The activity of ADAMTS13 (a von Willebrand factor–cleaving metalloprotease) is decreased in congenital and acquired thrombotic thrombocytopenic purpura (TTP) either because of a gene defect or because of transient inhibition by an autoantibody.1,2 Current diagnosis in patients is based on various activity assays, most of which are time-consuming. Not only would a quicker diagnostic method be advantageous, but such a method that differentiates between congenital and antibody-induced TTP would be additionally useful because different treatment strategies might be more appropriate for congenital than antibody-induced TTP.
ADAMTS13 recently has been characterized and cloned.3,4 Here we describe a highly sensitive assay using a recombinant ADAMTS135 (rADAMTS13) to screen plasma samples for antibodies against ADAMTS13. This antibody test also may allow treatment to be monitored, especially for potential anamnestic responses caused by replacement of the protease.
ADAMTS13 activity and the inhibitor titer of the plasma samples were determined as described earlier.6 To visualize the inhibitors, 3 μL (100 mU) of rADAMTS13 containing concentrated cell supernatant was electrophoresed on gradient polyacrylamide gels (4%-12%) in the presence of sodium dodecyl sulfate under nonreducing conditions and subjected to a standard Western blotting procedure.7 The blots were incubated for 2 hours at room temperature with diluted plasma samples from TTP patients or from healthy donors, or with a monoclonal mouse anti–human ADAMTS13 antibody (242Q2).
The blots were developed by further incubation with an alkaline phosphatase (ALP)–labeled donkey F(ab′)2 fragment anti–human IgG for the plasma samples, and with an ALP-labeled rabbit F(ab′)2 fragment anti–mouse IgG for the monoclonal antibody (both from Accurate, Westbury, NY). The blots were stained with an ALP-substrate kit (BioRad, Hercules, CA). Quantitative densitometry analysis and molecular mass determination of the stained protein bands were performed from scanned images of the immunoblots using a calibrated scanner and image processing software (Image Master; Amersham Pharmacia, Uppsala, Sweden).
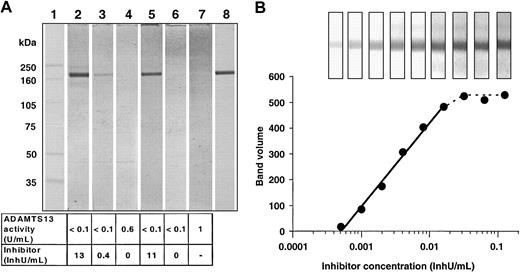
In the plasma samples in which an inhibitor was detectable with the activity assay, a band appeared with a molecular mass of 190 kDa (Figure 1A, lanes 2, 3, and 5), but no band was found in plasma pooled from healthy donors (lane 7), a TTP patient after treatment (lane 4), or a patient with hereditary TTP (lane 6). An equivalent 190-kDa band was obtained when the immunoblot was developed with the monoclonal antibody against the rADAMTS13 preparation (lane 8).
Immunoblot for detecting autoantibodies against ADAMTS13 in plasma samples. (A) Comparison of different plasma samples with a monoclonal anti–human ADAMTS13 antibody. Lane 1: molecular mass marker (full-range rainbow marker, Amersham Pharmacia). Lanes 2-4: plasma samples from a patient6 with antibody-induced TTP taken at different stages during her disease course. Lane 5: plasma sample from a patient with antibody-induced TTP. Lane 6: plasma sample from a patient with hereditary TTP. Lane 7: pooled normal plasma. Lane 8: monoclonal anti–human ADAMTS13 antibody applied in 1/1000 dilution. The plasma samples used for staining lanes 2-7 were diluted 1/200 with 20 mM Tris (tris(hydroxymethyl)aminomethane), 130 mM NaCl, pH 7.2 buffer, containing 0.05% (wt/vol) Tween 20 and 10% (vol/vol) blocker casein in Tris-buffered saline (TBS; Pierce, Rockford, IL). The bottom panel shows the ADAMTS13 activities and the inhibitor concentrations measured by the collagen-binding assay. (B) Quantitative evaluation of the immunoblots. The intensity of the ADAMTS13 bands in the Western blots developed with serial dilutions of a plasma sample from patient A were plotted against the applied inhibitor concentration. The inset shows the ADAMTS13 bands as they appeared on the immunoblots.
Immunoblot for detecting autoantibodies against ADAMTS13 in plasma samples. (A) Comparison of different plasma samples with a monoclonal anti–human ADAMTS13 antibody. Lane 1: molecular mass marker (full-range rainbow marker, Amersham Pharmacia). Lanes 2-4: plasma samples from a patient6 with antibody-induced TTP taken at different stages during her disease course. Lane 5: plasma sample from a patient with antibody-induced TTP. Lane 6: plasma sample from a patient with hereditary TTP. Lane 7: pooled normal plasma. Lane 8: monoclonal anti–human ADAMTS13 antibody applied in 1/1000 dilution. The plasma samples used for staining lanes 2-7 were diluted 1/200 with 20 mM Tris (tris(hydroxymethyl)aminomethane), 130 mM NaCl, pH 7.2 buffer, containing 0.05% (wt/vol) Tween 20 and 10% (vol/vol) blocker casein in Tris-buffered saline (TBS; Pierce, Rockford, IL). The bottom panel shows the ADAMTS13 activities and the inhibitor concentrations measured by the collagen-binding assay. (B) Quantitative evaluation of the immunoblots. The intensity of the ADAMTS13 bands in the Western blots developed with serial dilutions of a plasma sample from patient A were plotted against the applied inhibitor concentration. The inset shows the ADAMTS13 bands as they appeared on the immunoblots.
The results from this immunoblot assay correlated well with the quantitative determination of inhibitors by the collagen-binding assay.
The sensitivity of inhibitor detection was determined by using serial dilutions from 1/100 to 1/25 600 of a plasma sample from a patient with an inhibitor titer of 13 inhibitor units (InhU)/mL (Figure 1B). When the blots were quantified by densitometry, there was a linear correlation between the inhibitor concentrations of the samples and the band intensities in the range of 0.0005 to 0.010 InhU/mL. Taking into account that the plasma samples were diluted at least 100-fold, we estimated that the method detected inhibitory antibodies at a plasma concentration of 0.05 InhU/mL, which is far below the detection limit of currently available assays. In addition to the functional inhibitor assays, the immunoblotting can also detect noninhibitory antibodies, which will further explain the role of ADAMTS13 proteases and the pathogeneses of TTP.
The high specificity and sensitivity of this immunoblotting procedure renders the assay a valuable tool complementary to quantitative ADAMTS13 inhibitor assays and thus facilitates screening patients for antibody-induced TTP.
We are grateful for the excellent technical assistance of Manfred Billwein.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal