 30 μL in volume with approximately 30 000 mononuclear cells. Therefore, if leukemic or preleukemic cells with a unique fusion gene sequence were to be present in at least 1 cell per 30 000 in blood, then they might be detectable by sensitive PCR methods with clonotypic primers specifically designed for each patient's leukemic cells. We first showed that this was possible using MLL-AF4 genomic sequences in 3 cases of non-twinned ALL in patients aged 2 months, 6 months, and 24 months.82 Encouraged by this result, we next examined the neonatal blood spots of a pair of twins diagnosed at age 3 years with ALL who shared an identical TEL-AML1 sequence (no. 14; Figure 7). Four segments of one blood spot for each twin was examined,70 and 2 of 4 blood spots for each patient registered a TEL-AML1 signal verified by sequencing (Figure 9).
Detection of clonotypic genomic fusion sequences of TEL-AML1 in the neonatal blood spots (Guthrie cards) of twins.
Detection of clonotypic genomic fusion sequences of TEL-AML1 in the neonatal blood spots (Guthrie cards) of twins.
Analysis of Guthrie cards of non-twinned children (aged 2-6 years) with ALL showed that most did have detectable, clonotypic TEL-AML1 sequences at birth.70 Some blood spots were negative, but this result is uninterpretable: It could mean that these cases were postnatal in origin, but, equally, it could indicate that in some prenatal cases, there are less than 1 in 30 000 leukemia cells in the blood. We regard the latter as a likely explanation in at least some “negative” cases, particularly, as in positive cases, the number of leukemic cells per spot is clearly low with some negative segments of spots. These data have now been backed up with the detection of clonotypic IGH sequences in blood spots of children with ALL, including cases of ALL that have the common hyperdiploid karyotype.83-85 In one case, there was evidence that triploidy of chromosome 14 (with 3 unique rearranged IGH alleles) was present in the blood spot.86 In a recent study, 50% of AML cases investigated had clonotypic AML1-ETO fusion sequences in their neonatal blood spots, including 2 children who were older than 10 years at diagnosis.87 It might have been anticipated that not all subtypes of pediatric leukemias would involve prenatal initiation, and this appears to be the case. Few if any cases of pre-B ALL with t(1;19) E2A-PBX1 have positive neonatal blood spots,88 and no pairs of identical twins concordant for that subtype of ALL have been described.
These data have 3 important implications: first, they provide direct confirmation of the prenatal interpretation for the concordant leukemias in twins. Second, they verify that this early developmental origin in fetal hemopoiesis applies to most, although probably not all, pediatric leukemias. And third, they indicate that discordance of ALL in older children can be ascribed in large measure, if not entirely, to the requirement for one or more additional postnatal events following in utero initiation.
Guthrie cards have proven to be an extraordinarily rich resource for leukemia research. It is, therefore, somewhat ironic that when Bob Guthrie was developing his blood spot technique (for PKU screening) in the 1950s, he was asked to stop working on it because of its lack of any relevance to leukemia (D. Pinkel, personal communication to M.F.G., October 2002).
Concordance rates in twins, latency, and the need for a second, postnatal “hit”
The concordance rates of leukemia in twins have important implications for our understanding of leukemogenesis in general. A very high concordance rate for infants implies that, by the time that the twins are born, the process of leukemogenesis is sufficiently complete that a clinical diagnosis is inevitable. This might signify that an MLL fusion gene is, in itself (in the appropriate stem cell type), sufficient for overt leukemia development. The corollary would be that MLL fusion genes have a powerful deregulatory effect on gene expression.89 This effect might be achieved by MLL fusion proteins disrupting gene transcription in a broad fashion, say by simultaneously corrupting 2 or more signal pathways in cells. An alternative and perhaps more plausible possibility is that, once formed, an MLL fusion protein somehow promotes the inevitable accumulation of additional genetic changes via, for example, very rapid clonal expansion, genetic instability, or inhibition of DNA damage repair. Either way, initiation of leukemogenesis via MLL gene fusion appears to be tightly coupled with rapid transition to full-blown disease in patients. The latency of secondary leukemias with MLL gene fusions associated with prior exposure to topo-II inhibiting drugs is similarly very short, averaging 26 months.90 Animal models might be expected to clarify the crucial issue of exceedingly brief latency with MLL fusions, but they have not so far done so. Acute myeloid leukemias have been generated both by retroviral transfer of MLL-ENL to hemopoietic progenitor cells91,92 and via MLL-AF9 knock-in.93 However, in neither case is the latency markedly brief, suggesting that a second, independent hit might be required. In accord with this view, MLL-ENL and MLL-AF9 cooperate with a weak oncogenic tyrosine kinase (v-SEA) to produce leukemia in chickens.94 Several genetic abnormalities have been detected, in addition to MLL fusions, at diagnosis, including p53,95 RAS,96 FLT3.97 But these data still beg the question of why, if these additional mutations are required, latency is so brief in infant and secondary leukemias with MLL fusions. One possibility is that MLL gene fusions render cells susceptible to further genetic damage and that this consequence is highly prevalent in de novo infant and secondary leukemias as a result of chronic exposure to genotoxic chemicals that induce the MLL fusion itself. The latter etiologic component is missing from the animal studies. This potential explanation is being assessed currently in model systems.
The concordance rate for those 60% of infant twin pairs with a monochorionic placenta may well be 100%. Although discordant cases are unlikely to be reported, we are aware of only 3 (unpublished) pairs of monozygotic twins worldwide who are discordant for leukemia with an MLL fusion gene. In one pair from the United Kingdom in which one twin had ALL with MLL-AF19, the placenta was dichorionic, which probably restrained the leukemic cells to one twin. In a second pair, from New Zealand, the placenta was monochorionic, but the leukemic child was 5 years old at diagnosis with an MLL-AF10-positive AML. It is likely that in this case, initiation was postnatal (“Implications for healthy co-twins of leukemic patients”). In a third case of infant twins, from Japan, in which one had an MLL-AF4-positive ALL, the placental status was unknown. We suspect, therefore, that rare cases of discordant MLL fusion gene-positive leukemia in twins arise either because of a dichorionic placenta or because of a postnatal initiation.
The situation is clearly different for older twin children with ALL. A discordance of 90% is generally regarded in twin studies of disease as indicating the requirement of a second postnatal exposure or other event. Twin discordance of leukemia can now be interpreted along these same lines in the light of the finding that most cases of ALL, at least in the subset with TEL-AML1, originate prenatally. This interpretation suggests that TEL-AML1 initiates leukemogenesis but is insufficient for overt disease, further genetic alterations being required. This interpretation is endorsed by the finding that TEL-AML1 is not overtly transforming in in vitro models98 and, as an in vivo transgene selectively expressed in the B-cell lineage, does not produce leukemia (A.M.F., C.A. Bennett, and M.F.G., unpublished observations, June 2003). The variable and occasionally very protracted latency of ALL in the concordant twin pairs also accords with this interpretation. Animal models with TEL-AML199 and AML1-ETO100,101 indicate, however, that expression of these genes does lead to the development of leukemia in the presence of cooperating mutations.
Some, albeit incomplete, insight into the nature of secondary, postnatal genetic events in childhood ALL is now available. Although diverse additional chromosomal abnormalities have been described in cases of ALL with TEL-AML1, by far the most frequent are deletions on chromosome 12p.102 The deletions vary in size, and, although they can encompass loss of up to 10 megabases of DNA, the minimally deleted region includes the normal (unrearranged) TEL allele with occasional small intragenic deletions of TEL.103 The frequency of TEL deletion in ALL cases with TEL-AML1 fusion may depend on the methodology used but appears to be very high (about 65%-80%). Although most cases of ALL with TEL-AML1 clearly do have a loss of normal TEL, the functional significance of this is unclear. As TEL proteins self-dimerize, it could be that the presence of normal TEL protein dimerizing with TEL-AML1 tends to quench its inhibitory function.104 Alternatively, TEL may function as a conventional suppressor gene whose loss has selective (clonal) advantage in the presence of TEL-AML1.105,106 Either way, there appears to be strong selective pressure favoring 12p deletion, including the normal TEL allele.
There is compelling evidence in both singleton patients and in twins with ALL that TEL deletion is secondary to TEL-AML1 fusion. In FISH analysis of leukemic cells from non-twin patients, TEL deletions appear to be subclonal (ie, a variable minority of cells in any one patient with TEL-AML1 still retain the normal TEL allele).107 Analysis of TEL deletions in patients with ALL and TEL-AML1 at diagnosis versus relapse with both microsatellite markers and FISH show that a TEL deletion at diagnosis can be “resurrected” at relapse or be present in relapse with an apparently decreased size of its deletion boundaries.108 These alterations in genotype are only possible if TEL deletion is a secondary or later event in leukemogenesis. In a pair of identical twins (no. 16 in Figure 7) with ALL and a shared, clonotypic TEL-AML1 fusion gene, the TEL deletion was subclonal by FISH (Figure 10) and with different 3′ genomic boundaries in leukemic cells of the 2 twins.68 These data are compatible with the notion that, in this twin pair, TEL deletions arose as secondary, clonally independent, and postnatal events. As AML1 gene fusions appear to primarily impede cell differentiation, it has been proposed that leukemogenesis requires a second complementary genetic change involving alterations in genes that provide survival or proliferative signals, eg, an activated kinase.109 TEL deletion may satisfy this requirement; alternatively another, cryptic alteration may be involved.
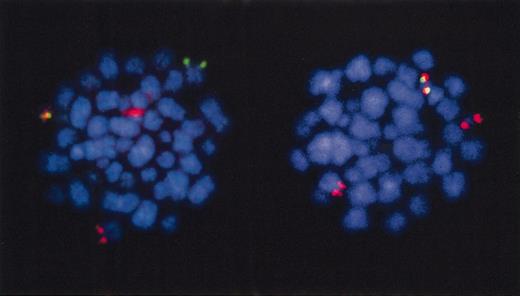
Fluorescence in situ hybridization to TEL and AML1 in leukemia from a twin patient.TEL deletion is subclonal. Red indicates AML1 probe; green, TEL probe. Metaphase cells have red plus green (=yellow), TEL-AML1 fusion signal. The cell on the left retains normal TEL allele (green signals). The cell on the right has lost normal TEL allele. Patient is twin in triplet set no. 16, data in Wiemels et al.68 FISH picture courtesy of Drs C Harrison and G Jalali (University of Southampton, United Kingdom).
Fluorescence in situ hybridization to TEL and AML1 in leukemia from a twin patient.TEL deletion is subclonal. Red indicates AML1 probe; green, TEL probe. Metaphase cells have red plus green (=yellow), TEL-AML1 fusion signal. The cell on the left retains normal TEL allele (green signals). The cell on the right has lost normal TEL allele. Patient is twin in triplet set no. 16, data in Wiemels et al.68 FISH picture courtesy of Drs C Harrison and G Jalali (University of Southampton, United Kingdom).
Collectively, these twin data, endorsed by studies on leukemic cells of singletons with ALL, have provided unique insights into the differing natural histories of pediatric leukemia subtypes. Figure 11 illustrates the models that have emerged and from which several important biologic and clinical implications follow.
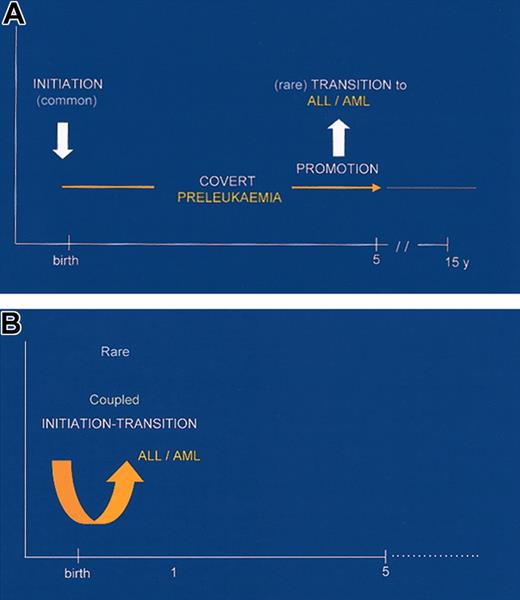
Minimal models for the natural histories of infant and childhood leukemias.
Silent preleukemia in newborns?
The minimal 2-step, prenatal-postnatal model for the natural history of childhood leukemia61 is strongly endorsed by these twin studies and parallels 2-step model of Knudson110 for noninherited pediatric solid tumors. In the leukemia case, we now have a prenatal-postnatal spacing of the 2 oncogenic events and a preferential order of particular chromosomal abnormalities. We can take the model one step further. The combined twin and Guthrie card data allow us to assume that in most cases in which a leukemic clone is initiated in utero by, say, TEL-AML1 fusions that the required postnatal second hit is not acquired; in the twin context, this “failure” would be at the approximate 90% level. From this assumption follows an important prediction: that for every child with ALL with TEL-AML1 (or other early genetic marker), there should be many more healthy children with clinically covert “fetal” preleukemic clones expanded, with a functional TEL-AML1 gene but still minus the critical TEL deletion and/or other essential secondary genetic event(s). The twin discordance rates imply that these healthy individuals should outnumber the leukemic cases by approximately 10:1, but it could be considerably more than this, given that the twins are genetically identical and probably share the same environment, exposures, immune response, and so forth.
This possibility can be assessed by the systematic screening of a large cohort of unselected neonatal cord blood samples for the presence of functional leukemia fusion genes. The way this experiment is designed, technically, is crucial, particularly as sensitive reverse transcriptase (RT)-PCR methods using generic primers, prone to contamination, are used. Also, inappropriate screens may detect low-level nonfunctional gene fusion products.111-113 For a leukemia gene to be regarded as functional and associated with a putative preleukemic clone requires that the following criteria are met: (1) that the fusion is invariably bona fide, with an in-frame fusion sequence; (2) that the positive cell population level is indicative of clonal expansion, say more than 10-5; and (3) that the fusion gene arises in an appropriate stem cell type for the leukemia with which it is normally associated and is demonstrably present, by FISH, in the corresponding, lineage-marked progeny of that cell.
These conditions have been applied in a recent survey of around 600 cases of neonatal cord blood.111 The striking result was 1% of cord blood samples (6 of 597) had a functional TEL-AML1 gene in B lineage cells. The latter included both CD10+ B precursors and λ or κ+ mature B cells, and, significantly, all retained the normal TEL allele. The fusion gene-positive cell population size was calculated to be present in cord blood at the level of 10-3 to 10-4. One cord blood sample (of 497 tested) had an AML1-ETO fusion gene in 10-4 cells. The cumulative incidence or risk of childhood leukemia is 1 in 2000, and the risk of ALL with TEL-AML1 is approximately 1 in 10 000. Therefore, these cord blood screening data suggest that childhood ALL (or at least the subset with TEL-AML1) is initiated prenatally at a rate that is 100 times that of overt, clinically diagnosed ALL. The same most probably applies to AML with AML1-ETO.
Could the same apply to MLL gene fusions in healthy fetal hemopoiesis? The available data are somewhat conflicting. Uckun et al114 first reported that some 25% of healthy newborns had MLL-AF4 fusions detectable by RT-PCR. We115 and others116,117 subsequently were unable to confirm that finding in a total of approximately 300 cord blood samples within which no single one was positive. In a larger sample size, or with greater sensitivity, one might expect to find nonfunctional MLL gene fusions, such as out of frame or in the incorrect cell type with no clonal expansion, for example. One might have to screen a very large number, approximating to the actual incidence rate of the disease (ie, 1 in approximately 60 000), to find a case with a functional MLL-AF4 fusion. However, if infant ALL cases do require an independent second hit, then clearly bona fide positives might be more frequent—perhaps 100 times the incidence rate, or 1 in 600.
Time windows of “exposure” to etiologic events
One clear implication of the twin data and the resultant models of the natural history of pediatric leukemia is that the critical time periods can be identified that delineate windows of opportunity for critical exposures or events that underlie the etiology of disease.118
For infant ALL, the twin data argue that all relevant exposures are likely to be in utero during pregnancy (ie, transplacental exposures of mother and fetus). MLL gene fusions have been associated, in secondary leukemias, with prior chemotherapeutic exposure to topoisomerase II (topo II)-inhibiting epidophyllotoxins and anthracycline reviewed in Felix.119 MLL gene rearrangement is a very early consequence of such exposure.60 With this association in mind, it was suggested that similar exposures during pregnancy might be responsible for infant leukemias with MLL gene fusions.58,120 Candidates would be natural or medicinal topo II inhibitors, including flavonoid substances. Some evidence for this suggestion is now available. First, the MLL gene has a functional topo II binding site close to exon 9 in proximity to breakpoints in secondary and infant leukemia.121,122 Exposure of cells in vitro to known topo II inhibitors, including bioflavonoids, can produce MLL gene rearrangements.123 Genetic evidence supports this link, at least for MLL-AF4. Vulnerability to infant leukemia, and particularly infant ALL with MLL-AF4, has been associated with inheritance of low function NQ01 alleles.124,125 NQ01 encodes NAD(P)H:quinone oxidoreductase, which detoxifies genotoxic benzene metabolites and chemicals with quinone rings, including flavonoids. But as NQ01 also exercises more general antioxidant functions and stabilizes p53, interpretation of the NQ01 allele linkage is not entirely straightforward.
Epidemiologic, case-control studies of infant leukemia have also focused on the pregnancy period. Such studies are difficult to design and execute successfully, and any positive associations require replication. Two positive links have been recorded. One was paradoxical perhaps: excess intake during pregnancy of fruit, possibly reflecting flavonoid excess.126 A second, international case-control study found a significant and selective association with infant leukemias (< 12 months) that had MLL fusion genes: either with pregnancy exposure to pesticides, and in particular propoxur (Baygon), or with consumption of the drug dipyrone, known colloquially as “Mexican aspirin.”127 The latter is known to cause myelotoxicity, is proscribed in the United States and the United Kingdom, and has been previously linked, in Brazil, with pediatric Wilm tumors.128 These studies are ongoing.
For older children with TEL-AML1-positive or hyperdiploid ALL, epidemiologic evidence currently favors a causal role for an abnormal response to infection.129-132 These data indicate that absence of exposure to common infections in the first year of life is a risk factor for common ALL. The rationale offered is that in the absence of this important, infection-driven modulation of the naive immune network in infants, subsequent infectious exposures may result in highly dysregulated responses in susceptible individuals.128 These responses, in turn, could impose proliferative and/or apoptotic stress to the “preleukemic” bone marrow61,129 (ie, “delayed” infection is providing the promotional effect and second or postnatal hit).118 Larger case-control epidemiologic studies are assessing further this hypothesis,66 incorporating also screens for genetic susceptibility alleles within the immune system.133
This leaves open the crucial question of what might be responsible for what now appears to be a high rate of leukemia initiation via chromosome translocation in utero. There is no association of common ALL in children to NQ01 alleles124 and no epidemiologic evidence to date clearly indicting genotoxic exposures during pregnancy for this major subtype of leukemia. We have earlier suggested that prenatal initiation of ALL might arise as a developmental error via endogenous oxidative stress61 (ie, in the absence of exogenous DNA-damaging exposures), and we still regard this as a plausible hypothesis. Recent data do indicate, however, that the risk of leukemia initiation in utero can be modified by both genetic and dietary factors. A report from Australia,134 which requires confirmation, provided striking evidence that folate supplementation during pregnancy protects offspring from ALL. Genetic studies have found that low-function methyltetrahydrofolate reductase (MTHFR) alleles are associated with reduced risk (odds ratios) for infant (MLL fusion gene-positive) and childhood ALL.135 These associations are plausible, as it is well established that deficient intracellular folate leads to uracil incorporation and excision from DNA with consequent DNA breaks.136 It is possible, therefore, that the risk of genetic and chromosome damage of any kind in active stem cells during fetal hemopoiesis can be modified by genetic and dietary factors in the folate pathway.
Implications for healthy co-twins of leukemic patients
We return finally to the issue of twins themselves and their risk assessment for leukemia. Although twins may present simultaneously with clinical symptoms of leukemia leading to a diagnosis and instigation of treatment, this is not always the case. Even when pairs are recorded with near synchronous diagnosis dates, as in Figure 1, the detailed medical history reveals a more complex story.137 What happens not infrequently is that one twin of a pair has clinical symptoms and is diagnosed with leukemia, but at that time the co-twin is apparently healthy. But because of the known risk to a co-twin, the healthy twin is investigated and followed up. Hematologic and molecular evidence for incipient leukemia is then discovered, and treatment commenced. These observations are partly anecdotal, but they bring into focus the issue of what investigations should be undertaken and what advice should be given to parents regarding the healthy co-twin of a patient with leukemia. First, the parents should be counseled with respect to risk. Although the risk estimates available are not very accurate, they can be regarded as a reasonable guide, approaching 100%, for infants and a risk of the order of 1 in 10 for older children. These calculated risk estimates apply only to monozygotic, identical twins, and it is likely that they apply only to twins with a single monochorionic placenta. The rate of concordance in identical twins with a double or dichorionic placenta is, unfortunately, unknown. We suspect that in identical twins with dichorionic placentas the risk of leukemia in a second co-twin, whether an infant or child, is substantially reduced, although probably still higher than the roughly doubled risk of non-twinned siblings. With increasing age of diagnosis in the first twin, the risk of leukemia in the co-twin is also likely to decline in parallel with the general decrease in age-associated incidence. These calculated risk levels are still substantial, and, therefore, clinical surveillance of the healthy co-twin of a leukemic patient throughout childhood may be justified. Whether the healthy co-twin should have regular hematologic and molecular investigations is a more difficult issue. We have conducted such monitoring assays, in one case following a healthy co-twin for 7 years using hematologic, immunologic, and molecular (clone-specific) markers. The justification we give for this is, first, that if the results are unambiguously negative, then this offers reassurance to the parents. Second, if a positive result is obtained and confirmed, then treatment could be commenced before white counts become highly elevated and the disease more advanced in its genetic complexity and drug resistance. Whether, in practice, this treatment would offer any benefit in improved outcome is difficult to substantiate, but we have recorded anecdotal evidence of such “early,” preclinical diagnosis in several infants whose subsequent long-term survival contrasts with that of their overtly leukemic co-twin.137
Molecular scrutiny of the blood of the healthy co-twin is particularly relevant if that individual is being considered as a bone marrow donor for a twin with leukemia. Recently, we had such an opportunity when the healthy co-twin of a 6-year-old child with MLL-AF10-positive AML was considered as a transplant donor (A.T.M., J. Cochrane, R. Corbett, M.G., unpublished observations, March 2003). We found that the unique genomic fusion sequence of the leukemic cells was undetectable in the healthy donor, and the transplantation was subsequently carried out successfully. We also found that the neonatal blood spot of the leukemic patient was negative for the fusion sequence. We concluded, therefore, that in this particular pair, who did share a single placenta, that MLL gene fusion probably occurred postnatally in one twin only.
Twins have provided an extraordinarily rich insight into the natural history and pathogenesis of pediatric leukemias, and they are likely to continue to do so. They are a vivid illustration of the often repeated dictum in medical research that much can be learned from studying rare conditions. We encourage those clinicians treating any concordant or discordant leukemia in twin pairs to be aware of its intrinsic importance and to continue to support local or international collaborative studies of this infrequent but exceptional condition. Finally, we wish to acknowledge the essential contribution of the families with twins to studies of this kind. They may face a doubly tragic diagnosis, but their willingness to contribute has made a real difference to our current knowledge of the diverse natural histories of pediatric leukemias (Figure 12).
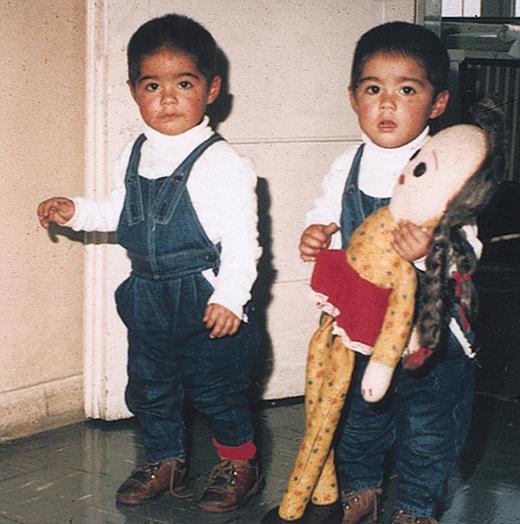
Twins who contributed to this study. These twins from Chile were the first to be recorded with a shared clonotypic leukemic marker, MLL-AF4 fusion. They are patients no. 1 in Figure 7. Reproduced with permission of the parents and the referring clinician.
Twins who contributed to this study. These twins from Chile were the first to be recorded with a shared clonotypic leukemic marker, MLL-AF4 fusion. They are patients no. 1 in Figure 7. Reproduced with permission of the parents and the referring clinician.
Appendix
Clinicians with concordant leukemias in twins are encouraged to register these cases by e-mailing details to m.greaves@icr.ac.uk under subject matter: TWINS.
Prepublished online as Blood First Edition Paper, June 5, 2003; DOI 10.1182/blood-2002-12-3817.
Supported by successive specialist program grants from the Leukaemia Research Fund, United Kingdom.
We thank the following colleagues for provision of twin samples: Drs M. Aluddin, M. E. Cabrera, M. Campbell, L. C. Chan, J. Chessells, W. Crist, O. B. Eden, A. Hirt, H. Kempski, S. Lie, S. Mizutani, M. Pombo-de-Oliveira, V. Saha, M. Steel, J. Trka, E. R. van Wering, A. Will, and M. Williams. Ms B. Deverson assisted with the preparation of the manuscript.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal