Abstract
The overproduction of red blood cells in patients with polycythemia vera (PV) is reflected in vitro by the formation of erythroid burst-forming units (BFU-Es) in the absence of exogenous erythropoietin. In contrast to other myeloproliferative disorders, the molecular mechanism of PV is unknown and no specific chromosomal abnormality has been described. We speculated that imatinib mesylate may reverse the pathological overproduction of red cells by inhibition of autonomous erythropoiesis. In the present study, imatinib mesylate was found to either block or strongly inhibit autonomous BFU-E formation in vitro in all patients tested. Moreover, autonomous BFU-E growth was also markedly reduced by exposure of PV cells to imatinib mesylate prior to cultivation in semisolid medium. The profound effect of imatinib mesylate on autonomous erythropoiesis suggests the involvement of an as yet unidentified kinase in the pathogenesis of PV and should provide the rationale for a forthcoming clinical trial.
Introduction
Polycythemia vera (PV) is a clonal disorder of the multipotential hematopoietic progenitor cell. The disease is characterized by increased red blood cells, and elevated numbers of granulocytes and platelets are not uncommon. Two distinct populations of erythroid progenitor cells in PV marrow have been described, indicating the coexistence of a malignant and a nonmalignant population of hematopoietic progenitors.1 Erythroid progenitors of the malignant clone can form hemoglobinized colonies (erythroid burst-forming units [BFU-Es]) in the absence of exogenous erythropoietin (EPO) in vitro.2 Such spontaneous BFU-E growth is never observed in healthy individuals and thus represents a highly characteristic feature in PV.3-5 EPO-independent BFU-E growth may either be due to the secretion of growth stimuli by the malignant clone or to the release of growth factors from accessory cells to hypersensitive PV precursors.6-9 In this respect we were able to demonstrate that the cytokine synthesis–inhibiting molecule interleukin-10 is able to profoundly inhibit the spontaneous formation of erythroid colonies in PV by suppression of endogenous granulocyte-macrophage colony-stimulating factor (GM-CSF) production.10 Although the mechanism for autonomous erythropoiesis remains unknown, mutations that involve tyrosine kinases may deregulate normal signaling pathways and render them constitutively activated without the requirement of the physiological stimulus. A growing number of oncogenes known to code for tyrosine kinases, including Bcr-Abl, c-Kit, epidermal growth factor receptor (EGF-R), PDGF-R, and Tel-Abl, are involved in the pathogenesis of various malignancies.11 Recently, a rearrangement of the platelet-derived growth factor receptor β (PDGF-RB) gene with TEL (ETV6), usually caused by a t(5;12)(q33;p13) translocation, in some cases of chronic myeloproliferative disease have been described.12,13 Although not known yet, similar mechanisms may cause growth alteration of a single aberrant pluripotent hematopoietic progenitor cell in PV, thus resulting in excess production of mature red cells.
Imatinib mesylate (STI571, Gleevec) is a potent inhibitor of Bcr-Abl tyrosine kinase activity and has recently been approved for the treatment of chronic myeloid leukemia (CML).14 This small molecule is a competitive antagonist of the adenosine triphosphate (ATP)–binding site of Bcr-Abl tyrosine kinase domain and reverses the growth advantage of immature and mature malignant progenitor cells in CML.15 Imatinib mesylate does not exclusively inhibit Bcr-Abl, but also other tyrosine kinases encoded by protooncogenes such as c-kit and PDGF-R. Recently, Apperley et al described the successful treatment of 4 cases of myeloproliferative disorders (MPDs) with imatinib mesylate.16 In these patients the causative molecular abnormality was likely to be a rearrangement of the PDGF-RB gene. We show here that in PV imatinib mesylate is a potent inhibitor of autonomous erythropoiesis in vitro. In contrast, imatinib mesylate exerts only minor effects on cytokine-stimulated colony growth derived from PV patients and healthy controls.
Study design
Patients
Thirteen patients with PV as established by the diagnostic criteria of the Polycythemia Vera Study Group were studied. Patients were treated by phlebotomy at the time of study, and none of them had previously received cytostatic drugs or radioactive phosphorus. Peripheral blood (PB) was collected from routine clinical controls after obtaining informed consent. Bone marrow was drawn from one patient who underwent bone marrow biopsy of the iliac crest at diagnosis.
Reagents
Imatinib mesylate was a kind gift from Novartis Pharmaceutical (Basel, Switzerland). Recombinant human interleukin-3 (rhIL-3) (Novartis, Basel, Switzerland), rhGM-CSF (R&D Systems, Minneapolis, MN), and rhEPO were purchased from Roche (Basel, Switzerland).
Colony assay
Peripheral blood mononuclear cells (PBMCs) (1 × 105/mL) from PV patients (n = 13) or healthy donors (n = 5) were cultured in semisolid medium either in the presence or absence of imatinib mesylate (0.01 to 10 μM). Autonomous colony growth was assessed in the absence of exogenous cytokines.17 Stimulated colony growth was investigated in the presence of EPO (2 U/mL), GM-CSF (10 ng/mL), and IL-3 (10 U/mL).18 Colonies containing more than 100 hemoglobinized cells were counted as BFU-Es.
Results and discussion
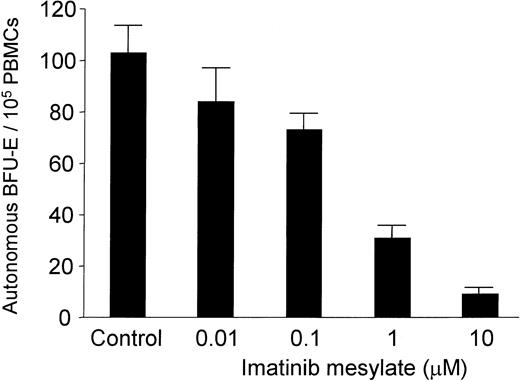
PBMCs of all PV patients enrolled in this study spontaneously formed erythroid colonies in the absence of exogenous EPO. As shown in Table 1, imatinib mesylate completely blocked autonomous BFU-E growth in 7 of 12 patients tested and suppressed burst formation by at least 62% in the remaining 5 PV patients. The median BFU-E number was 8.5 (range, 2 to 126) per 105 PBMCs in cultures without imatinib mesylate compared with 0 (range, 0 to 48) BFU-Es per 105 PBMCs in cultures containing 10 μM imatinib mesylate (P = .002). Spontaneous BFU-E formation was also completely suppressed in one patient from whom BMMCs could be obtained for in vitro studies (Table 1; patient C). The amount of suppression was dose dependent and, importantly, occurred at concentrations of imatinib mesylate that are also achieved in patients (Figure 1; patient S.E.).19 Thus, imatinib mesylate at a concentration of 1 μM suppressed autonomous BFU-E growth with a mean inhibition of 73% (n = 7; range of inhibition, 51% to 100%; P = .004).
In vitro effect of imatinib mesylate on autonomous BFU-E growth in PV patients
. | . | Autonomous BFU-Es ± SD per dish* . | . | . | . | |
|---|---|---|---|---|---|---|
| Patient . | Source . | Control . | Imatinib mesylate, 10 μM . | Percent inhibition . | P . | |
| A | PB | 126 ± 24.6 | 48 ± 5.6 | 62 | .008 | |
| B | PB | 2 ± 0.6 | 0 ± 0 | 100 | .007 | |
| C | BM | 19 ± 8 | 0 ± 0 | 100 | .015 | |
| D | PB | 8 ± 1.5 | 0 ± 0 | 100 | .001 | |
| E | PB | 8 ± 3 | 0 ± 0 | 100 | .009 | |
| F | PB | 31 ± 8 | 2 ± 1 | 94 | .004 | |
| G | PB | 54 ± 6.1 | 5 ± 1 | 91 | <.001 | |
| H | PB | 7 ± 2 | 0 ± 0.3 | 100 | .005 | |
| I | PB | 4 ± 2.5 | 0 ± 0 | 100 | .041 | |
| J | PB | 9 ± 0.6 | 0 ± 0 | 100 | <.001 | |
| K | PB | 4 ± 1.1 | 0 ± 0.3 | 100 | .038 | |
| L | PB | 21 ± 2 | 1 ± 0.5 | 95 | <.001 | |
| M | PB | 103 ± 13 | 9 ± 3 | 91 | <.001 | |
. | . | Autonomous BFU-Es ± SD per dish* . | . | . | . | |
|---|---|---|---|---|---|---|
| Patient . | Source . | Control . | Imatinib mesylate, 10 μM . | Percent inhibition . | P . | |
| A | PB | 126 ± 24.6 | 48 ± 5.6 | 62 | .008 | |
| B | PB | 2 ± 0.6 | 0 ± 0 | 100 | .007 | |
| C | BM | 19 ± 8 | 0 ± 0 | 100 | .015 | |
| D | PB | 8 ± 1.5 | 0 ± 0 | 100 | .001 | |
| E | PB | 8 ± 3 | 0 ± 0 | 100 | .009 | |
| F | PB | 31 ± 8 | 2 ± 1 | 94 | .004 | |
| G | PB | 54 ± 6.1 | 5 ± 1 | 91 | <.001 | |
| H | PB | 7 ± 2 | 0 ± 0.3 | 100 | .005 | |
| I | PB | 4 ± 2.5 | 0 ± 0 | 100 | .041 | |
| J | PB | 9 ± 0.6 | 0 ± 0 | 100 | <.001 | |
| K | PB | 4 ± 1.1 | 0 ± 0.3 | 100 | .038 | |
| L | PB | 21 ± 2 | 1 ± 0.5 | 95 | <.001 | |
| M | PB | 103 ± 13 | 9 ± 3 | 91 | <.001 | |
BFU-E indicates erythroid burst-forming unit; PV, polycythemia vera; SD, standard deviation; PB, peripheral blood; BM, bone marrow.
Mean number of erythroid bursts per 105 mononuclear cells ± SD from triplicates.
Dose-dependent effect of imatinib mesylate on autonomous BFU-E formation. PB mononuclear cells (MNCs) (1 × 105/mL) from 4 patients with PV were cultured in semisolid medium together with graded amounts of imatinib mesylate (0.01 to 10 μM) in the absence of exogenous growth factors. Autonomous BFU-E formation was assessed after a culture period of 14 days. The mean BFU-E numbers of triplicates (+ standard deviation) are given from 1 representative experiment (patient M).
Dose-dependent effect of imatinib mesylate on autonomous BFU-E formation. PB mononuclear cells (MNCs) (1 × 105/mL) from 4 patients with PV were cultured in semisolid medium together with graded amounts of imatinib mesylate (0.01 to 10 μM) in the absence of exogenous growth factors. Autonomous BFU-E formation was assessed after a culture period of 14 days. The mean BFU-E numbers of triplicates (+ standard deviation) are given from 1 representative experiment (patient M).
The effect of imatinib mesylate on PV precursor cell growth was also assessed in the presence of the growth-promoting molecules IL-3, GM-CSF, and EPO. The median stimulated BFU-E number was 81.5 (range, 16 to 302) per 105 PBMCs in methylcellulose cultures without imatinib mesylate and 53.5 (range, 13 to 137) in the presence of imatinib mesylate (P > .05). The minor suppression of erythroid colony formation in the presence of exogenous cytokines may be explained by a coexistence of both malignant and nonmalignant populations of hematopoietic progenitors. The addition of exogenous cytokines may thus enhance the outgrowth of normal BFU-Es while PV progenitors are suppressed by imatinib mesylate. Alternatively, pharmacologic doses of exogenous growth stimulators, as used in this study, may at least in part overcome imatinib mesylate–induced inhibition of PV progenitors. In agreement with published data, we observed no relevant inhibition of cytokine-stimulated colony growth from normal PBMCs by imatinib mesylate in concentrations up to 10 μM (data not shown).20
We next investigated the effect of preincubation of PV cells with imatinib mesylate at a concentration of 10 μM. Following pretreatment with imatinib mesylate for 24 to 96 hours, cells were washed and autonomous BFU-E growth was subsequently assessed in semisolid medium. Exposure to the drug resulted in a time-dependent inhibition of autonomous BFU-E growth. Interestingly, culturing with imatinib mesylate for 24 and 48 hours, respectively, did not sufficiently inhibit spontaneous burst formation. In contrast, a prolonged exposure up to 96 hours led to a 59% reduction of BFU-E growth as compared with untreated cells (data not shown). Cell numbers after precultivation did not differ between culture flasks, and there was no evidence of increased cell death as evaluated by morphology and trypan blue exclusion. Therefore, it is unlikely that the observed effects on autonomous BFU-E growth were due to increased proliferation in the untreated cell cultures or, alternatively, a toxic effect of imatinib mesylate on treated cells. It is therefore tempting to speculate that the drug specifically inhibits erythroid progenitor cells of the malignant clone.
In summary, this study demonstrates significant and dose-dependent in vitro effects of imatinib mesylate on spontaneous burst formation of erythroid progenitors in patients with PV. Recently, Silver reported the results of imatinib mesylate treatment in 7 patients with PV.21 These patients experienced a suppression of erythropoiesis as evidenced by the cessation of the need for phlebotomy. Regardless of the mechanism involved, those encouraging clinical findings and the results of our in vitro study should form the basis of a larger clinical trial for imatinib mesylate treatment in patients with PV.
Prepublished online as Blood First Edition Paper, May 22, 2003; DOI 10.1182/blood-2003-03-0676.
Supported by a grant from the Medizinisch-Wissenschaftlicher Fonds des Bürgermeister der Bundeshauptstadt Wien no. 2078.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal