Abstract
Most patients with acute myeloid leukemia (AML) enter complete remission (CR) after treatment with chemotherapy, but a large number of them experience relapse with resistant disease. To identify genes that are associated with their prognoses, we analyzed gene expression in 54 pediatric patients with AML using an oligonucleotide microarray that contained 12 566 probe sets. A supervised approach using the Student t test selected a prognostic set of 35 genes, some of which are associated with the regulation of cell cycle and apoptosis. Most of these genes had not previously been reported to be associated with prognosis and were not correlated with morphologically classified French-American-British (FAB) subtypes or with karyotypes. These results indicate the existence of prognosis-associated genes that are independent of cell lineage and cytogenetic abnormalities, and they can provide therapeutic direction for individual risk-adapted therapy for pediatric AML patients.
Introduction
Acute myeloid leukemia (AML) is a heterogeneous group of genetically diverse hematopoietic malignancies.1 Current chemotherapy enables a high percentage of patients with AML to enter complete remission (CR), but many remain refractory to therapy or experience relapse with resistant disease. Because of the wide heterogeneity of this disease, predicting a patient's risk for treatment failure or relapse at the time of diagnosis is important for the optimum selection of treatment strategies. AML is classified morphologically according to the French-American-British (FAB) classification subtypes of M0 to M7.2 FAB classification is generally associated with patient outcome. The M2, M3, and M4Eo (a specific type in M4) subtypes are considered favorable, whereas the M7 subtype is considered unfavorable.3,4 Many clinical features are associated with poor outcome and include advanced age, high leukocyte count, extramedullary mass, and history of a hematologic disorder such as myelodysplastic syndrome.1,5,6 Immunophenotyping and cytogenetic analysis are also used for clinical diagnosis. Cytogenetic abnormalities in leukemic cells are now recognized as highly important prognostic determinants in AML. The t(8;21), t(15;17), and inv(16) abnormalities, which are usually found in AML with the M2, M3, and M4Eo subtypes, respectively, are associated with relatively good outcome.1,5,7-9 In contrast, the presence of a complex karyotype, –5, del(5q), –7, or a 3q abnormality defines a group with relatively poor prognosis.1,8-10 The t(9;11) translocation, which is associated with the M5 subtype, predicts a favorable outcome.10-12 In addition, many biologic and genetic features, which include internal tandem duplication of the FLT3 gene13-15 and aberrant expression of drug-resistance transporter genes16,17 and of BCL2 family genes,18,19 are reportedly associated with outcome and are useful prognostic factors. Although many prognostic factors are now available, the accurate prediction of risk for treatment failure or relapse is still difficult. To improve risk assignment and develop new therapeutic strategies, we must learn more about the biologic characteristics of leukemic cells.
Recently, gene expression profiling with DNA microarray technology has been successfully used to predict outcome in some types of malignant diseases.20-26 We also used microarray analysis to predict clinical outcomes in AML and to find genes whose aberrant expression leads to poor prognosis. Although AML occurs in the very young and the very old in an age-independent manner, some distinct characteristics have been described in childhood and adult AML.27 Pediatric AML, which develops within a relatively short lifetime, may have fewer gene mutations and may show less variation in its gene expression than adult AML. In this study, we focused on pediatric AML to investigate genes associated with prognosis.
Patients, materials, and methods
Patient samples
Characteristics of the 54 pediatric AML patients (younger than 15 years) enrolled in this study are shown in Table 1. All leukemic samples (bone marrow or peripheral blood) were obtained at the time of diagnosis and were subjected to morphologic study. The median percentage of leukemic blast cells was 67% (32%-99%). FAB classifications were performed on bone marrow smears stained with Wright-Giemsa and other cytochemical procedures. Bone marrow smears were reviewed at the study center base. All patients were treated with chemotherapy. Infants (younger than 1.5 years) were treated according to the regimen of the Japan infant leukemia study group,28 and most of the childhood patients (older than 1.5 years) were treated based on the protocol of the Japan multi-institutional chemotherapy regimen.29 Fifty-three patients entered CR, 16 of whom were treated with stem cell transplantation. CR status was designated as less than 5% of blasts in the bone marrow, and induction failure (IF) was defined as no CR achievement within 3 months of the start of treatment. Nine patients with good prognosis (GP; GP1-GP9) were randomly selected from among 19 patients in CR for more than 3 years, and 9 patients with poor prognosis (PP; PP1-PP9) were selected from among 18 patients who experienced induction failure or relapse within 1 year of the first CR. Event-free survival (EFS) was defined as the time from the start of treatment to relapse, death, or last follow-up.
Fifty-four pediatric AML patients enrolled in this study
Patient . | Age . | Sex . | FAB subtype . | Karyotype . | Outcome . | EFS, mo . | SCT . |
|---|---|---|---|---|---|---|---|
| GP1 | 10 y | M | M4 | 46XY | CR | 61.9 | + |
| GP2 | 6 mo | M | M4 | 46XY, t(11;12)(q23;q13) | CR | 40.8 | + |
| GP3 | 10 y 7 mo | M | M2 | 46XY,t(8;21) | CR | 85.5 | - |
| GP4 | 6 y 6 mo | M | M2 | 46XY,t(8;21) | CR | 37.3 | - |
| GP5 | 3 mo | M | M4 | 46XY,t(11;19)(q23;p13) | CR | 68.7 | + |
| GP6 | 3 d | M | M5 | 46XY | CR | 57.3 | + |
| GP7 | 10 mo | M | M4 | 46XY,inv(16)(p13q22) | CR | 49.9 | - |
| GP8 | 7 mo | M | M4 | 46XY | CR | 41.9 | - |
| GP9 | 12 y | M | M2 | 46XY,t(6;9)(p23;q34),add(21)(q22) | CR | 48.9 | + |
| PP1 | 10 y 1 mo | M | M4 | 46XY,inv(16) | R | 12.0 | + |
| PP2 | 22 d | F | Mixed | 46XX,t(4;11) | R | 1.0 | - |
| PP3 | 8 y 2 mo | M | M5 | 47XY,+8 | R | 9.5 | + |
| PP4 | 5 mo | M | Mixed | 47XY,+19 | IF | 1.0 | - |
| PP5 | 7 mo | M | M7 | 45XY,-16 | R | 3.0 | - |
| PP6 | 1 y | M | M2 | 46XY,inv(1)(p12;q36),addX(q26) | R | 3.4 | + |
| PP7 | 0 d | F | M5 | 46XX,t(4;11)(q21;q23) | R | 1.0 | - |
| PP8 | 1 y 7 mo | M | M7 | 46XY,t(6;22;17)(p23;q11;q12),t(12;21)(p11.2;q22) | R | 4.9 | - |
| PP9 | 13 y 6 mo | M | M2 | 46XY,t(8;21) | R | 11.7 | + |
| S1 | 4 y | M | M2 | 46XY,t(8;21) | R | 11.6 | + |
| S2 | 14 y 2 mo | F | M5 | 47XX,+21 | R | 18.2 | - |
| S3 | 6 y 7 mo | M | M5 | 46XY,t(6;11)(q25;q23) | R | 15.1 | + |
| S4 | 15 y | M | M2 | 46XY,t(9;22)(q34;q11) | R | 7.4 | + |
| S5 | 1 y | F | M5 | 46XX,t(11;19)(q23;p13.1),+der(6)t(6;8)(p21.3;q22), -6 | R | 29.1 | - |
| S6 | 11 mo | F | M7 | 46XX | R | 27.0 | - |
| S7 | 5 y 10 mo | F | M2 | 46XX,t(8;21) | R | 19.0 | + |
| S8 | 6 mo | M | M7 | 46XY,del(6)(q13) | R | 4.1 | + |
| S9 | 4 mo | F | M4 | 46XX,t(9;11)(p22;q23) | R | 21.2 | - |
| S10 | 17 d | F | M5 | NA | CR | 36.4 | - |
| S11 | 8 y | M | M2 | 45X, -Y,t(8;21)(q22;q22) | CR | 70.2 | - |
| S12 | 2 y | F | M4 | 46XX,inv(16) | CR | 22.3 | - |
| S13 | 3 mo | M | M5 | 46XY,t(9;11)(p22;q23) | CR | 20.2 | - |
| S14 | 12 y | M | M2 | 46XY,t(8;21) | CR | 11.1 | - |
| S15 | 12 y | F | M2 | 46XX,t(8;21) | CR | 3.7 | - |
| S16 | 9 mo | M | M4 | 46XY,inv(16)(p13;q22) | R | 4.9 | + |
| S17 | NA | M | M5 | NA | R | 4.0 | - |
| S18 | 1 y 9 mo | M | M7 | 47XY, +21 | R | 0.6 | - |
| S19 | 7 mo | F | M4 | 46XX | CR | 41.0 | - |
| S20 | 1 y 11 mo | M | M5 | NA | CR | 52.9 | - |
| S21 | 10 mo | M | M4 | 46XY,t(6;11)(q27;q23) | R | 4.2 | + |
| S22 | 3 mo | F | M5 | 46XX,t(11;19) | CR | 41.0 | - |
| S23 | 9 mo | F | M5 | 46XX,t(9;11)(p22;q23) | CR | 62.2 | - |
| S24 | 4 y | F | M5 | 50XXX, +6, +8, +18,del(11)(q21;q23)—11q23(-)* | R | 9.6 | - |
| S25 | 9 mo | M | M5 | 46XY,add(X)(q27)—11q23(+)* | R | 1.0 | - |
| S26 | 10 y | F | M4 | 46XX,inv(9)(p11;q13),inv(16)(p13;q22) | CR | 68.4 | - |
| S27 | 1 y 11 mo | F | M7 | NA | CR | 18.4 | - |
| S28 | NA | F | M5 | NA | R | 19.0 | - |
| S29 | 5 mo | M | M5 | NA | CR | 66.1 | - |
| S30 | 6 d | F | M0 | 46XX,add(7)(q22),del(12)(p13) | CR | 31.1 | - |
| S31 | 2 mo | M | M7 | 49XY,+19,+21,+22 | CR | 68.7 | - |
| S32 | 1 y 4 mo | F | M5 | 46XX,t(9;11) | R | 17.1 | - |
| S33 | 10 mo | M | M7 | 47XY,+21 | CR | 54.9 | - |
| S34 | NA | F | M5 | NA | CR | 13.9 | - |
| S35 | 6 mo | M | M5 | NA | CR | 27.2 | - |
| S36 | NA | F | M2 | NA | CR | 24.0 | - |
Patient . | Age . | Sex . | FAB subtype . | Karyotype . | Outcome . | EFS, mo . | SCT . |
|---|---|---|---|---|---|---|---|
| GP1 | 10 y | M | M4 | 46XY | CR | 61.9 | + |
| GP2 | 6 mo | M | M4 | 46XY, t(11;12)(q23;q13) | CR | 40.8 | + |
| GP3 | 10 y 7 mo | M | M2 | 46XY,t(8;21) | CR | 85.5 | - |
| GP4 | 6 y 6 mo | M | M2 | 46XY,t(8;21) | CR | 37.3 | - |
| GP5 | 3 mo | M | M4 | 46XY,t(11;19)(q23;p13) | CR | 68.7 | + |
| GP6 | 3 d | M | M5 | 46XY | CR | 57.3 | + |
| GP7 | 10 mo | M | M4 | 46XY,inv(16)(p13q22) | CR | 49.9 | - |
| GP8 | 7 mo | M | M4 | 46XY | CR | 41.9 | - |
| GP9 | 12 y | M | M2 | 46XY,t(6;9)(p23;q34),add(21)(q22) | CR | 48.9 | + |
| PP1 | 10 y 1 mo | M | M4 | 46XY,inv(16) | R | 12.0 | + |
| PP2 | 22 d | F | Mixed | 46XX,t(4;11) | R | 1.0 | - |
| PP3 | 8 y 2 mo | M | M5 | 47XY,+8 | R | 9.5 | + |
| PP4 | 5 mo | M | Mixed | 47XY,+19 | IF | 1.0 | - |
| PP5 | 7 mo | M | M7 | 45XY,-16 | R | 3.0 | - |
| PP6 | 1 y | M | M2 | 46XY,inv(1)(p12;q36),addX(q26) | R | 3.4 | + |
| PP7 | 0 d | F | M5 | 46XX,t(4;11)(q21;q23) | R | 1.0 | - |
| PP8 | 1 y 7 mo | M | M7 | 46XY,t(6;22;17)(p23;q11;q12),t(12;21)(p11.2;q22) | R | 4.9 | - |
| PP9 | 13 y 6 mo | M | M2 | 46XY,t(8;21) | R | 11.7 | + |
| S1 | 4 y | M | M2 | 46XY,t(8;21) | R | 11.6 | + |
| S2 | 14 y 2 mo | F | M5 | 47XX,+21 | R | 18.2 | - |
| S3 | 6 y 7 mo | M | M5 | 46XY,t(6;11)(q25;q23) | R | 15.1 | + |
| S4 | 15 y | M | M2 | 46XY,t(9;22)(q34;q11) | R | 7.4 | + |
| S5 | 1 y | F | M5 | 46XX,t(11;19)(q23;p13.1),+der(6)t(6;8)(p21.3;q22), -6 | R | 29.1 | - |
| S6 | 11 mo | F | M7 | 46XX | R | 27.0 | - |
| S7 | 5 y 10 mo | F | M2 | 46XX,t(8;21) | R | 19.0 | + |
| S8 | 6 mo | M | M7 | 46XY,del(6)(q13) | R | 4.1 | + |
| S9 | 4 mo | F | M4 | 46XX,t(9;11)(p22;q23) | R | 21.2 | - |
| S10 | 17 d | F | M5 | NA | CR | 36.4 | - |
| S11 | 8 y | M | M2 | 45X, -Y,t(8;21)(q22;q22) | CR | 70.2 | - |
| S12 | 2 y | F | M4 | 46XX,inv(16) | CR | 22.3 | - |
| S13 | 3 mo | M | M5 | 46XY,t(9;11)(p22;q23) | CR | 20.2 | - |
| S14 | 12 y | M | M2 | 46XY,t(8;21) | CR | 11.1 | - |
| S15 | 12 y | F | M2 | 46XX,t(8;21) | CR | 3.7 | - |
| S16 | 9 mo | M | M4 | 46XY,inv(16)(p13;q22) | R | 4.9 | + |
| S17 | NA | M | M5 | NA | R | 4.0 | - |
| S18 | 1 y 9 mo | M | M7 | 47XY, +21 | R | 0.6 | - |
| S19 | 7 mo | F | M4 | 46XX | CR | 41.0 | - |
| S20 | 1 y 11 mo | M | M5 | NA | CR | 52.9 | - |
| S21 | 10 mo | M | M4 | 46XY,t(6;11)(q27;q23) | R | 4.2 | + |
| S22 | 3 mo | F | M5 | 46XX,t(11;19) | CR | 41.0 | - |
| S23 | 9 mo | F | M5 | 46XX,t(9;11)(p22;q23) | CR | 62.2 | - |
| S24 | 4 y | F | M5 | 50XXX, +6, +8, +18,del(11)(q21;q23)—11q23(-)* | R | 9.6 | - |
| S25 | 9 mo | M | M5 | 46XY,add(X)(q27)—11q23(+)* | R | 1.0 | - |
| S26 | 10 y | F | M4 | 46XX,inv(9)(p11;q13),inv(16)(p13;q22) | CR | 68.4 | - |
| S27 | 1 y 11 mo | F | M7 | NA | CR | 18.4 | - |
| S28 | NA | F | M5 | NA | R | 19.0 | - |
| S29 | 5 mo | M | M5 | NA | CR | 66.1 | - |
| S30 | 6 d | F | M0 | 46XX,add(7)(q22),del(12)(p13) | CR | 31.1 | - |
| S31 | 2 mo | M | M7 | 49XY,+19,+21,+22 | CR | 68.7 | - |
| S32 | 1 y 4 mo | F | M5 | 46XX,t(9;11) | R | 17.1 | - |
| S33 | 10 mo | M | M7 | 47XY,+21 | CR | 54.9 | - |
| S34 | NA | F | M5 | NA | CR | 13.9 | - |
| S35 | 6 mo | M | M5 | NA | CR | 27.2 | - |
| S36 | NA | F | M2 | NA | CR | 24.0 | - |
EFFS indicates event-free survival; SCT, stem cell transplantation; NA, no available data; CR, not relapsed (complete remission), R, relapsed; IF, induction failure; and -, patients without SCT.
S24 had del(11)(q21;q23), but MLL gene rearrangement was not detected by Southern hybridization analysis; thus, this patient was judged as 11q23 rearrangement-negative. S25 did not show any 11q23 rearrangement by karyotyping, but MLL gene rearrangement was detected by Southern hybridization analysis; thus, this patient was judged as 11q23 rearrangement-positive.
Our study was approved by the ethics committee of the National Cancer Center. Patient identities were masked, and study numbers were assigned to all collected samples. To maintain anonymity, only clinical outcome, age, sex, FAB subtype, and chromosomal abnormalities of the patients were linked with gene expression profile results. This study was conducted according to tenets of the Declaration of Helsinki, and informed consent was provided.
Gene expression profiling
Mononuclear cells were collected from the peripheral blood or bone marrow of AML patients at the time of diagnosis and were stored in liquid nitrogen until used. Total RNA was isolated using RNeasy columns (Qiagen, Hilden, Germany). Total RNA from bone marrow mononuclear cells of 3 healthy volunteers was used for the controls. In addition, total RNA of bone marrow purchased from BD Biosciences Clontech (Palo Alto, CA) was also used as a control. The integrity of the total RNA was confirmed using RNA LabChip and 2100 Bioanalyzer (Agilent Technologies, Waldbronn, Germany). For gene expression profiling, a GeneChip Human Genome U95Av2 oligonucleotide microarray (Affymetrix, Santa Clara, CA) was used that contained 12 566 probe sets. Target cRNA preparations from total RNA, hybridization to the microarray, washing and staining with the antibody amplification procedure, and scanning were all carried out according to the manufacturer's instructions. The expression value (average difference; AD) of each gene was calculated and normalized (that is, multiplied by scaling factor [Data Set 1; see the Blood web site for the Supplemental Materials link at the top of the online article]) using Affymetrix Microarray Suite software version 4.0 so that the mean of AD values in each experiment was 100 to adjust for minor differences between the experiments.
Statistical analysis
The Student t test was used to examine whether each gene was expressed differentially between the 2 comparison groups of patients. To identify the FAB subtype-specific genes, we compared 12 patients with the M2 subtype and 42 patients with the other FAB subtypes, 31 patients with the M4 or M5 subtypes and 23 patients with the other FAB subtypes, and 8 patients with the M7 subtype and 46 patients with the other FAB subtypes. We then selected probe sets with P < .001 in one comparison but not in the other comparisons. To identify chromosome-rearrangement–specific genes, we compared 8 patients with t(8;21) and 37 patients known not to have t(8;21), 5 patients with inv(16) and 40 patients known not to have inv(16), 4 patients with t(9;11) and 41 patients known not to have t(9;11), and 13 patients with the 11q23 rearrangement and 32 patients known not to have the 11q23 rearrangement. We then selected probe sets with P < .001 in each comparison among the 45 patients for which karyotyping data are available.
Hierarchical clustering was performed using Cluster and TreeView software.30 For this analysis, all values for each gene were normalized by the median. Principal component analysis (PCA) was performed using GeneMaths software (Applied Maths, Saint-Martens-Latem, Belgium). Support vector machine (SVM) analysis was performed using NeuroSolutions (NeuroDimension, Gainesville, FL). The Kaplan-Meier method and log-rank test were used for comparison of EFS between 2 groups.
Results
Gene expression profiling of pediatric AML
We analyzed 54 pediatric patients with AML (Table 1)—1 with the M0 subtype, 12 with M2, 12 with M4, 19 with M5, 8 with M7, and 2 unclassified with mixed lineage. Patients with the M3 subtype were excluded from this study because they received a substantially distinct therapy. Among the 45 patients whose karyotypes were analyzed, 8 patients with the M2 subtype had t(8;21), 5 with M4 had inv(16), and 1 with M4 and 3 with M5 had t(9;11). Thirteen (42%) of the 31 patients with the M4 or M5 subtype had the 11q23 rearrangement. Although 1 patient was resistant to chemotherapy, all the others entered CR. Sixteen patients underwent stem cell transplantation. Among the 53 patients who achieved CR, 25 (47%), including 11 who had undergone stem cell transplantation, experienced relapse within 0.6 to 29.1 months (median, 9.5 months) from the first CR.
By using the diagnostic samples of bone marrow or peripheral blood, we investigated the gene expression of these 54 patients with an oligonucleotide microarray containing 12 566 probe sets. We also obtained gene expression data of 4 normal bone marrow (NBM) samples as controls. As an introductory approach to analyze these gene expression data, we used unsupervised hierarchical clustering analysis30 to cluster patients and genes on the basis of similarity of expression pattern. In this 2-dimensional clustering analysis, patients with t(8;21), inv(16), M4/M5 subtype, and M7 subtype formed clusters (an example is shown in Supplemental Figure S1), suggesting the existence of gene expression patterns specific to t(8;21), inv(16), M4/M5 subtype, and M7 subtype. However, we could not find any expression pattern related to prognosis by this unsupervised clustering analysis.
Prediction of outcome by gene expression profiling
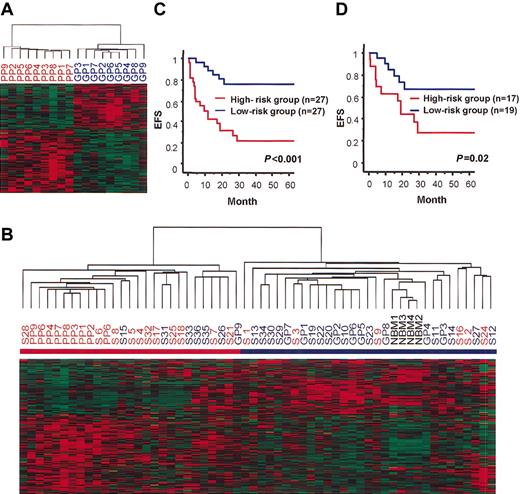
The gene expression signature related to prognosis may be hidden under the gene expression patterns determined by chromosomal abnormalities and FAB subtypes. Thus, to find out the genes associated with prognosis, we adopted the Student t test to directly examine whether the expression of each gene differed between patients with good outcomes and those with poor outcomes. We first selected 18 representative patients (9 with good prognosis and 9 with poor prognosis) from the 54 AML patients (see “Patients, materials, and methods” and Table 1). The GP patients (GP1-GP9) survived for more than 3 years without relapse; this group consisted of 3 patients with M2, 5 with M4, and 1 with M5. The PP patients (PP1-PP9) did not enter CR (induction failure; IF) or they experienced relapse within 1 year of the first CR. This group consisted of 2 patients with M2, 1 with M4, 2 with M5, 2 with M7, and 2 unclassified with mixed lineage. Then we selected genes with P < .01 in the t test to identify 133 genes (135 probe sets) that were differentially expressed between the GP and PP patients (see Data Set 2). Hierarchical clustering analysis using these 133 genes separated the GP and PP patients clearly (Figure 1A). To confirm that these 133 genes are actually related to prognosis, we added the remaining 36 patients and 4 NBM controls to the analysis as test samples. These 36 patients consisted of 17 patients who experienced relapse 0.6 to 29.1 months after the first CR and 19 patients who had not experienced relapse within a follow-up duration of 3.7 to 70.2 months. Hierarchical clustering analysis with the 133 genes clearly separated the 54 patients and 4 NBM controls into 2 groups (Figure 1B). One group consisted of 27 patients, including all the PP patients and 1 GP patient, and was designated the high-risk group. The other group consisted of 27 patients and 4 NBM controls, including 8 GP patients, and was designated the low-risk group. The Kaplan-Meier plot and log-rank test showed that EFS was significantly different between these 2 groups (P < .001) (Figure 1C). In addition, even when GP and PP patients were excluded from the analysis, a significant difference in EFS was still observed between these 2 groups (P = .02) (Figure 1D). Thus, at least some of the 133 genes we selected are associated with the prognosis of pediatric AML, and we can predict outcome to some extent by analyzing the expression of these genes.
Separation of pediatric AML patients based on the expression data of the 133 prognosis-associated genes. (A) Hierarchical clustering of 9 GP and 9 PP patients based on the expression of the 133 prognosis-associated genes. Each row represents a separate gene, and genes were also hierarchically clustered. Expression levels are normalized for each gene and are indicated by color, with red representing high expression and green representing low expression. The dendrogram at the top shows the degree to which each patient is related to the others with respect to gene expression. (B) Hierarchical clustering of 54 patients and 4 NBM controls based on the expression of the 133 prognosis-associated genes. Patients were divided into 2 major clusters indicated by red and blue bars (high-risk and low-risk groups, respectively). All 4 NBM controls were included in the low-risk group. The patients with red and blue letters represent those who experienced relapse or who had induction failure and those who did not experience relapse, respectively. (C-D) Kaplan-Meier plots of EFS of patients divided into high-risk and low-risk groups based on the expression data of the 133 prognosis-associated genes. EFS was significantly different between the 2 groups when compared in the 54 patients including GP and PP patients (panel C, P < .001 in log-rank test) and in the 36 patients excluding the GP and PP patients (panel D, P = .02 in log-rank test).
Separation of pediatric AML patients based on the expression data of the 133 prognosis-associated genes. (A) Hierarchical clustering of 9 GP and 9 PP patients based on the expression of the 133 prognosis-associated genes. Each row represents a separate gene, and genes were also hierarchically clustered. Expression levels are normalized for each gene and are indicated by color, with red representing high expression and green representing low expression. The dendrogram at the top shows the degree to which each patient is related to the others with respect to gene expression. (B) Hierarchical clustering of 54 patients and 4 NBM controls based on the expression of the 133 prognosis-associated genes. Patients were divided into 2 major clusters indicated by red and blue bars (high-risk and low-risk groups, respectively). All 4 NBM controls were included in the low-risk group. The patients with red and blue letters represent those who experienced relapse or who had induction failure and those who did not experience relapse, respectively. (C-D) Kaplan-Meier plots of EFS of patients divided into high-risk and low-risk groups based on the expression data of the 133 prognosis-associated genes. EFS was significantly different between the 2 groups when compared in the 54 patients including GP and PP patients (panel C, P < .001 in log-rank test) and in the 36 patients excluding the GP and PP patients (panel D, P = .02 in log-rank test).
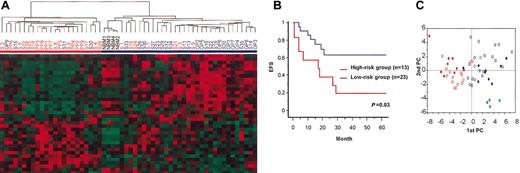
However, we expected that some of the 133 genes were selected erroneously in the microarray experiments and by chance in the t test and were not associated with prognosis. To exclude them as much as possible, we further selected the highly expressed genes from among the 133 genes because they were expected to have relatively low experimental errors. Thirty-five genes (36 probe sets) were selected as prognosis associated after genes with a median expression value (see “Patients, materials, and methods”) lower than 200 in the PP and GP groups were excluded (Table 2). Hierarchical clustering analysis using these 35 genes again separated the 54 AML patients into high-risk and low-risk groups (Figure 2A). By this 35-gene analysis, 6 patients (S7, S21, S26, S33, S35, S36) from the high-risk group according to the 133-gene analysis were reclassified to the low-risk group, and 2 patients (S2, S30) in the low-risk group according to the 133-gene analysis were reclassified to the high-risk group. However, the Kaplan-Meier plot and log-rank test again showed significant differences in EFS between the 2 groups separated by the 35-gene analysis, not only when all patients were included (P < .001) (data not shown) but also when the GP and PP patients were excluded (P = .03) (Figure 2B). This indicates that these 35 genes can aid in predicting the outcomes of pediatric AML patients. To visualize differences in the expression of these 35 genes among the patients, we carried out a principle component analysis (PCA) to reduce multidimensional data to a few specified dimensions. This analysis also showed that patients were distributed by the expression of these 35 genes, but patients with poor outcomes (PP and other patients in relapse) and those with good outcomes (GP and other patients not in relapse) were substantially separated by 2 major components (Figure 2C).
Thirty-five genes (36 probe sets) associated with prognosis of pediatric AML
. | . | . | P . | Median expression value . | . | |
|---|---|---|---|---|---|---|
| Gene symbol . | Gene name . | Probe set ID . | t test . | PP patients . | GP patients . | |
| VDAC1 | Voltage-dependent anion channel 1 | 40198_at | .0002 | 365.6 | 201.3 | |
| ZYX | Zyxin | 36958_at | .0005 | 403.4 | 905.5 | |
| VAT1 | Vesicle amine transport protein 1 | 40147_at | .0007 | 342.0 | 757.1 | |
| 605_at | .0042 | 239.4 | 479.5 | |||
| NPC2 | Niemann-Pick disease type C2 | 39345_at | .0008 | 560.6 | 881.3 | |
| AZU1 | Azurocidin 1 | 33963_at | .0015 | -149.8 | 3509.6 | |
| ECGF1 | Endothelial cell growth factor 1 | 36879_at | .0018 | -7.5 | 372.3 | |
| HOMER-3 | Homer-3 | 38233_at | .0020 | 104.0 | 290.3 | |
| PGD | Phosphogluconate dehydrogenase | 36963_at | .0022 | 1041.7 | 1866.1 | |
| ENSA | Endosulfine α | 39011_at | .0025 | 467.7 | 393.4 | |
| TKT | Transketolase | 38789_at | .0028 | 892.2 | 1981.9 | |
| BST1 | Bone marrow stromal cell antigen 1 | 32675_at | .0030 | 70.5 | 219.6 | |
| STK17B | Serine/threonine kinase 17b | 33390_at | .0033 | 252.7 | 946.8 | |
| CDK6 | Cyclin-dependent kinase 6 | 1207_at | .0034 | 217.8 | 138.0 | |
| RAB32 | Rab32 | 41523_at | .0035 | 94.5 | 236.1 | |
| PTP4A2 | Protein tyrosine phosphatase type 4A member 2 | 1241_at | .0040 | 582.7 | 745.4 | |
| APLP2 | Amyloid β A4 precursor-like protein 2 | 33944_at | .0041 | 602.8 | 1023.1 | |
| CYLN2 | Cytoplasmic linker 2 | 41396_at | .0041 | 261.7 | 139.9 | |
| OGT | O-linked N-acetylglucosamine transferase | 38614_s_at | .0044 | 211.7 | 116.3 | |
| HNRPD | Heterogeneous nuclear ribonucleoprotein D | 38016_at | .0059 | 701.6 | 546.4 | |
| (W28504) | EST | 36338_at | .0060 | 450.5 | 251.6 | |
| (D50525) | EST | 33683_at | .0061 | 243.4 | 192.9 | |
| POLR2H | RNA polymerase 2 polypeptide H | 35631_at | .0064 | 200.2 | 144.0 | |
| TIAL1 | TIA1 cytotoxic granule-associated RNA binding protein-like 1 | 41763_g_at | .0067 | 316.2 | 238.5 | |
| ATP6F | Lysosomal H(+)-transporting ATPase subunit F | 36167_at | .0075 | 844.2 | 1113.3 | |
| NME1 | Protein expressed in nonmetastatic cells 1 | 1985_s_at | .0076 | 822.4 | 455.2 | |
| GRIK5 | Ionotropic glutamate receptor kainate 5 | 34905_at | .0078 | 209.8 | 103.8 | |
| CD14 | CD14 antigen | 36661_s_at | .0078 | 2.4 | 583.8 | |
| GABARAP | GABA(A) receptor-associated protein | 37298_at | .0080 | 911.4 | 1728.7 | |
| NFKBIA | NF-κB inhibitor α | 1461_at | .0080 | 1012.4 | 1738.7 | |
| GCN5L2 | GCN5-like 2 | 38628_at | .0080 | 406.8 | 258.9 | |
| HSPE1 | Heat shock protein 1, 10 kDa | 39353_at | .0083 | 540.6 | 330.4 | |
| LBR | Laminin B receptor | 288_s_at | .0088 | 222.6 | 430.3 | |
| ECE2 | Endothelin converting enzyme 2 | 35536_at | .0090 | 248.8 | 170.7 | |
| BZRP | Benzodiazepine receptor | 32806_at | .0093 | 1690.0 | 2937.9 | |
| XPO1 | Exportin 1 | 37729_at | .0094 | 733.5 | 443.0 | |
. | . | . | P . | Median expression value . | . | |
|---|---|---|---|---|---|---|
| Gene symbol . | Gene name . | Probe set ID . | t test . | PP patients . | GP patients . | |
| VDAC1 | Voltage-dependent anion channel 1 | 40198_at | .0002 | 365.6 | 201.3 | |
| ZYX | Zyxin | 36958_at | .0005 | 403.4 | 905.5 | |
| VAT1 | Vesicle amine transport protein 1 | 40147_at | .0007 | 342.0 | 757.1 | |
| 605_at | .0042 | 239.4 | 479.5 | |||
| NPC2 | Niemann-Pick disease type C2 | 39345_at | .0008 | 560.6 | 881.3 | |
| AZU1 | Azurocidin 1 | 33963_at | .0015 | -149.8 | 3509.6 | |
| ECGF1 | Endothelial cell growth factor 1 | 36879_at | .0018 | -7.5 | 372.3 | |
| HOMER-3 | Homer-3 | 38233_at | .0020 | 104.0 | 290.3 | |
| PGD | Phosphogluconate dehydrogenase | 36963_at | .0022 | 1041.7 | 1866.1 | |
| ENSA | Endosulfine α | 39011_at | .0025 | 467.7 | 393.4 | |
| TKT | Transketolase | 38789_at | .0028 | 892.2 | 1981.9 | |
| BST1 | Bone marrow stromal cell antigen 1 | 32675_at | .0030 | 70.5 | 219.6 | |
| STK17B | Serine/threonine kinase 17b | 33390_at | .0033 | 252.7 | 946.8 | |
| CDK6 | Cyclin-dependent kinase 6 | 1207_at | .0034 | 217.8 | 138.0 | |
| RAB32 | Rab32 | 41523_at | .0035 | 94.5 | 236.1 | |
| PTP4A2 | Protein tyrosine phosphatase type 4A member 2 | 1241_at | .0040 | 582.7 | 745.4 | |
| APLP2 | Amyloid β A4 precursor-like protein 2 | 33944_at | .0041 | 602.8 | 1023.1 | |
| CYLN2 | Cytoplasmic linker 2 | 41396_at | .0041 | 261.7 | 139.9 | |
| OGT | O-linked N-acetylglucosamine transferase | 38614_s_at | .0044 | 211.7 | 116.3 | |
| HNRPD | Heterogeneous nuclear ribonucleoprotein D | 38016_at | .0059 | 701.6 | 546.4 | |
| (W28504) | EST | 36338_at | .0060 | 450.5 | 251.6 | |
| (D50525) | EST | 33683_at | .0061 | 243.4 | 192.9 | |
| POLR2H | RNA polymerase 2 polypeptide H | 35631_at | .0064 | 200.2 | 144.0 | |
| TIAL1 | TIA1 cytotoxic granule-associated RNA binding protein-like 1 | 41763_g_at | .0067 | 316.2 | 238.5 | |
| ATP6F | Lysosomal H(+)-transporting ATPase subunit F | 36167_at | .0075 | 844.2 | 1113.3 | |
| NME1 | Protein expressed in nonmetastatic cells 1 | 1985_s_at | .0076 | 822.4 | 455.2 | |
| GRIK5 | Ionotropic glutamate receptor kainate 5 | 34905_at | .0078 | 209.8 | 103.8 | |
| CD14 | CD14 antigen | 36661_s_at | .0078 | 2.4 | 583.8 | |
| GABARAP | GABA(A) receptor-associated protein | 37298_at | .0080 | 911.4 | 1728.7 | |
| NFKBIA | NF-κB inhibitor α | 1461_at | .0080 | 1012.4 | 1738.7 | |
| GCN5L2 | GCN5-like 2 | 38628_at | .0080 | 406.8 | 258.9 | |
| HSPE1 | Heat shock protein 1, 10 kDa | 39353_at | .0083 | 540.6 | 330.4 | |
| LBR | Laminin B receptor | 288_s_at | .0088 | 222.6 | 430.3 | |
| ECE2 | Endothelin converting enzyme 2 | 35536_at | .0090 | 248.8 | 170.7 | |
| BZRP | Benzodiazepine receptor | 32806_at | .0093 | 1690.0 | 2937.9 | |
| XPO1 | Exportin 1 | 37729_at | .0094 | 733.5 | 443.0 | |
Shown are the 35 genes (36 probe sets) with P < .01 according to the t test and with median expression values greater than 200 in the PP or GP patients.
Separation of patients based on their expression of the 35 prognosis-associated genes. (A) Hierarchical clustering of 54 patients and 4 NBM controls based on the expression of 35 prognosis-associated genes. Patients were divided into 2 major clusters indicated by red and blue bars (high-risk and low-risk groups, respectively). (B) Kaplan-Meier plots of EFS of patients divided into high-risk and low-risk groups based on the expression of the 35 prognosis-associated genes (GP and PP patients were excluded). EFS differed significantly between the 2 groups (P = .03 in log-rank test). (C) PCA of 54 patients and 4 NBM controls based on the expression of 35 prognosis-associated genes. The first and second components, which represent 31.8% and 12.5%, respectively, are shown. Red and blue closed circles indicate the PP and GP patients, respectively. Red and blue open circles indicate other patients who experienced relapse and those who did not experience relapse, respectively. Green closed circles indicate NBM controls. Patients who had relapses, including PP patients, those who did not have relapses, including GP patients, and NBM controls were clustered separately.
Separation of patients based on their expression of the 35 prognosis-associated genes. (A) Hierarchical clustering of 54 patients and 4 NBM controls based on the expression of 35 prognosis-associated genes. Patients were divided into 2 major clusters indicated by red and blue bars (high-risk and low-risk groups, respectively). (B) Kaplan-Meier plots of EFS of patients divided into high-risk and low-risk groups based on the expression of the 35 prognosis-associated genes (GP and PP patients were excluded). EFS differed significantly between the 2 groups (P = .03 in log-rank test). (C) PCA of 54 patients and 4 NBM controls based on the expression of 35 prognosis-associated genes. The first and second components, which represent 31.8% and 12.5%, respectively, are shown. Red and blue closed circles indicate the PP and GP patients, respectively. Red and blue open circles indicate other patients who experienced relapse and those who did not experience relapse, respectively. Green closed circles indicate NBM controls. Patients who had relapses, including PP patients, those who did not have relapses, including GP patients, and NBM controls were clustered separately.
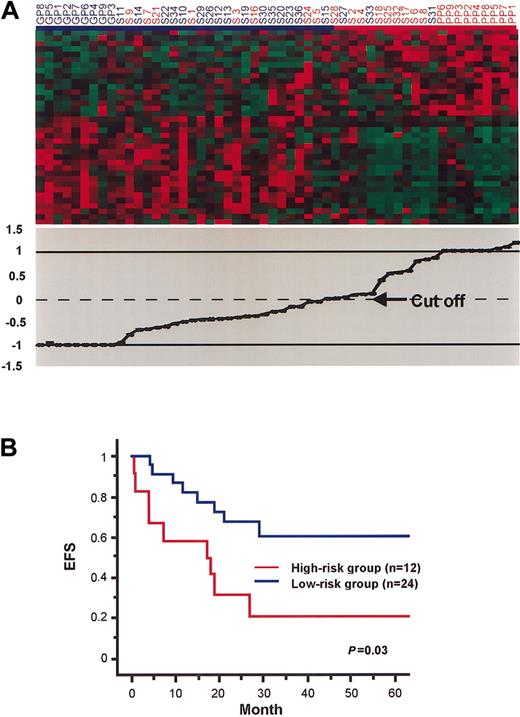
In addition, as another approach to predict the outcomes of patients based on the expression of the 35 genes, we used a support vector machine (SVM). GP and PP patients were used as training samples, and the other 36 patients were used as test samples. When GP and PP patients were initially assigned as –1 and 1, respectively, values of the test samples were calculated to be –0.954 to 0.950. As the cut-off value was set to 0, 12 of the 36 patients were judged to be in the high-risk group, and the others were in the low-risk group (Figure 3A). This classification using the SVM was also significantly correlated with actual clinical outcomes (Figure 3B).
Separation of patients by SVM based on their expression of the 35 prognosis-associated genes. (A) Patients are ordered according to their values calculated by SVM, when PP and GP patients were used as training samples and set as 1 and –1, respectively (lower panel). Patients with values greater than 0 were classified as the high-risk group (indicated by red bar), and the remaining patients were classified as the low-risk group (indicated by blue bar). Expression of the 35 genes in these patients is also shown (upper panel). Each column represents a patient, and each row represents a gene. (B) Kaplan-Meier plots of EFS of patients divided into the high-risk and low-risk groups by SVM based on the 35 prognosis-associated genes (PP and GP patients were excluded). EFS differed significantly between the 2 groups (P = .03 in log-rank test).
Separation of patients by SVM based on their expression of the 35 prognosis-associated genes. (A) Patients are ordered according to their values calculated by SVM, when PP and GP patients were used as training samples and set as 1 and –1, respectively (lower panel). Patients with values greater than 0 were classified as the high-risk group (indicated by red bar), and the remaining patients were classified as the low-risk group (indicated by blue bar). Expression of the 35 genes in these patients is also shown (upper panel). Each column represents a patient, and each row represents a gene. (B) Kaplan-Meier plots of EFS of patients divided into the high-risk and low-risk groups by SVM based on the 35 prognosis-associated genes (PP and GP patients were excluded). EFS differed significantly between the 2 groups (P = .03 in log-rank test).
Genes associated with prognosis
The 35 genes we selected as prognosis-associated genes are shown in Table 2. Among them, only NME1 was previously reported to be associated with AML prognosis.31 Drug-resistance transporter genes and BCL2 family genes, which are often involved in chemotherapy resistance, were not included. Although their association with prognosis has not been reported, a few of the genes function in cell cycle and apoptosis regulation. CDK6, which encodes a cyclin-dependent protein kinase, was included (P = .0034), and its expression was higher in the PP patients. In addition, though they were not included in our group of 35 prognosis-associated genes, the expression of CCND1, which encodes cyclin D1, and CDC25A, which encodes a protein tyrosine phosphatase to activate CDK4 and CDK6, was significantly higher in the PP patients (P = .031 and P = .048, respectively). Thus, increased cell cycle progression of leukemic cells might lead to poor prognosis for AML patients.
The expression of STK17B, which encodes a protein serine/threonine kinase that induces apoptosis when expressed in NIH3T3 cells,32 was lower in the PP patients (P = .0033). In addition, the NF-κB signal, which usually represses apoptosis, was expected to be enhanced. The expression of NFKBIA, which encodes an inhibitor (IκB) of NF-κB, was lower in the PP patients (P = .0080), and the expression of TRAF2, which encodes a signal transducer to activate an NF-κB signal, was higher in the PP patients (P = .0031). Thus, apoptotic potential may be repressed by aberrant expression of these genes in the PP patients.
Genes related to FAB subtypes and chromosomal translocations
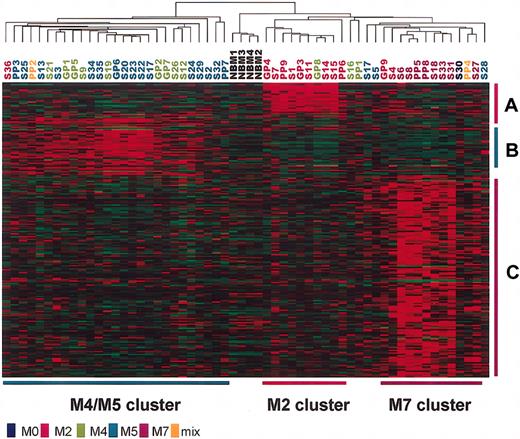
Previous studies25,33,34 showed that gene expression profiles of acute leukemia are closely related to the specific chromosomal translocations of the disease, which are often associated with FAB subtypes. In addition, our unsupervised hierarchical clustering analysis of the 54 pediatric AML patients also detected gene expression patterns related to the t(8;21) and inv(16) chromosomal aberrations and to the M4/M5 and M7 subtypes. On the other hand, FAB subtypes and some chromosomal translocations of AML are related to clinical outcome. Therefore, we compared genes associated with FAB subtypes and some chromosomal translocations with the 35 prognosis-associated genes that we identified. Fifty-two AML patients with known clinical FAB subtypes were subjected to analysis for identification of the FAB subtype-specific genes. By comparing patients with specific FAB subtypes (M2, M4/M5, M7) with the others, we selected 213 probe sets (21 M2-specific, 46 M4/M5-specific, 146 M7-specific probe sets) with P < .001 in the t test (see “Patients, materials, and methods” and Data Set 3). Hierarchical clustering analysis of the 54 patients and 4 NBM controls using the 213 FAB subtype-specific probe sets separated most of the patients with the M2, M4, M5, and M7 subtypes into the appropriate clusters (M2, M4/M5, M7 clusters), though the M4/M5 cluster included one patient with M2 and one with mixed lineage, and the M2 cluster included one patient with M4 (Figure 4). Similarly, we also identified 424 t(8;21)–specific, 471 inv(16)-specific, 144 t(9;11)–specific, and 388 11q23 rearrangement-specific probe sets with P < .001 in the t test (see “Patients, materials, and methods” and Data Set 4). Surprisingly, only 3 (ECGF1, STK17B, ATP6F) of the 35 prognosis-associated genes overlapped with the FAB subtype-specific genes and chromosome rearrangement-specific genes. ECGF1 and STK17B were included in the M4/M5-specific genes, and ATP6F was in the t(9;11)–specific genes. In addition, we did not find statistically significant differences in survival between 2 clusters that were separated by a hierarchical clustering analysis with FAB subtype-specific probe sets or with chromosome rearrangement-specific probe sets (data not shown). Thus, the gene expression signature related to prognosis that we identified was substantially independent of the FAB subtypes and chromosome rearrangements in patients.
Clustering of patients based on the expression data of the 213 FAB subtype-specific genes. Each column represents a patient, and each row represents a gene. Patient numbers are colored according to their FAB subtypes. M2-, M4/M5-, and M7-specific genes are indicated by red (A), green (B), and purple (C) vertical bars, respectively. M2, M4/M5, and M7 clusters of patients are indicated by red, green, and purple horizontal bars, respectively.
Clustering of patients based on the expression data of the 213 FAB subtype-specific genes. Each column represents a patient, and each row represents a gene. Patient numbers are colored according to their FAB subtypes. M2-, M4/M5-, and M7-specific genes are indicated by red (A), green (B), and purple (C) vertical bars, respectively. M2, M4/M5, and M7 clusters of patients are indicated by red, green, and purple horizontal bars, respectively.
Discussion
In this study, we identified 35 genes associated with prognosis of pediatric AML by selecting genes differentially expressed between representative patients with unfavorable outcomes and those with good outcomes. Then we confirmed that expression of these genes is actually associated with prognosis by analyzing the remaining patients with relatively intermediate phenotypes. Thus, we concluded that these genes are useful to predict outcomes of pediatric AML patients at the time of diagnosis.
Many approaches have been undertaken to predict the risk for treatment failure and relapse at the time of AML diagnosis. Diagnostic predictions have been based on clinical data such as age of onset, sex, leukocyte count, immunophenotype, FAB subtype, and cytogenetic abnormality. In addition, several genes are reportedly associated with the outcome of AML, including drug resistance-associated genes such as ABCB1 (MDR1),16 ABCG2 (BCRP),17 NT5C2,35 and the BCL2 family of genes.18,19 ABCB1 and ABCG2 encode adenosine triphosphate (ATP)–binding cassette transporters that confer resistance to multiple drugs. NT5C2 encodes a cytoplasmic 5′-nucleotidase involved in cytarabine resistance. However, the expression of these genes was not significantly different between the PP and GP patients (ABCB1, P = .79; ABCG2, P = .10; NT5C2, P = .45). The expression of the BCL2 family of genes, which regulate apoptosis, was not significantly different in this study either (BCL2, P = .65; BAX, P = .30; BCL2L1 [BCLXL and BCLXS], P = .46; BCL2L11 [BIM], P = .37; BAD, P = .20; BIK, P = .42). However, the expression of STK17B, which has potential to induce apoptosis, was lower in the PP patients than in the GP patients. The expression of NFKBIA, which encodes an inhibitor of NF-κB, was lower, and that of TRAF2, which encodes a signal transducer to activate NF-κB, was higher in the PP patients. Recent studies show that NF-κB is constitutively activated in most primary AML cells and that the inhibition of NF-κB induces apoptosis.36,37 In AML patients with poor prognosis, leukemic cells might escape apoptosis after chemotherapy because of the low expression of STK17B and enhancement of the NF-κB signal. In addition, because higher expression of CDK6, CCND1 (cyclin D1), and CDC25A was observed in the PP patients than in the GP patients, increased cell cycle progression of leukemic cells might lead to poor prognosis.
Most of the 35 genes we selected did not correspond to known prognostic genes or to genes associated with FAB subtypes or chromosome rearrangements. Because more than half the patients analyzed in this study received a recently improved intensive chemotherapy that produced excellent therapeutic results,28 the previously reported prognostic factors might not have been relevant. In fact, age of onset, sex, chromosome rearrangements such as t(8;21), inv(16), and t(9;11) and FAB subtypes other than M7 were not significant prognostic factors for patients enrolled in this study (data not shown). By analyzing such patients, we identified previously unknown prognosis-associated genes. These newly identified prognosis-associated genes and pathways, such as the NF-κB signal, may serve as molecular targets for new therapeutic strategies to repress the relapse of pediatric AML.
In this study, we successfully classified pediatricAML patients based on their risk for relapse, but the gene expression signature associated with prognosis was faint. Unsupervised analysis did not detect this expression signature from gene expression profiles largely reflecting morphologic subtypes and chromosome aberrations. Even when a supervised approach was used, probe sets with P < .01 in the t test were only 135 (1.1%) of the 12 566 probe sets. However, as clearly shown above, at least some of the genes represented by these probe sets are associated with prognosis. Thus, DNA microarray technology is useful to find such a tiny gene expression signature.
In summary, we demonstrated the existence of a gene expression signature associated with prognosis of pediatric AML that is independent of FAB subtypes and cytogenetic abnormalities. Microarray-based gene expression profiling can be used to predict outcomes in pediatric AML and to formulate individual risk-adjusted treatment strategies. Because we analyzed only pediatric patients in this study, it is unclear whether these genes are also useful for predicting the outcomes of adult patients. In addition, more accurate prediction is desirable even for pediatric patients. Further analysis is essential to identify a universal gene set to predict outcomes of all AML patients accurately.
Prepublished online as Blood First Edition Paper, May 8, 2003; DOI 10.1182/blood-2003-02-0578.
Supported by the Program for Promotion of Fundamental Studies in Health Sciences of the Organization for Drug ADR Relief, R&D Promotion, and Product Review; by a Grant-in-Aid for Cancer Research from the Ministry of Health, Labor and Welfare; and by a Grant-in-Aid for Scientific Research on Priority Areas from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
The authors declare that they have no competing financial interests.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal