Abstract
The intracellular pathways by which inflammatory mediators transmit their angiogenic signals is not well studied. The effects of a potent inflammatory mediator, bacterial lipopolysaccharide (LPS), are transmitted through Toll-like receptors (TLRs). A major, although not exclusive, LPS/TLR intracellular signaling pathway is routed through TNF (tumor necrosis factor) receptor associated factor 6 (TRAF6). In this report we demonstrate that LPS directly stimulates endothelial sprouting in vitro. By blocking TRAF6 activity using retroviral expression of a dominant-negative TRAF6 in endothelial cells, we show that TRAF6 is absolutely required for the LPS-initiated angiogenic response in vitro and in vivo. Inhibition of either c-Jun N-terminal kinase (JNK) activity or nuclear factor κB (NF-κB) activity, downstream of TRAF6, is sufficient to inhibit LPS-induced endothelial sprouting. In contrast, only inhibition of NF-κB, but not JNK, activity blocks basic fibroblast growth factor (bFGF)–induced angiogenesis. Our findings thus demonstrate a direct endothelial-stimulatory role of LPS in initiating angiogenesis through activation of TRAF6-dependent signaling pathways.
Introduction
Although several angiogenic cytokines have been described, the understanding of signaling pathways through which these factors transduce their message remains in its infancy. In particular, little has been reported on the intracellular angiogenic signaling mediated by inflammatory mediators. There is controversy as to whether inflammatory mediators such as tumor necrosis factor (TNF) and bacterial lipopolysaccharide (LPS) directly stimulate endothelial sprouting and angiogenesis, or whether these mediators act by stimulating the release of intermediary growth factors/cytokines.1-4 Several studies have demonstrated the angiogenic potential of LPS.2,5-7 However, in some cases it has been suggested that the angiogenic effect of LPS may be secondary to the release of angiogenic factors from adjacent nonendothelial cells, rather than a direct effect on the endothelium.2,8 In this report we demonstrate that, in contrast to TNF, LPS directly stimulates endothelial sprouting in vitro. Because TNF receptor associated factor 6 (TRAF6) is a major route of the LPS/Toll-like receptor (TLR) signaling pathway, we have used dominant-negative constructs of TRAF6 and downstream signaling molecules to dissect the intracellular signal transduction pathway required for LPS-mediated induction of angiogenesis in vitro and in vivo.9-11
Study design
Reagents and cell lines
Generation and characterization of retrovirally transduced human dermal microvascular endothelial cell lines (HMEC-vector, HMEC-TRAF6-C, HMEC-IκBmt, HMEC-JNK-APF) has been reported previously.11 The avian retroviral packaging cell line Q2bn was a gift from K. McNagny (University of British Columbia, Vancouver, Canada).
Endothelial sprouting assay
Endothelial sprouting was assessed as previously described.12 Briefly, microcarrier beads coated with gelatin were seeded with HMEC lines and embedded in fibrin gels in 96-well plates. Fibrin gels were supplemented with basic fibroblast growth factor (bFGF; 1 ng/mL), LPS (100 ng/mL), or TNF (10 ng/mL) according to the experiment. The overlying medium contained either MCDB medium + 2% fetal bovine serum (FBS) alone or was supplemented with bFGF (1 ng/mL), LPS (100 ng/mL), or TNF (10 ng/mL). After 3 days of incubation with daily medium changes, the number of capillary-like tubes formed was quantitated by counting the number of tubelike structures more than 150 μm in length per microcarrier bead (sprouts per bead).
Chick chorioallantoic membrane (CAM) assay
The chick CAM assay was performed as previously described.12 Briefly, on embryonic day 8, the developing CAM was separated from the shell by opening a small circular window at the broad end of the egg. On day 10, Q2bn avian retroviral producer cell lines were transfected with either empty vector (CK) or dominant-negative TRAF6 (CK-TRAF6-C), resuspended in phosphate-buffered saline (PBS) alone or supplemented with bFGF (30 ng/mL) or LPS (10 ng/mL), and placed onto nylon meshes on the CAM. Expression of the TRAF6-C construct was confirmed by immunoblotting. The retroviral producer cells distribute throughout the mesh and secrete virus (control or TRAF6-C) which mainly infects the proliferating endothelial cells.12,13 On day 14, images of the CAMs were captured digitally, and neovascularization was quantitated for each CAM by counting the number of vessels that entered the mesh area and dividing by the perimeter of the mesh (vessels per millimeter).
Statistics
Results were analyzed by analysis of variance (ANOVA) to ascertain differences between groups, followed by a Tukey test for multiple comparisons.
Results and discussion
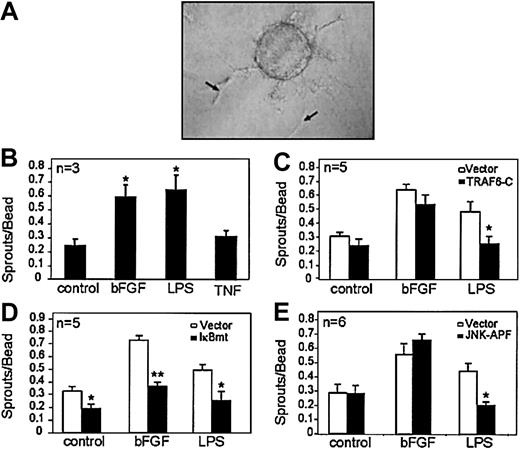
Tissues exhibiting inflammation show increased vascularity.14,15 We first attempted to determine whether the inflammatory mediators TNF and LPS could directly stimulate endothelial sprouting using an in vitro tube-forming morphogenesis assay. Microcarrier beads seeded with human microvascular endothelial cells (HMECs) were embedded in fibrin gels and stimulated with LPS, TNF, or bFGF (positive control). Interestingly, LPS but not TNF was able to directly stimulate endothelial sprouting in this assay. Figure 1A demonstrates endothelial sprout formation in response to LPS. Figure 1B shows the number of sprouts per bead in response to LPS or TNF, with bFGF used as a positive control. These findings suggest that TLRs activate specific downstream signals promoting angiogenesis, which are distinct from TNF receptors.
LPS signals endothelial sprouting through a TRAF6-mediated pathway that activates NF-κB and JNK. Microcarrier beads seeded with endothelial cells were embedded in fibrin gels and exposed to various stimuli. (A) An example of endothelial sprouts (arrows) formed in this assay following LPS (100 ng/mL) stimulation. (B) Quantitation of the number of sprouts formed following stimulation by serum-only (control), bFGF (1 ng/mL), LPS (100 ng/mL), or TNF (10 ng/mL). Effect of inhibiting (C) TRAF6 activation, (D) NF-κB activation, or (E) JNK activation on endothelial sprouting in response to bFGF (1 ng/mL) or LPS (100 ng/mL). Each experiment was done in triplicate, and the total number of experiments done in each case is indicated on the graph. Results shown are the mean + SEM of the total number of experiments. *P ≤ .05, **P ≤ .01.
LPS signals endothelial sprouting through a TRAF6-mediated pathway that activates NF-κB and JNK. Microcarrier beads seeded with endothelial cells were embedded in fibrin gels and exposed to various stimuli. (A) An example of endothelial sprouts (arrows) formed in this assay following LPS (100 ng/mL) stimulation. (B) Quantitation of the number of sprouts formed following stimulation by serum-only (control), bFGF (1 ng/mL), LPS (100 ng/mL), or TNF (10 ng/mL). Effect of inhibiting (C) TRAF6 activation, (D) NF-κB activation, or (E) JNK activation on endothelial sprouting in response to bFGF (1 ng/mL) or LPS (100 ng/mL). Each experiment was done in triplicate, and the total number of experiments done in each case is indicated on the graph. Results shown are the mean + SEM of the total number of experiments. *P ≤ .05, **P ≤ .01.
A critical intermediary signaling molecule used by the TLRs is TRAF6.9,11,16,17 To determine whether TRAF6 is involved in signaling the endothelial sprouting response, we used endothelial cells that overexpress a dominant-negative TRAF6 construct that lacks amino acids 1-289 (TRAF6-C).11 HMEC-TRAF6-C or vector-transduced cells were seeded onto microcarrier beads, and sprouting in response to LPS or bFGF was assessed. Figure 1D demonstrates that TRAF6-C inhibited LPS-induced endothelial sprouting, indicating the importance of TRAF6 activation in LPS-induced sprouting. The lack of effect of TRAF6-C on bFGF-induced endothelial sprouting (Figure 1D) confirms the specificity of the inhibitory effect of this dominant-negative construct as previously shown.11
We have shown that LPS-induced NF-κB activation and c-Jun N-terminal kinase (JNK) activation lie downstream of TRAF6 in endothelial cells.11 To determine whether NF-κB activation was required for LPS-induced sprouting, we used endothelial cells that express a super-repressor IκBα protein that is resistant to degradation and, thus, retains NF-κB in the cytoplasm despite activating signals.18 Figure 1E demonstrates that abrogation of NF-κB activation inhibited endothelial sprouting in response to either LPS or bFGF. Interestingly, blockade of NF-κB also inhibited baseline sprouting in response to serum stimulation only (control, Figure 1E), highlighting the general importance of this pathway in endothelial sprouting. However, we did not find that IκBmt inhibited endothelial cell proliferation (data not shown), suggesting that NF-κB activation is required for morphogenesis rather than endothelial proliferation in this context.
To test whether JNK activation was similarly necessary for LPS-induced sprouting, we used endothelial cells transduced with a dominant-negative JNK (JNK-APF) construct.11 We have previously shown that this mutant JNK specifically blocks JNK activation without affecting other mitogen-activated protein (MAP) kinase pathways.11 Figure 1F demonstrates that, although JNK activation is required for LPS-induced endothelial sprouting, bFGF-induced sprouting can proceed independently of JNK. Thus, in contrast to the critical role of NF-κB in signaling endothelial morphogenesis in response to diverse stimuli, the role of JNK appears to be limited to specific angiogenic activators.
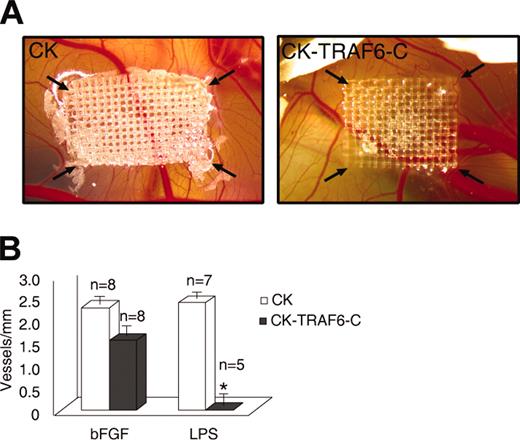
To confirm that our findings can be translated to angiogenesis in vivo, we used the chick CAM assay to test whether LPS can induce angiogenesis. We found that LPS (10 ng/mL) was able to induce angiogenesis on the chick CAM to a degree similar to bFGF (30 ng/mL) (data not shown). Next, we used an avian retroviral producer line to infect the chick CAM with the TRAF6-C construct. We and others have previously shown that most of the cells transduced using this technique are vascular endothelial cells.12,13 As seen in Figure 2A-B, angiogenesis on the chick CAM is blocked by the dominant-negative TRAF6 when LPS is the stimulus, but not when bFGF is the angiogenic agent, thereby corroborating our in vitro findings.
Inhibition of TRAF6 signaling on the chick CAM inhibits LPS- but not bFGF-induced angiogenesis. Avian retroviral producer lines were used to infect the proliferating cells (mainly endothelial) on the chick CAM with either empty vector (CK) or a dominant-negative TRAF6 (CK-TRAF6-C). (A) A representative CAM showing LPS-induced angiogenesis following infection of the CAM by empty vector (left panel) or TRAF6-C (right panel). The arrows point to the corners of the mesh on which the retroviral producer lines are seeded. (B) Quantitation of vessel number entering the mesh following retroviral infection and stimulation with bFGF (30 ng/mL) or LPS (10 ng/mL). Results shown are the number of vessels entering the mesh perimeter over baseline control (medium only). The number of experiments for each condition is shown over the bars. Results shown are the mean + SEM. *P ≤ .05.
Inhibition of TRAF6 signaling on the chick CAM inhibits LPS- but not bFGF-induced angiogenesis. Avian retroviral producer lines were used to infect the proliferating cells (mainly endothelial) on the chick CAM with either empty vector (CK) or a dominant-negative TRAF6 (CK-TRAF6-C). (A) A representative CAM showing LPS-induced angiogenesis following infection of the CAM by empty vector (left panel) or TRAF6-C (right panel). The arrows point to the corners of the mesh on which the retroviral producer lines are seeded. (B) Quantitation of vessel number entering the mesh following retroviral infection and stimulation with bFGF (30 ng/mL) or LPS (10 ng/mL). Results shown are the number of vessels entering the mesh perimeter over baseline control (medium only). The number of experiments for each condition is shown over the bars. Results shown are the mean + SEM. *P ≤ .05.
Our findings demonstrate that LPS-induced activation of TRAF6 in endothelial cells signals angiogenesis through activation of both NF-κB and JNK. LPS has been demonstrated to promote tumor angiogenesis and metastasis in mouse models.2,6 Thus, our results may be of relevance in cancer therapeutics, given that clinical trials with a lipid A analog, the bioactive domain of LPS, are in progress.19
Prepublished online as Blood First Edition Paper, April 24, 2003; DOI 10.1182/blood-2003-01-0288.
Supported by the Canadian Institutes of Health Research, the Heart and Stroke Foundation of British Columbia and the Yukon, and the National Cancer Institute of Canada with funds from the Canadian Cancer Society. K.G.L. is supported by a Doctoral Research Award from the Canadian Institutes of Health Research, and a Predoctoral Fellowship Award from the U.S. Department of the Army (DAMD17-01-1-0164).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
A.K. is a Clinician-Scientist of the Canadian Institutes of Health Research and a Scholar of the Michael Smith Foundation for Health Research.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal