Abstract
Homozygous mutant (Shp-2Δ46-110) embryonic stem (ES) cells exhibit decreased hematopoiesis; however, the point at which Shp-2 is critical for ES cell differentiation to hematopoietic cells is unknown. We characterized the differentiation defect of Shp-2Δ46-110 ES cells by examining early points of differentiation, conducting leukemia inhibitory factor (LIF)–stimulated biochemical analysis, and performing in vitro reconstitution studies with wild-type (WT) Shp-2. ES cell in vitro differentiation assays were used to compare the differentiation of WT, Shp-2Δ46-110, and reconstituted ES cells to mesoderm, by measuring brachyury expression, to hemangioblasts, by measuring blast colony-forming cell (BL-CFC) formation and flk-1 expression, and to hematopoietic progenitor colony-forming cells, by performing secondary plating assays. LIF-stimulated phospho-Stat3 (known to be critical for ES cell self-renewal and maintenance of an undifferentiated state) and phospho-Erk levels were examined by immunoblotting. ES cell survival, using annexin V staining, and secondary embryoid body (EB) formation were also evaluated. Differentiation to both mesoderm and hemangioblasts was lower in Shp-2Δ46-110 cells compared to WT cells. On reconstitution with WT Shp-2, expression of brachyury and flk-1 and differentiation to hemangioblasts and primitive and definitive hematopoietic progenitors were restored. LIF-stimulated phospho-Stat3 levels were higher, whereas phospho-Erk levels were lower in Shp-2Δ46-110 ES cells than in WT and reconstituted cells. The increased phospho-Stat3 levels correlated with increased Shp-2Δ46-110 ES cell secondary EB formation and survival. We conclude that normal Shp-2 function is critical for the initial step of ES cell differentiation to mesoderm and to hemangioblasts and acts within the LIF-gp130-Stat3 pathway to maintain a proper balance of ES cell differentiation, pluripotency, and apoptosis.
Introduction
Murine embryonic stem (ES) cells are pluripotent cells with the capacity to self-renew and differentiate into all tissues, including germ cells.1,2 In suspension culture, ES cells grow into spheres termed embryoid bodies (EBs). Within this context, these cells differentiate into ectoderm-, endoderm-, and mesoderm-derived tissues3-6 and are useful for the study of specific gene products in ES cell function. Stem cells execute a limited repertoire of activities including differentiation, self-renewal divisions, or programmed cell death.7 The Shp-2 homozygous mutant (Shp-2Δ46-110) ES cells bear an exon 3 deletion from the murine Shp-2 locus resulting in an in-frame deletion of amino acids 46 to 110 within the N-terminal SH2 domain of the mature Shp-2 protein (Figure 1A); therefore, the Shp-2Δ46-110 ES cells express a truncated (57-kDa) form of the Shp-2 protein, albeit at a lower level of expression compared to wild-type (WT) cells.8,9 Mice homozygous for this mutation are embryonic lethal (day 8.5-10.5) due to abnormal gastrulation and malformation of axial mesoderm-derived structures, including the notochord and somites.8 Whether the abnormality in mesoderm-derived structures in the Shp-2Δ46-110 mouse embryo is due to a cell autonomous defect in mesodermal differentiation or to a field defect due to abnormal cell migration is unknown.10,11 Additionally, Shp-2Δ46-110 ES cells demonstrate decreased differentiation to erythroid and myeloid progenitors in vitro9 and in vivo12 ; however, the point at which Shp-2Δ46-110 ES cells fail to proceed with normal differentiation, that is, from ES cells to mesodermal precursors, or from mesoderm to hematopoietic cells, is unknown.
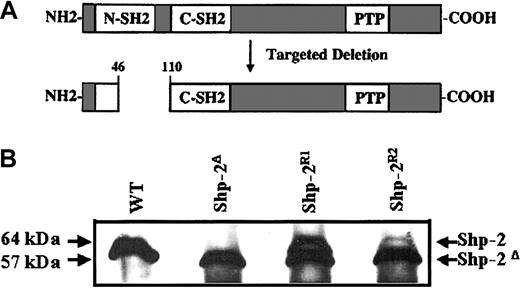
WT Shp-2 is expressed in Shp-2R cells. (A) Schematic diagram of WT Shp-2 protein and resultant mutant Shp-2 protein after targeted deletion of the Shp-2 allele. (B) Protein lysate from WT, Shp-2Δ, and Shp-2R1, and Shp-2R2 cells was immunoprecipitated and blotted with anti-SH-PTP2C (C18), which recognizes both the WT Shp-2 and Shp-2Δ46-110.
WT Shp-2 is expressed in Shp-2R cells. (A) Schematic diagram of WT Shp-2 protein and resultant mutant Shp-2 protein after targeted deletion of the Shp-2 allele. (B) Protein lysate from WT, Shp-2Δ, and Shp-2R1, and Shp-2R2 cells was immunoprecipitated and blotted with anti-SH-PTP2C (C18), which recognizes both the WT Shp-2 and Shp-2Δ46-110.
The role of Shp-2 in leukemia inhibitory factor (LIF)–stimulated signal transduction is of interest because ES cells are grown in LIF to maintain an undifferentiated, self-renewing state.13 LIF signals through gp130, the common subunit for the interleukin 6 (IL-6) family of cytokines,14 resulting in activation of the Jak kinases with recruitment and phosphorylation of Shp-2 and signal transducer and activator of transcription 3 (Stat3).15 The Jak-Stat pathway is critical for stem cell self-renewal in Drosophila16,17 and mammals.18,19 Additionally, activated Stat3 has been shown to up-regulate prosurvival molecules resulting in decreased apoptosis.20,21 The function of Shp-2 in ES cell self-renewal and apoptosis is unclear; however, previous studies demonstrated that ES cells expressing a granulocyte colony-stimulating factor (G-CSF)–gp130 chimeric receptor bearing a mutation at the Shp-2 binding tyrosyl residue (Y757) required lower levels of gp130 stimulation for the maintenance of pluripotency and demonstrated increased gp130-stimulated levels of activated Stat3 compared to ES cells bearing a WT chimeric receptor,22 suggesting that Shp-2 participation is required in the LIF-gp130-Stat3 pathway to maintain a proper balance of ES cell self-renewal and differentiation.
We have defined further the role of Shp-2 in the ES cell functions of differentiation, self-renewal, and apoptosis. We examined early points of WT and Shp-2Δ46-110 ES cell differentiation to mesoderm by measuring levels of brachyury expression. We also examined differentiation to hemangioblasts (blast colony-forming cells [BL-CFCs]), a multipotential precursor that has the capacity to differentiate into primitive and definitive erythroid cells as well as endothelial cells,23,24 by measuring flk-1 expression and BLCFC formation. Lack of normal Shp-2 function resulted in decreased and delayed expression of brachyury and flk-1 as well as decreased differentiation to hemangioblasts. On reconstitution with WT Shp-2, differentiation to hemangioblasts as well as to primitive and definitive progenitor colony-forming cells was restored. Shp-2Δ46-110 ES cells have increased LIF-stimulated phospho-Stat3 levels and decreased LIF-stimulated phospho-Erk levels, thereby defining aberrant signaling pathways that account, at least in part, for the abnormal differentiation observed. Functionally, the Shp-2Δ46-110 cells preferentially maintain an undifferentiated state, as assayed by secondary EB formation, and have increased survival, as assayed by annexin V binding. Taken together, these results demonstrate that normal Shp-2 function is critical in mediating the appropriate levels of activated Erk and Stat3 necessary to maintain the proper balance of ES cell differentiation, self-renewal, and apoptosis.
Materials and methods
ES cell culture and cell lines
All ES cell lines were maintained on gelatinized plates in Dulbecco modified Eagle medium (DMEM) with 15% ES cell–qualified fetal calf serum (FCS; Hyclone, Logan, UT), 55 μM β-mercaptoethanol, and 1000 U/mL LIF (ESGRO; CHEMICON, Temecula, CA). WT (R1) ES cells were used to generate the Shp-2 mutant allele as described previously.8 This original parental R1 ES cell line was used throughout the experiments in this study. The homozygous mutant Shp-2Δ46-110 ES cell line, IC3, was generated by selecting heterozygous mutant Shp-2Δ46-110 ES cells in G418 at 2 mg/mL, as described.9 The mammalian vector phβA-Shp-2, used to express the Shp-2 cDNA in IC3 cells, was prepared by replacing the cytomegalovirus (CMV) promoter of pcDNA3.1/hygro (Invitrogen, Carlsbad, CA) with approximately 3 kilobase (kb) of the human β-actin promoter from the vector pBAP (a kind gift of Dr Mark Kelley25 ). The Shp-2 cDNA as previously described26 was sequenced and subcloned into the multiple cloning site. Reconstituted cells were generated by mixing 5.6 × 106 IC3 cells with 40 μg linearized phβA-Shp-2 followed by electroporation (240 V, 500 μF) and selection in 0.3 mg/mL hygromycin. Expression of WT Shp-2 was demonstrated by immunoblotting.
Colony differentiation assay
ES cell lines were cultured (250-500 cells/mL) on gelatinized tissue culture plates for 6 to 8 days in ES cell LIF-containing media. The resulting colonies were cultured for an additional 48 hours in LIF-free media. The colonies were fixed and stained with Giemsa. Colonies were scored as differentiated when surrounded by flattened, fibroblast-like outgrowths.
ES cell differentiation into EBs
For primary differentiation assays, ES cells were plated in bacterial grade Petri dishes at 1000 to 2000 cells/mL in 0.9% methylcellulose-based differentiation media that included Iscove modified Dulbecco medium (IMDM), 2 mM glutamine, penicillin/streptomycin (100 U/mL/100 μg/mL), 5% protein-free hybridoma medium (PFHM-II) (Gibco BRL), 200 mg/mL iron-saturated holo-transferrin (Sigma, St Louis, MO), 5 mg/mL ascorbic acid, 450 μM monothioglycerol (Sigma), and 15% differentiation FCS (StemCell Technologies, Vancouver, BC, Canada) and incubated for 8 to 10 days at 37°C in 5% CO2. EBs were viewed by light microscopy and scored for the presence or absence of hemoglobin. For the formation of EBs used for secondary assays, ES cells were plated either in liquid-based differentiation media for day 3 to 6 EBs or in methylcellulose-based differentiation media for day 10 EBs.
Secondary plating assays
At days 3, 4, and 5 (for the hemangioblast assay), days 5 to 6 (for secondary EB or primitive erythroid assays), or day 10 (for definitive erythroid, mixed, and granulocyte-macrophage assays) of differentiation, first-degree EBs were digested with 0.25% trypsin followed by passaging through a 20-gauge needle 2 to 10 times. Cell concentrations and viability were performed using trypan blue. For the hemangioblast assay, cells were plated at 12 500 cells/mL in the presence of 10% differentiation serum, 25% D4T cell-conditioned media (D4T cells kindly provided by Dr Gordon Keller24 ), stem cell factor (SCF; 100 ng/mL), and vascular endothelial growth factor (VEGF; 5 ng/mL). Cells were plated at 50 000 cells/mL with erythropoietin (5 U/mL), SCF (100 ng/mL), and IL-3 (1 ng/mL) for definitive erythroid assays or with erythropoietin (5 U/mL), SCF (100 ng/mL), IL-3 (1 ng/mL), granulocyte-macrophage colony-stimulating factor (GM-CSF; 10 ng/mL), and macrophage colony-stimulating factor (M-CSF; 5 ng/mL) for mixed and granulocyte-macrophage assays. For the detection of primitive erythroid progenitors, cells were plated with 15% plasma-derived serum (Animal Technologies, Antech, TX) and erythropoietin (5 U/mL).
Immunoblot analysis and antibodies
Control and LIF-stimulated cell lysates were prepared as previously described.27 ES cells were cultured in serum-free, LIF-free maintenance media containing 0.5% bovine serum albumin (Sigma) for 6 hours followed by stimulation for various time points with 1000 U/mL LIF. Clarified total cell lysates were electrophoresed on a 10% polyacrylamide gel followed by transfer to a nitrocellulose membrane. To detect both WT and mutant Shp-2, anti-SH-PTP2C (C18) from Santa Cruz Biotechnology (Santa Cruz, CA) was used. Antiphospho-Stat3, anti-Stat3, antiphospho-Erk, and anti-Erk were from New England Biolabs (Beverly, MA). Signals were detected by enhanced chemiluminescence and bands were quantitated using NIH Image.
RT-PCR analysis
Total cellular RNA was prepared from day 3, 4, and 5 first-degree EBs using QIAamp Blood (Qiagen, Valencia, CA). Total cellular RNA preparations were treated with RNase-free DNase I (Promega, Madison, WI) followed by quantitation by UV spectroscopy. First-strand synthesis was performed using 2 μg (1 ×), 200 ng (.1 ×), or 20 ng (.01 ×) total cellular RNA with poly dT primer and reverse transcriptase (RT; SuperScript, Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. Primer pairs for brachyury,28 flk-1,28 and β-actin29 (internal control) were used as previously described. Polymerase chain reaction (PCR) amplification was 1 minute denaturation at 94°C, 1 minute annealing at 58°C for flk-1 or 52°C for β-actin and brachyury, and 1 minute 30 seconds for extension at 72°C using puReTaq Ready-To-Go PCR beads (Amersham Pharmacia, Piscataway, NJ) according to the manufacturer's instructions. To achieve semiquantitative analysis, PCRs were terminated after 10, 20, or 30 cycles. PCR products were subjected to agarose gel electrophoresis, stained with ethidium bromide for visualization, and quantitated using NIH Image.
Apoptosis assay
ES cell lines were plated at 500 000 cells/3.5-cm gelatinized plate and cultured for 24 hours in standard ES cell media. The media was changed and cells were cultured for an additional 96 hours without change of or addition to the media. The cells were collected by trypsinization, stained with annexin V–fluorescein isothiocyanate (FITC) and propidium iodide (BD PharMingen, San Diego, CA), and analyzed by fluorescence-activated cell sorting (FACS) analysis.
Statistical analysis
Groups were compared using the 2-tailed Student t test.
Results
Reintroduction of WT Shp-2 into Shp-2Δ46-110 ES cells
To examine whether the observed abnormal differentiation phenotype of the Shp-2Δ46-110 cells can be corrected by adding back WT Shp-2, in vitro reconstitution studies were performed. The parental Shp-2 mutant ES cell line, IC3, was transfected with the plasmid phβA-Shp-2 and selected in hygromycin. Two clones were used for functional and biochemical analysis. Figure 1B demonstrates the level of WT (64 kDa) and mutant (57 kDa) Shp-2 expression in ES cells of WT, homozygous mutant (Shp-2Δ46-110 labeled as Shp-2Δ in the figures), 2 independent cell lines that express the WT Shp-2 at different levels (Shp-2R1 and Shp-2R2). The Shp-2R2 cell line expressed a low level of WT Shp-2 as compared to Shp-2R1 and expression of the Shp-2 protein was diminished on differentiation. In contrast, the Shp-2R1 cell line continued to express WT Shp-2 throughout differentiation (data not shown) and therefore we used Shp-2R1 cell line as well as the WT and Shp-2Δ46-110 ES cell lines in most of the experiments in this study.
Shp-2 is required for mesoderm and hemangioblast formation
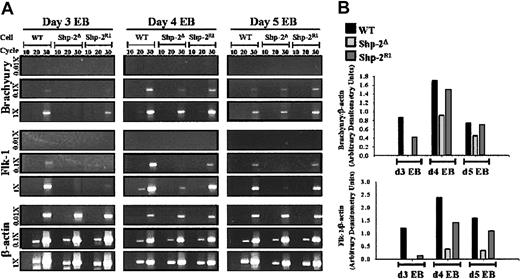
Previous work has demonstrated that lack of normal Shp-2 results in defective differentiation of ES cells to mature hematopoietic cells; however, the point of ES cell differentiation at which Shp-2 is needed is unknown. We examined the very first step of ES cell differentiation to hematopoietic cells, progression to mesoderm, by examining the level of the mesoderm-specific molecular marker, brachyury,24,30,31 in day 3 EBs derived from the Shp-2Δ46-110 and Shp-2R1 cell lines. We found that the brachyury message was lower in the Shp-2Δ46-110 cells compared to the WT cells and was modestly increased on reconstitution with WT Shp-2 (Figure 2A; brachyury band intensities normalized to β-actin band intensities shown in Figure 2B). We next examined later time points to determine if differentiation to mesoderm is merely delayed in the Shp-2 mutant EBs. We found that brachyury levels were higher in Shp-2 mutant EBs at day 4 than that at day 3, but still lower than that seen in the WT and Shp-2 reconstituted EBs (Figure 2). Similar results were observed following 5 days of EB differentiation. The lower level of brachyury expression in the Shp-2 mutant EBs at all time points tested demonstrates that differentiation to mesoderm is decreased, but not completely blocked. These results suggest that the abnormal formation of mesoderm-derived structures in the Shp-2Δ46-110 murine embryo is due in part to a cell autonomous defect in Shp-2Δ46-110 ES cell differentiation to mesoderm.
Shp-2 is necessary for brachyury and flk-1 expression in EBs. (A) Total cellular RNA was prepared from day 3, 4, and 5 EBs, reverse transcription was performed using 2 μg (1 ×), 200 ng (.1 ×), or 20 ng (.01 ×) as template, and amplification of brachyury, flk-1, and the constitutively expressed β-actin was conducted for 10, 20, or 30 cycles. PCR products were electrophoresed on agarose gels and stained with ethidium bromide for visualization. (B) Brachyury and flk-1 band intensities (1 ×, 30 cycles of amplification) were quantitated by densitometry using NIH Image and normalized to β-actin band intensities (0.01 ×, 30 cycles of amplification).
Shp-2 is necessary for brachyury and flk-1 expression in EBs. (A) Total cellular RNA was prepared from day 3, 4, and 5 EBs, reverse transcription was performed using 2 μg (1 ×), 200 ng (.1 ×), or 20 ng (.01 ×) as template, and amplification of brachyury, flk-1, and the constitutively expressed β-actin was conducted for 10, 20, or 30 cycles. PCR products were electrophoresed on agarose gels and stained with ethidium bromide for visualization. (B) Brachyury and flk-1 band intensities (1 ×, 30 cycles of amplification) were quantitated by densitometry using NIH Image and normalized to β-actin band intensities (0.01 ×, 30 cycles of amplification).
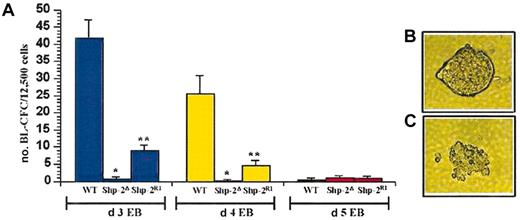
We next examined expression of the hemangioblast-associated marker, flk-1,23,24 to determine if differentiation to the hemangioblast stage is compromised in the Shp-2Δ46-110 EBs. At all time points examined, expression of flk-1 in the Shp-2Δ46-110 EBs was significantly lower than that observed in the WT or Shp-2R1 EBs (Figure 2A-B). To corroborate flk-1 expression levels with cell function, we compared the ability of WT, Shp-2Δ46-110, and the Shp-2 reconstituted ES cell lines, Shp-2R1, to differentiate to hemangioblasts (BL-CFCs). As predicted by the flk-1 expression results, we observed minimal differentiation of the Shp-2Δ46-110 cells to hemangioblasts following 3, 4, or 5 days of EB differentiation (Figure 3A). This was in contrast to that observed with the WT and Shp-2R1 cells following 3 and 4 days of EB differentiation. By day 5 of EB differentiation, very few hemangioblast colonies were observed from any of the cell lines, consistent with the original description that BL-CFCs are most abundant at 3.5 days of EB differentiation.24 Given that there is a defect, but not a complete block, in Shp-2Δ46-110 ES cell differentiation to mesoderm based on the brachyury expression levels described, these results demonstrate that Shp-2 is required to promote differentiation of mesodermal precursors to the hematopoietic lineage and that the decrease in Shp-2Δ46-110 ES cell-derived hematopoietic cells is not due merely to a defect in differentiation to mesoderm.
Shp-2 is necessary for hemangioblast (BL CFC) formation. (A) Day 3, 4, or 5 EBs were dissociated and plated into hemangioblast cultures. At least 2 independent experiments were conducted for each comparison and cultures were plated in duplicate or triplicate. Day 3: *P < .0001 comparing WT to Shp-2Δ; **P < .0001 comparing Shp-2R1 to Shp-2Δ. Day 4: *P < .006 comparing WT to Shp-2Δ and **P = .01 comparing Shp-2R1 and Shp-2Δ. (B) Representative secondary EB. (C) Representative hemangioblast (BL-CFC) colony. Error bars represent SEM.
Shp-2 is necessary for hemangioblast (BL CFC) formation. (A) Day 3, 4, or 5 EBs were dissociated and plated into hemangioblast cultures. At least 2 independent experiments were conducted for each comparison and cultures were plated in duplicate or triplicate. Day 3: *P < .0001 comparing WT to Shp-2Δ; **P < .0001 comparing Shp-2R1 to Shp-2Δ. Day 4: *P < .006 comparing WT to Shp-2Δ and **P = .01 comparing Shp-2R1 and Shp-2Δ. (B) Representative secondary EB. (C) Representative hemangioblast (BL-CFC) colony. Error bars represent SEM.
Shp-2 rescues ES cell differentiation and hematopoiesis
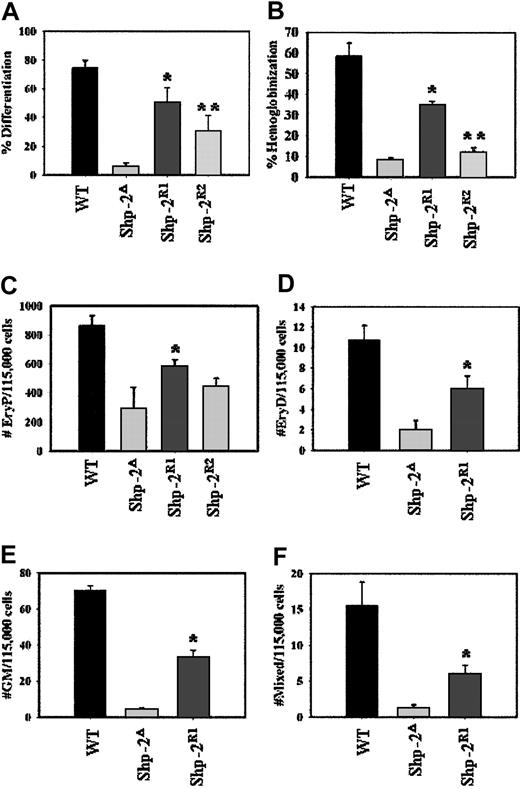
One measure of ES cell differentiation capacity is the development of flattened, fibroblast-like outgrowths from ES cell colonies on the withdrawal of LIF. Wild-type ES cells consistently produced 60% to 70% of colonies with differentiated morphology in contrast to that of Shp-2Δ46-110 cells, which produced less than 5% differentiated colonies (Figure 4A). As seen using both the Shp-2R1 and Shp-2R2 lines, on reconstitution with WT Shp-2, the defective differentiation phenotype was corrected. Regarding differentiation to hematopoietic cells, 50% to 60% of the EBs developed from WT cells contained hemoglobinized cells compared to only 10% of the EBs developed from the Shp-2Δ46-110 cells (Figure 4B). Again, we observed a significant correction of this defective phenotype on reintroducing WT Shp-2 as seen with the Shp-2R1 cell line, and to a lesser extent with the Shp-2R2 cell line.
Reconstitution with Shp-2 rescues ES cell differentiation and hematopoiesis. (A) ES cell colonies were scored following 48 hours of culture without LIF. The number of differentiated colonies was divided by the total number of colonies to yield percent differentiated colonies. Two independent experiments, cultures plated in duplicate; *P = .009 comparing Shp-2R1 to Shp-2Δ and **P = .05 comparing Shp-2R2 to Shp-2Δ. (B) EBs were scored for the presence or absence of hemoglobinized cells at day 8 to 10 of differentiation. The number of hemoglobinized EBs was divided by the total number of EBs to yield percent hemoglobinization. Three independent experiments, cultures were plated in duplicate; *P < .0001 comparing Shp-2R1 to Shp-2Δ and **P = .06 comparing Shp-2R2 to Shp-2Δ. (C) Primitive erythroid (EryP) progenitors; 2 independent experiments, cultures plated in duplicate; *P = .04 comparing Shp-2R1 to Shp-2Δ. All assays for definitive progenitors were conducted 2 independent times and all cultures were in duplicate. (D) Definitive erythroid (EryD) progenitors; *P = .03 comparing Shp-2R1 to Shp-2Δ. (E) Mixed progenitors; *P = .002 comparing Shp-2R1 to Shp-2Δ. (F) Granulocyte-macrophage (GM) progenitors; *P < .0001 comparing Shp-2R1 to Shp-2Δ. Error bars represent SEM.
Reconstitution with Shp-2 rescues ES cell differentiation and hematopoiesis. (A) ES cell colonies were scored following 48 hours of culture without LIF. The number of differentiated colonies was divided by the total number of colonies to yield percent differentiated colonies. Two independent experiments, cultures plated in duplicate; *P = .009 comparing Shp-2R1 to Shp-2Δ and **P = .05 comparing Shp-2R2 to Shp-2Δ. (B) EBs were scored for the presence or absence of hemoglobinized cells at day 8 to 10 of differentiation. The number of hemoglobinized EBs was divided by the total number of EBs to yield percent hemoglobinization. Three independent experiments, cultures were plated in duplicate; *P < .0001 comparing Shp-2R1 to Shp-2Δ and **P = .06 comparing Shp-2R2 to Shp-2Δ. (C) Primitive erythroid (EryP) progenitors; 2 independent experiments, cultures plated in duplicate; *P = .04 comparing Shp-2R1 to Shp-2Δ. All assays for definitive progenitors were conducted 2 independent times and all cultures were in duplicate. (D) Definitive erythroid (EryD) progenitors; *P = .03 comparing Shp-2R1 to Shp-2Δ. (E) Mixed progenitors; *P = .002 comparing Shp-2R1 to Shp-2Δ. (F) Granulocyte-macrophage (GM) progenitors; *P < .0001 comparing Shp-2R1 to Shp-2Δ. Error bars represent SEM.
To examine primitive and definitive hematopoiesis, specifically, we collected primary EBs on days 5 and 10 of EB differentiation, dissociated them into single-cell suspension, and plated them into primitive and definitive progenitor assays, respectively. We observed significantly lower differentiation to all progenitors from the Shp-2Δ46-110 cells (Figure 4C-F), consistent with our previous results.9 On reconstitution with WT Shp-2, rescue of differentiation to primitive erythroid progenitors was observed in Shp-2R1 and Shp-2R2 cells at different levels that are consist with the amounts of Shp-2 protein expression (Figure 4C). Rescue of definitive hematopoiesis was also observed in Shp-2R1 cells, which stably express WT Shp-2 protein, as demonstrated by increased numbers of definitive erythroid, mixed, and granulocyte-macrophage colonies compared to the Shp-2Δ46-110 cell line (Figure 4D-F). However, the Shp-2R2 cell line, which expresses a low level of WT Shp-2, demonstrated minimal differentiation to definitive progenitors (data not shown), suggesting a stringent requirement of Shp-2 for definitive hematopoiesis.
Shp-2 modulates LIF-stimulated Stat3 and Erk activity
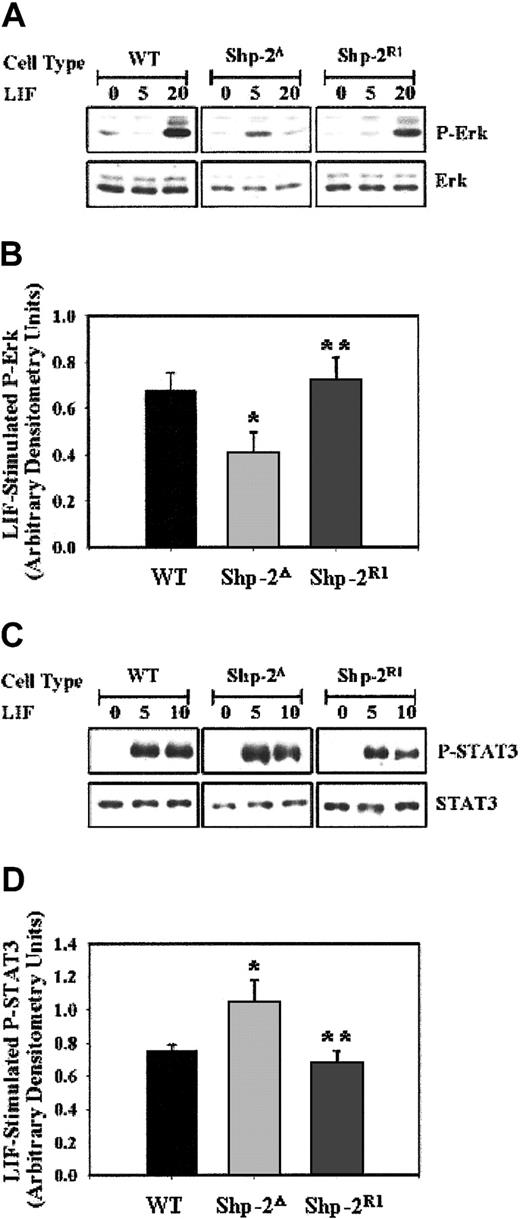
These studies demonstrate that WT Shp-2 function is required for proficient hematopoiesis and that a major component of the abnormal hematopoietic phenotype observed in the Shp-2Δ46-110 ES cells is secondary to an early differentiation defect from ES cells to mesoderm and to hemangioblasts. We next examined potential molecular mechanisms that may account for this differentiation defect. Activation of the Ras-Raf-Mek-Erk signaling cascade is known to be of particular importance in inducing cellular differentiation. As shown in Figure 5A-B, Shp-2Δ46-110 exhibited lower LIF-induced phospho-Erk compared to WT cells, as observed previously.9 Correlating with the functional data presented, on reintroduction of WT Shp-2, LIF-stimulated phospho-Erk levels were more strongly induced.
LIF-stimulated phospho-Erk is lower and phospho-Stat3 is greater in Shp-2Δ cells. (A) WT, Shp-2Δ, and Shp-2R1 ES cells were either unstimulated or stimulated with LIF (1000 U/mL) for 5 or 20 minutes followed by immunoblot analysis for p-Erk and Erk. The blot is a representation of 4 independent experiments. (B) Compilation of normalized p-Erk to Erk levels from 4 independent experiments following LIF stimulation in WT, Shp-2Δ, and Shp-2R1 cells; *P = .04 comparing Shp-2Δ to WT, and **P = .03 comparing Shp-2R1 to Shp-2Δ. (C) WT, Shp-2Δ, and Shp-2R1 ES cells were either unstimulated or stimulated with LIF (1000 U/mL) for 5 or 10 minutes followed by immunoblot analysis for p-Stat3 and Stat3. The blot is a representation of 4 independent experiments. (D) Compilation of normalized p-Stat3 to Stat3 from 4 independent experiments following LIF stimulation in WT, Shp-2Δ, and Shp-2R1 cells; *P = .05 comparing Shp-2Δ to WT, and **P = .05 comparing Shp-2R1 to Shp-2Δ.
LIF-stimulated phospho-Erk is lower and phospho-Stat3 is greater in Shp-2Δ cells. (A) WT, Shp-2Δ, and Shp-2R1 ES cells were either unstimulated or stimulated with LIF (1000 U/mL) for 5 or 20 minutes followed by immunoblot analysis for p-Erk and Erk. The blot is a representation of 4 independent experiments. (B) Compilation of normalized p-Erk to Erk levels from 4 independent experiments following LIF stimulation in WT, Shp-2Δ, and Shp-2R1 cells; *P = .04 comparing Shp-2Δ to WT, and **P = .03 comparing Shp-2R1 to Shp-2Δ. (C) WT, Shp-2Δ, and Shp-2R1 ES cells were either unstimulated or stimulated with LIF (1000 U/mL) for 5 or 10 minutes followed by immunoblot analysis for p-Stat3 and Stat3. The blot is a representation of 4 independent experiments. (D) Compilation of normalized p-Stat3 to Stat3 from 4 independent experiments following LIF stimulation in WT, Shp-2Δ, and Shp-2R1 cells; *P = .05 comparing Shp-2Δ to WT, and **P = .05 comparing Shp-2R1 to Shp-2Δ.
The LIF-stimulated activation of Stat3 has been shown to be important in maintaining ES cells in an undifferentiated and self-renewing state.15,18,19,32 Based on these previous studies and the observation that Shp-2 mutant ES cells have a severe defect in differentiation and have been shown to be hypersensitive functionally to LIF,33 Stat3 activation was examined as a potential target for Shp-2 modulation. We found that the level of LIF-stimulated phospho-Stat3 in the Shp-2Δ46-110 cells is increased compared to that observed in WT cells (Figure 5C-D). Reconstitution with WT Shp-2 resulted in decreased LIF-stimulated phospho-Stat3 levels in the Shp-2R1 cells, similar to WT levels. Together, these biochemical data demonstrate that normal Shp-2 function is essential for the proper balance of Erk and Stat3 activation in ES cells, which play critical roles in maintaining the proper balance of ES cell differentiation and self-renewal, respectively.
Shp-2 modulates secondary EB formation and ES cell survival
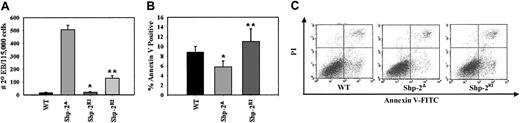
We next investigated ES cell functions shown previously to be modulated by LIF and activated Stat3 including maintenance of an undifferentiated phenotype18,19 and programmed cell death.20,21 When primary EBs are dissociated and replated in secondary culture, cells that fail to commit and differentiate in the primary culture, yet retain pluripotency and the capacity to undergo cell division, grow into new EBs, termed secondary EBs. The number of secondary EBs reflects the ES cell capacity to maintain an undifferentiated state and provides insight into ES cell self-renewal capacity.33 We observed that the Shp-2Δ46-110 cells had a dramatically higher number of secondary EBs compared with the WT cell line (Figure 6A). On reconstitution with WT Shp-2, the number of secondary EBs returned to WT levels as seen with Shp-2R1 and Shp-2R2 cell lines. As predicted from the previous experiments, the reconstitution of a normal phenotype was not as pronounced using the Shp-2R2 cell line as compared with Shp-2R1 cells.
Shp-2 modulates secondary EB formation and survival. (A) EBs grown for 7 days in primary differentiation culture were harvested, dissociated, and plated into secondary culture for secondary EBs. Secondary EBs were scored on day 7 of secondary culture. Two independent experiments, cultures plated in duplicate; *P < .0001 comparing Shp-2R1 to Shp-2Δ and **P = .0004 comparing Shp-2R2 to Shp-2Δ. Error bars represent SEM. (B) ES cells were cultured on gelatinized plates for 96 hours without change or supplementation of media followed by trypsinization, staining with annexin V-FITC, propidium iodide (PI), and FACS analysis. Graphic representation of 4 independent experiments. Values for percent annexin V+ cells were calculated by adding the values of the upper right quadrant (annexin V+/PI+) and lower right quadrant (annexin V+/PI–) of the FACS dot plots; *P = .03 comparing WT to Shp-2Δ cells, and **P = .07 comparing Shp-2R1 to Shp-2Δ cells. (C) FACS analysis dot plots from a representative experiment.
Shp-2 modulates secondary EB formation and survival. (A) EBs grown for 7 days in primary differentiation culture were harvested, dissociated, and plated into secondary culture for secondary EBs. Secondary EBs were scored on day 7 of secondary culture. Two independent experiments, cultures plated in duplicate; *P < .0001 comparing Shp-2R1 to Shp-2Δ and **P = .0004 comparing Shp-2R2 to Shp-2Δ. Error bars represent SEM. (B) ES cells were cultured on gelatinized plates for 96 hours without change or supplementation of media followed by trypsinization, staining with annexin V-FITC, propidium iodide (PI), and FACS analysis. Graphic representation of 4 independent experiments. Values for percent annexin V+ cells were calculated by adding the values of the upper right quadrant (annexin V+/PI+) and lower right quadrant (annexin V+/PI–) of the FACS dot plots; *P = .03 comparing WT to Shp-2Δ cells, and **P = .07 comparing Shp-2R1 to Shp-2Δ cells. (C) FACS analysis dot plots from a representative experiment.
To investigate the increase in secondary EBs seen with the Shp-2Δ46-110 cells, we compared the apoptosis levels between various cell lines by staining with the fluorochrome-conjugated phospholipid-binding protein, annexin V.34 We observed that the Shp-2Δ46-110 cells had a modest but significant decrease in apoptosis compared to the WT cells following continuous culture for 96 hours without change of or supplementation to media (Figure 6A-B). The level of apoptosis returned to WT levels on reconstitution with WT Shp-2 as seen with the Shp-2R1 cells. These data suggest that absence of normal Shp-2 function confers a survival advantage to ES cells. This survival advantage is likely contributing to the increased frequency of secondary EBs observed. However, because the increase in survival for the Shp-2Δ46-110 cells is approximately 1.5-fold greater than the WT cells, whereas the increase in secondary EB frequency is more than 10-fold greater, survival alone cannot account for the increase in second-degree EB frequency. It is likely that other LIF- and Stat3-regulated functions in addition to survival are enhanced resulting in the increased maintenance of an undifferentiated state.
Discussion
Shp-2Δ46-110 ES cells, which have a decreased capacity to differentiate into hematopoietic progenitors, provide an excellent model to study the role of Shp-2 in the cellular and molecular mechanisms that determine the fate of a stem cell. To study the mechanism of Shp-2 function in ES cell differentiation, we sought to elucidate the point at which Shp-2 is acting by examining ES cell differentiation to mesoderm and to hemangioblasts. We found that Shp-2 is necessary for the earliest step examined, because expression of brachyury transcripts is delayed and decreased in the Shp-2Δ46-110 EBs compared to WT EBs. These data demonstrate that normal Shp-2 function is required at the cellular level for proper ES cell differentiation to mesoderm. Previous studies of Shp-2Δ46-110 murine embryos have demonstrated abnormal gastrulation and notochord formation8 and a decreased contribution of Shp-2Δ46-110/Rosa26 LacZ ES cells to the somites and limb buds in chimeric mice11 ; however, it has been difficult to determine if these abnormalities are due exclusively to migration defects or are also caused by cell autonomous abnormalities of ES cell differentiation to mesoderm. Our studies using in vitro ES cell differentiation techniques suggest that an intrinsic defect in Shp-2Δ46-110 ES cell differentiation to mesoderm also likely contributes to the malformation of mesoderm-derived structures in the Shp-2Δ46-110 murine embryos.
We also examined differentiation to the multipotential precursor for both hematopoietic and endothelial cells, the hemangioblast. We observed lower levels of the hemangioblast-associated molecular marker, flk-1, and, consistently, observed lower levels of hemangioblast (BL-CFC) formation derived from the Shp-2Δ46-110 EBs. These data demonstrate that the decreased level of hematopoietic progenitors derived from Shp-2Δ46-110 ES cells is due to an initial defect in ES cell differentiation to mesodermal precursors and in a secondary defect in differentiation to hemangioblasts. These mesodermal and hemangioblast abnormalities, along with the abnormal vascular morphology of the Shp-2Δ46-110 yolk sac, which shows the development of the capillary plexus, but abnormal vasculogenesis into thick-walled vessels,8 is reminiscent of the hematopoietic and vascular abnormalities found in the scl/tal-135-37 and runx138 knock-out mice. It will be of interest to study the role of Shp-2 in arteriogenesis, about which little is known.
Based on the findings that phospho-Stat3 is necessary to maintain ES cells in an undifferentiated state,15 promotes ES cell pluripotency,15,32 and self-renewal18,19 and that Shp-2Δ46-110 ES cells require lower concentrations of LIF for maintenance of an undifferentiated state,33 we reasoned that LIF-stimulated phospho-Stat3 would be greater in ES cells lacking normal Shp-2 function. We did indeed observe increased LIF-stimulated phospho-Stat3 in the Shp-2Δ46-110 compared to the WT cells. We next evaluated known phospho-Stat3–mediated functions, such as maintenance of undifferentiated state and apoptosis. The Shp-2 mutant ES cells demonstrated increased secondary EB formation and increased survival as assayed by annexin V staining. Based on these data, we propose that one of the crucial signaling mechanisms regulated by Shp-2 in ES cells is the level of phospho-Stat3.
In all experiments conducted, we examined the effect of reintroducing WT Shp-2 into the mutant Shp-2Δ46-110 cell line to determine if the observed abnormal phenotypes could be normalized in the presence of WT Shp-2 expression. This question is of particular significance in this model because the mutant cells express a truncated Shp-2 molecule. Whether the residual mutant Shp-2 protein contributes to the abnormal phenotype observed has been a question of contention; however, from our observations, it is safe to conclude that the presence of WT Shp-2 protein, even at low levels as observed in our reconstituted ES cell lines, is capable of restoring the early stages of differentiation to the hemangioblast and primitive erythroid stages. However, we also observed that sustained WT Shp-2 expression is necessary for differentiation to definitive hematopoietic progenitors because the Shp-2R1 ES cell line, which continued to express detectable levels of WT Shp-2 throughout differentiation, was able to give rise to definitive hematopoietic progenitors, whereas the Shp-2R2 ES cell line, which ceased to express WT Shp-2 on EB differentiation, was unable to differentiate to definitive hematopoietic progenitors. These results at least suggest that if the residual mutant Shp-2 protein does act aberrantly to maintain Shp-2Δ46-110 ES cells in an undifferentiated state, the WT Shp-2 protein very effectively competes with this mutant protein to normalize the observed biochemical and cellular abnormalities. However, it appears more likely that Shp-2Δ46-110 is a recessive mutant allele encoding a loss-of-function molecule, because WT Shp-2, expressed at a low level, efficiently rescued the phenotype of Shp-2Δ46-110 ES cells.
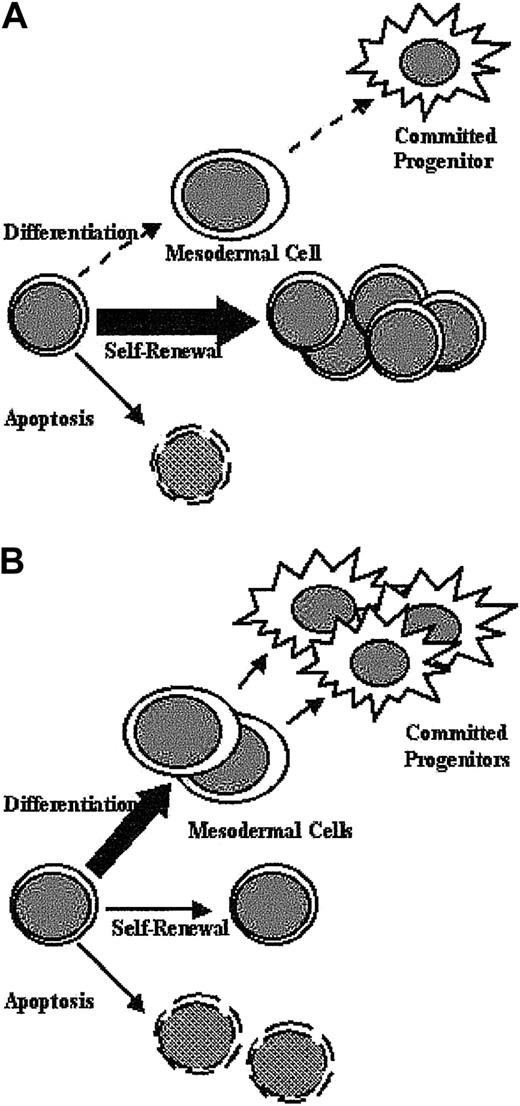
We show schematically (Figure 7A) the observed abnormalities in Shp-2Δ46-110 ES cell function. Lack of normal Shp-2 function causes ES cells to remain uncommitted and undifferentiated and to exhibit a lower level of apoptosis. The overall result is the maintenance of ES cells in an undifferentiated, replicative stem cell compartment. The biochemical abnormalities observed in the aberrantly functioning Shp-2Δ46-110 ES cells include decreased LIF-stimulated phospho-Erk and increased LIF-stimulated phospho-Stat3. Our findings are consistent with the model generated by Burdon and colleagues in that the balance between Stat3 activation, which promotes ES cell self-renewal, and Erk activation, which is dispensable for self-renewal and likely promotes differentiation, determines stem cell fate.19,22 On reconstitution with WT Shp-2 (Figure 7B), all of these functional defects are normalized in conjunction with normalization of the LIF-stimulated Erk and Stat3 activation. Taken together, these results suggest that normal Shp-2 function is crucial for the proper balance of activated Erk and activated Stat3 resulting in the appropriate level of ES cell differentiation, self-renewal, and apoptosis.
Schematic diagram. The diagram shows aberrant Shp-2Δ ES cell function (A) and correction on reintroduction of WT Shp-2 (B).
Schematic diagram. The diagram shows aberrant Shp-2Δ ES cell function (A) and correction on reintroduction of WT Shp-2 (B).
Prepublished online as Blood First Edition Paper, June 5, 2003; DOI 10.1182/blood-2003-04-1171.
Supported by grants NIH F32CA84677 (R.J.C.), NIH RO1HL63169 (M.C.Y.), NIH RO1CA78606, and RO1GM53660 (G.-S. F.) and by the generous philanthropic contribution of Alan and Dorothy Klineman to the Indiana University School of Medicine.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors wish to thank Drs Marion Kennedy, Gordon Keller, and Min You for advice and helpful discussions about stem cell experiments and Pat Fox for help with preparation of the manuscript.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal