Abstract
Embryonic stem (ES) cells can differentiate into most blood cells in vitro, providing a powerful model system to study hematopoiesis. However, ES cell–derived T lymphocytes have not been generated in vitro, and it was unresolved whether such potential is absent or merely difficult to isolate. Because the latter case might result from rapid commitment to non–T-cell fates, we isolated ES cell–derived prehematopoietic precursors for reconstitution of fetal thymic organ cultures. We found a transient Flk1+CD45– subset of these precursors generated T lymphocytes in vitro, and the use of reaggregate thymic organ cultures greatly enhanced reconstitution frequency. These findings reveal that ES cells can exhibit in vitro T-cell potential, but this is restricted to early stages of ES cell differentiation. Moreover, the results support the notion that the thymic microenvironment can induce T-cell differentiation from a subset of prehematopoietic progenitors and suggest deficient migration into intact thymi hindered previous attempts to generate T cells in vitro from ES cell–derived progenitors. These findings demonstrate that a defined subset of ES cells has the potential to generate T cells in vitro and could contribute to greater understanding of the molecular events of hematopoietic induction and T-cell lineage commitment.
Introduction
Embryonic stem (ES) cells are totipotent cells isolated from the inner cell mass of a blastocyst.1 In vivo, they can contribute to all the tissues of an animal, including germ cells, when transplanted back into a developing blastocyst.2,3 In vitro, their differentiation provides a model system to study the development of various tissues and lineages.4 In particular, ES cells can differentiate into most blood cell lineages, facilitating the study of hematopoietic differentiation.4,5
In the thymus, T cells are generated from hematopoietic stem cell (HSC)–derived lymphoid progenitors, which can also give rise to B cells and natural killer (NK) cells.6,7 Thus, the reports demonstrating that B and NK lymphocytes can be generated from ES cells in vitro5,8-11 suggest that ES cell–derived developmental intermediates should also possess the ability to become T lymphocytes. While there was evidence that ES cell–derived precursors exhibit T-cell potential in vivo,12-14 a specific precursor population had not been defined and such potential had not yet been demonstrated in vitro.
We have employed an in vitro ES cell differentiation system that is ideally suited to address these questions. This experimental approach takes advantage of the macrophage colony-stimulating factor (M-CSF)–deficient stromal cell line, OP9, which efficiently supports in vitro lymphopoiesis from ES cell–derived progenitors.8,10 We have shown that ES cells differentiated on OP9 cells (ES/OP9 cocultures) undergo progressive commitment to the hematopoietic lineage and that the cells with hematopoietic potential can be identified by cell surface expression of Flk1.11 Flk1 (vascular endothelial growth factor receptor-2, or VEGFR-2) is a receptor tyrosine kinase expressed on subsets of mesoderm and on the earliest endothelial and hematopoietic precursors.15-18 Flk1 deficiency is embryonically lethal, and Flk1-deficient mice exhibit defects in endothelial and hematopoietic development.19 Further, existing evidence indicates that Flk1 expression serves as a marker of hemangioblast cells,20-22 which are the proposed common developmental progenitors of both endothelial and hematopoietic lineages.
Using the ES/OP9 coculture system, we report that ES cell–derived Flk1+CD45– prehematopoietic progenitors give rise to T lymphocytes in vitro when transferred into reaggregate thymic organ cultures (RTOCs). The capacity of this defined prehematopoietic subset of differentiated ES cells for in vitro T-cell generation suggests that a wholly in vitro system using these cells could help elucidate the mechanisms that induce and support T-cell–lineage commitment and differentiation.
Materials and methods
Mice
Timed-pregnant Swiss.NIH and C57BL/6 CD45.1 congenic mice were obtained from the National Cancer Institute, Frederick Cancer Research and Development Center (Frederick, MD). RAG-2–/– CD45.1 congenic mice were bred and maintained in our animal facility.
Cell culture and differentiation of ES cells
ES/OP9 differentiation cocultures were performed as previously described.8,10 Briefly, 104 ES cells were seeded onto OP9 monolayers in 6-well plates, or 5 × 104 ES cells were seeded onto OP9 monolayers in 10-cm dishes. After 5 or 6 days of coculture, cells were harvested and made into single-cell suspensions by 0.25% trypsin treatment and vigorous pipetting. Cells were then washed and directly reseeded onto new OP9 cell monolayers or transferred into fetal thymic organ cultures (FTOCs). Alternatively, cells were either sorted for the expression of Flk1 or enriched for Flk1-expressing cells by magnetic-assisted cell sorting (MACS) (Miltenyi Biotec, Bergisch Gladbach, Germany) prior to reseeding onto OP9 monolayers or transfer into FTOCs or RTOCs. For continued ES/OP9 coculture, media were changed or cells were passaged (without trypsin) every 3 to 5 days. For FTOCs/RTOCs, media were changed every 4 to 6 days. Cells were harvested on various days for analysis by flow cytometry. In all experiments the R1 ES cell line was used.
Flow cytometry
Preparations of samples and flow cytometric analysis were performed as previously described.23 Postsort analyses showed a purity of at least 95%. Conjugated antibodies for flow cytometry were purchased from BD Biosciences (San Diego, CA). For analysis, live cells were gated based on forward- and side-scatter and lack of propidium iodide uptake.
Fetal thymic organ cultures (FTOCs) and reaggregate thymic organ cultures (RTOCs)
Lymphocyte-depleted thymic lobes were prepared by culturing day 14 to day 16 fetal thymic lobes from timed-pregnant mice in FTOC medium (Dulbecco modified Eagle medium [DMEM] supplemented with 12% fetal bovine serum [FBS], 2 mM glutamine, 10 U/mL penicillin, 100 μg/mL streptomycin, 100 μ g/mL gentamicin, 110 μg/mL sodium pyruvate, 50 μM 2-mercaptoethanol [2-ME], and 10 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], pH 7.4) containing 1 mM 2-deoxyguanosine, as previously described.24,25 Alternatively, thymic lobes were depleted by irradiation (25 to 30 Gy). For FTOCs, donor cells were washed with media, resuspended in 30 μL media, and added to individual wells in Terasaki plates to which host thymocyte–depleted fetal thymic lobes were also added. After adding donor cells or medium alone, the plates were inverted (“hanging drop”) and cultures were incubated at 37°C in a humidified incubator containing 5% CO2 in air for 24 to 48 hours. Lobes were then transferred to standard FTOCs for approximately 2 weeks. For RTOCs, deoxyguanosine-treated lobes were treated with 0.05% trypsin, 0.53 mM EDTA (ethylenediaminetetraacetic acid) for 30 to 40 minutes before being disaggregated into a single-cell suspension. A cell slurry was created from a mixture of stromal and progenitor cells, which was then deposited in freestanding drops on standard FTOC rafts. RTOCs were cultured in the presence of Flt3L and interleukin-7 (IL-7) (R&D Systems, Minneapolis, MN) at a final concentration of 5 ng/mL and 1 ng/mL, respectively, and analyzed after 19 days.
In the case of fetal liver–reconstituted RTOCs, thymic stroma was prepared as described above. Livers were removed from day 14 fetal mice, and single-cell suspensions were prepared. CD24lo fetal liver progenitor cells were enriched by anti-CD24 antibody-/complement-mediated depletion23 and then sorted for expression of CD117 and CD45.2.
Results
ES cell and OP9 cell cocultures
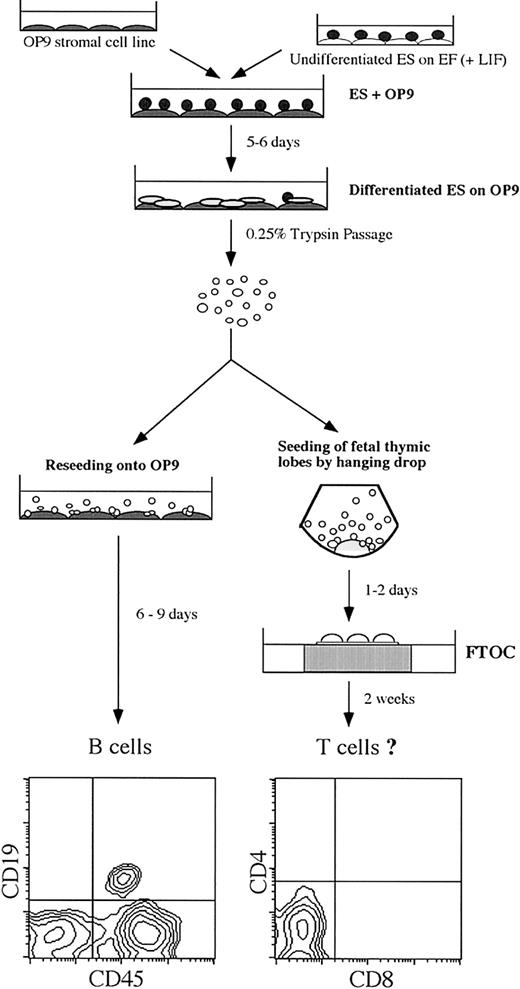
During embryogenesis, the hematopoietic lineage is established from mesodermal precursors.26 Similarly, mesoderm-like colonies are observed by day 5 from ES cells cocultured on OP9 cell monolayers (ES/OP9 cocultures), which give rise to hematopoietic clusters that, when reseeded back onto OP9 cells, differentiate into B lymphocytes (Figure 1, lower left panel).8,10,11 This led us to examine whether progenitor cells present in day 5 ES/OP9 cocultures could give rise to T lymphocytes when transferred into FTOCs (Figure 1). However, after 14 days in FTOCs, analysis by flow cytometry showed no evidence of donor-derived T cells (Figure 1, lower right panel). This indicated that although this population contains a subset of ES cell–derived progenitors that can efficiently give rise to lymphohematopoietic cells when reseeded back onto OP9 cell monolayers10 (Figure 1), the total population from day 5 ES/OP9 cocultures appears to contain a small subset that is very inefficient at giving rise to T lympyhocytes as well as cells that could disrupt the FTOC microenviroment. With this in mind, we reasoned that a defined subset of differentiated ES cells should be amenable to the thymic microenvironment.
Schematic for the differentiation of ES cells into lymphocytes. ES cells were induced to differentiate on the bone marrow–derived stromal cell line OP9. Initial seeding of ES cells onto OP9 cells is designated as day 0. After 5 to 6 days of coculture, mesoderm-like colonies were observed and were disaggregated by treatment with 0.25% trypsin. Reseeding onto fresh OP9 cell monolayers allows for the generation of B lymphocytes. For the generation of T lymphocytes, cells were seeded into fetal thymic lobes by “hanging drop” for 1 to 2 days and then transferred to fetal thymic organ cultures (FTOCs) for approximately 2 weeks. Flow cytometric analysis of B-cell lineage markers (CD19 and CD45) and T-cell lineage markers (CD4 and CD8) are shown for a day 19 ES/OP9 coculture and a day 14 FTOC, respectively. Using this protocol, the generation of B lymphocytes is routinely observed, whereas T lymphocytes are not generated.
Schematic for the differentiation of ES cells into lymphocytes. ES cells were induced to differentiate on the bone marrow–derived stromal cell line OP9. Initial seeding of ES cells onto OP9 cells is designated as day 0. After 5 to 6 days of coculture, mesoderm-like colonies were observed and were disaggregated by treatment with 0.25% trypsin. Reseeding onto fresh OP9 cell monolayers allows for the generation of B lymphocytes. For the generation of T lymphocytes, cells were seeded into fetal thymic lobes by “hanging drop” for 1 to 2 days and then transferred to fetal thymic organ cultures (FTOCs) for approximately 2 weeks. Flow cytometric analysis of B-cell lineage markers (CD19 and CD45) and T-cell lineage markers (CD4 and CD8) are shown for a day 19 ES/OP9 coculture and a day 14 FTOC, respectively. Using this protocol, the generation of B lymphocytes is routinely observed, whereas T lymphocytes are not generated.
T-cell differentiation from ES cell–derived Flk1+ cells
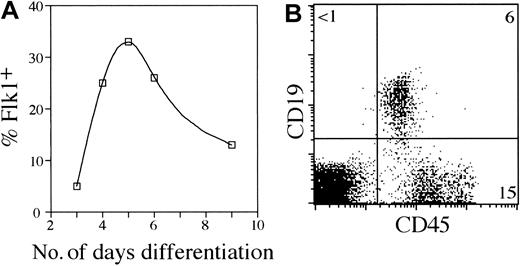
Kabrun et al suggested that a transient population of Flk1+ progenitors might represent the onset of embryonic hematopoiesis.16 Therefore, we hypothesized that isolating Flk1+ cells from ES/OP9 cocultures would include cells that could successfully reconstitute an FTOC. Moreover, the Flk1+CD45– population contains prehematopoietic precursors that are uncommitted to any particular hematopoietic lineage and are potentially permissive to T-cell commitment and differentiation within the thymus. Cell surface expression of Flk1 during the course of ES cell differentiation was analyzed by flow cytometry (Figure 2A). The results in Figure 2A indicate that the greatest percentage of Flk1+ cells occurs at approximately day 5 during ES/OP9 coculture. To assess the ability of Flk1+ cells to serve as potential lymphoid progenitors and clarify whether the Flk1+ cells generated in ES/OP9 cocultures resembled the Flk1+ population described by Nishikawa et al,18 Flk1+CD45– cells were sorted and reseeded back onto an OP9 monolayer (Figure 2B). Figure 2B shows that Flk1+ progenitors were able to give rise to B lymphocytes with continued coculture on OP9 cells, demonstrating that the Flk1+ subset contained precursors that can generate lymphocytes in vitro.
Lymphopoietic potential of ES cell–derived Flk1+ precursors. (A) A total of 104 ES cells were seeded onto OP9 cell monolayers in 6-well plates. Cells were harvested from ES/OP9 cocultures on various days and analyzed by flow cytometry for Flk1 surface expression. The percent of cells expressing Flk1 is shown. Cocultures were disaggregated with trypsin on day 6. (B) A total of 2 × 104 ES cell–derived Flk1+ cells was isolated by flow cytometric cell sorting (day 5 ES/OP9 coculture) and reseeded onto fresh OP9 cell monolayers. Evidence of B lymphopoiesis on day 14 was determined by flow cytometric analysis for the surface expression of CD45 and CD19. The total cell yield was approximately 1.5 × 105 cells for the coculture shown.
Lymphopoietic potential of ES cell–derived Flk1+ precursors. (A) A total of 104 ES cells were seeded onto OP9 cell monolayers in 6-well plates. Cells were harvested from ES/OP9 cocultures on various days and analyzed by flow cytometry for Flk1 surface expression. The percent of cells expressing Flk1 is shown. Cocultures were disaggregated with trypsin on day 6. (B) A total of 2 × 104 ES cell–derived Flk1+ cells was isolated by flow cytometric cell sorting (day 5 ES/OP9 coculture) and reseeded onto fresh OP9 cell monolayers. Evidence of B lymphopoiesis on day 14 was determined by flow cytometric analysis for the surface expression of CD45 and CD19. The total cell yield was approximately 1.5 × 105 cells for the coculture shown.
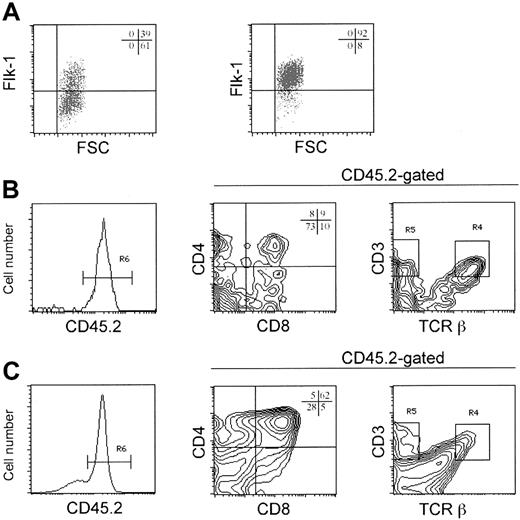
To determine whether Flk1+CD45– progenitors from day 5 to day 6 ES/OP9 cocultures could generate T lymphocytes in vitro, these cells were isolated by fluorescence-activated cell sorting (FACS) (Figure 3A) and seeded into host lymphocyte-depleted FTOCs that were derived from RAG-2–/–/CD45.127,28 congenic mice. After 13 to 16 days in FTOCs, donor-derived thymocyte reconstitution was determined by flow cytometric analysis for the surface expression of CD45.2, which was not present in control host FTOCs receiving only media (Figure 3B). The presence of donor-derived CD45.2+ cells indicated that ES cell–derived Flk1+ CD45– precursors successfully reconstituted the host thymus (Figure 3B). In contrast, reconstitution of FTOCs with Flk1– CD45– cells failed to give rise to donor-derived CD45.2+ cells (data not shown). Although the total number of cells recovered from FTOCs reconstituted with Flk1+CD45– cells was low, varying between 1.2 × 103 to 29 × 103 cells per lobe, the ES cell–derived thymocytes were able to differentiate into all the normal CD4 and CD8 thymic subsets (Figure 3C). The in vitro generation of T lymphocytes from ES cells for 3 independent experiments is shown in Figure 3C. The presence of donor-derived thymocytes in FTOCs reconstituted with ES cell–derived Flk1+CD45– precursors served to validate our initial hypothesis; however, not all reconstituted FTOCs gave rise to T lymphocytes, with approximately 60% of independent experiments (more than 10) showing ES cell–derived thymocytes. Our finding that T lymphocytes can be generated in FTOCs from prehematopoietic precursors is consistent with the results reported by Nishikawa et al, in which CD45– endothelial-like cells derived from embryonic tissues (day 9.5 to 10) gave rise to T cells in thymic organ cultures.29
In vitro generation of T lymphocytes from ES cell–derived Flk1+ progenitors. (A) Cells were harvested from day 5 or day 6 ES/OP9 cocultures, and Flk1+CD45– cells, as shown, were isolated by flow cytometric cell sorting. (B) Sorted Flk1+ cells were seeded into host cell–depleted fetal thymic lobes (day 14 to 16) from RAG-2–deficient CD45.1 congenic mice by hanging drop, cultured overnight, and then transferred to FTOCs. Two weeks later, donor-derived reconstitution was determined by flow cytometric analysis for CD45.2 surface expression. Negative control (no donor) lobes received only media. In 2 additional experiments CD45.2+ cells were undetectable in control lobes (no donor) and represented 91% and 89% in lobes that received ES cell–derived Flk1+ precursors. (C) Evidence of donor-derived reconstitution was determined by flow cytometry in 3 independent experiments. Analysis of CD4 and CD8 surface expression is shown for CD45.2+ gated cells, percentages as indicated. Individual lobes were pooled for analysis. Experiments 1 and 2 were analyzed on day 14 FTOCs, and experiment 3 was analyzed on day 16 FTOCs.
In vitro generation of T lymphocytes from ES cell–derived Flk1+ progenitors. (A) Cells were harvested from day 5 or day 6 ES/OP9 cocultures, and Flk1+CD45– cells, as shown, were isolated by flow cytometric cell sorting. (B) Sorted Flk1+ cells were seeded into host cell–depleted fetal thymic lobes (day 14 to 16) from RAG-2–deficient CD45.1 congenic mice by hanging drop, cultured overnight, and then transferred to FTOCs. Two weeks later, donor-derived reconstitution was determined by flow cytometric analysis for CD45.2 surface expression. Negative control (no donor) lobes received only media. In 2 additional experiments CD45.2+ cells were undetectable in control lobes (no donor) and represented 91% and 89% in lobes that received ES cell–derived Flk1+ precursors. (C) Evidence of donor-derived reconstitution was determined by flow cytometry in 3 independent experiments. Analysis of CD4 and CD8 surface expression is shown for CD45.2+ gated cells, percentages as indicated. Individual lobes were pooled for analysis. Experiments 1 and 2 were analyzed on day 14 FTOCs, and experiment 3 was analyzed on day 16 FTOCs.
ES cell–derived T cells in reaggregate thymic organ cultures
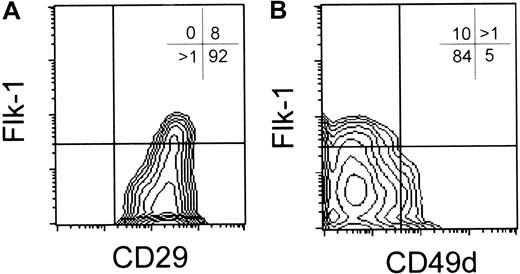
We reasoned that the efficiency of reconstitution observed might be impaired by a defect in the ability of the Flk1+ CD45– progenitor cells to migrate into the thymic environment. To address this issue, we examined Flk1+ cells for the expression of integrin molecules. Specifically, we assessed the expression of CD29 (β1) and CD49d (α4) integrins, because Potocnik et al30 found that CD29-deficient hematopoietic precursors are severely impaired in their ability to home to sites of primary hematopoiesis, including the thymus, and Arroyo et al31,32 demonstrated that CD49d was obligatory for postnatal migration of hematopoietic precursors into the thymus. We found that CD29 was ubiquitously expressed at high levels on day 5 Flk1+ CD45– cells (Figure 4A). However, these cells uniformly lacked expression of CD49d (Figure 4B). In contrast, a subset of day 5 Flk1– cells showed expression of both CD29 and CD49d (Figure 4). The lack of CD49d expression by Flk1+ cells suggested that these ES cell–derived prehematopoietic cells may not be capable of migrating into the thymus in an efficient manner.31,32 Thus, to obviate the requirement for day 5 ES-derived precursors to migrate into the thymic environment, we took advantage of the reaggregate thymic organ culture (RTOC) approach, in which progenitor cells are directly combined with thymic stromal cells, which then reform the thymus environment around the progenitors.33
ES cell–derived Flk1+ cells lack CD49d (α4 integrin) cell surface expression. Flow cytometric analysis for cell surface expression of Flk1 and CD29 (A) or CD49d (B) from day 5 ES/OP9 coculture cells.
ES cell–derived Flk1+ cells lack CD49d (α4 integrin) cell surface expression. Flow cytometric analysis for cell surface expression of Flk1 and CD29 (A) or CD49d (B) from day 5 ES/OP9 coculture cells.
Flk1+ cells were combined with disaggregrated thymic stroma obtained from CD45.1 congenic mice and placed in culture for 19 days (Figure 5A). Using this approach, CD45.2+ donor-derived cells were present in RTOCs reconstituted with Flk1+ cells (Figure 5B), and all the normal CD4 and CD8 double- and single-positive thymocyte subsets were observed. The presence of mature donor-derived T cells was demonstrated by the appearance of αβ TCRhi cells coexpressing CD3 molecules on the cell surface. Moreover, cells expressing CD3 in the absence of T-cell–receptor β (TCRβ), corresponding to γδ T cells, were also present (Figure 5B). Strikingly, the efficiency of successful reconstitutions was increased to 5 of 5 independent experiments by the use of RTOCs (Figure 5), with the levels of reconstitution varying between 1.7 × 103 to 24 × 103 cells per thymic reaggregate. In support of our earlier findings,11 a subset of day 5 ES/OP9 coculture–derived Flk1+ cells that coexpress CD105 also reconstituted RTOCs (data not shown). These cells also expressed CD3, CD4, CD8, and TCRβ in the appropriate distributions, although no increase in yield was achieved by sorting for this population.
Use of RTOCs to generate T cells from ES cell–derived Flk1+ progenitors. (A) Cells were harvested from day 5 ES/OP9 coculture, and CD45– Flk1+ progenitors were enriched by magnetic-assisted cell sorting to about 97% purity from about 33% of the starting population. (B) Flk1+ cells were combined with a thymic stromal cell suspension and deposited onto FTOC rafts (see “Materials and methods”). After 19 days, RTOC reconstitution was determined by flow cytometric analysis for CD45.2 surface expression. ES cell–derived CD45.2+ thymocytes were present in all RTOCs from 5 independent experiments. Flow cytometric analysis is also shown for CD4 and CD8 and for CD3 and TCRβ surface expression on CD45.2+ gated cells, with individual reaggregates pooled for analysis. (C) CD117+CD24loCD45.2+ fetal liver cells from day 14 fetal mice were combined with thymic stromal cells and placed in RTOCs and cultured for 12 days prior to flow cytometric analysis for CD45.2, CD4 and CD8, and CD3 and TCRβ surface expression.
Use of RTOCs to generate T cells from ES cell–derived Flk1+ progenitors. (A) Cells were harvested from day 5 ES/OP9 coculture, and CD45– Flk1+ progenitors were enriched by magnetic-assisted cell sorting to about 97% purity from about 33% of the starting population. (B) Flk1+ cells were combined with a thymic stromal cell suspension and deposited onto FTOC rafts (see “Materials and methods”). After 19 days, RTOC reconstitution was determined by flow cytometric analysis for CD45.2 surface expression. ES cell–derived CD45.2+ thymocytes were present in all RTOCs from 5 independent experiments. Flow cytometric analysis is also shown for CD4 and CD8 and for CD3 and TCRβ surface expression on CD45.2+ gated cells, with individual reaggregates pooled for analysis. (C) CD117+CD24loCD45.2+ fetal liver cells from day 14 fetal mice were combined with thymic stromal cells and placed in RTOCs and cultured for 12 days prior to flow cytometric analysis for CD45.2, CD4 and CD8, and CD3 and TCRβ surface expression.
To assess whether the cell yields observed in RTOCs reconstituted with ES/OP9 coculture–derived Flk1+ progenitors were the result of an intrinsic limitation of the RTOC approach, we performed the same procedure using the same ratio of CD24loCD117+CD45.2+ day 14 fetal liver cells to thymic stroma. As shown in Figure 5C, after 12 days in culture these hematopoietic progenitors were able to reconstitute RTOCs with cell yields similar to those observed with normal FTOCs (0.3 × 104 to 12.3 × 104 cells per aggregate), although with delayed kinetics (data not shown). Because the fetal liver–reconstituted lobes were cultured for a shorter period than the RTOCs reconstituted with Flk1+ cells, they contained a higher fraction of CD4+CD8+ double-positive cells.
Discussion
Our results demonstrate that ES cell–derived T cells can be generated from Flk1+ CD45– prehematopoietic progenitors. This finding provides a “proof of principle” for the in vitro differentiation potential of ES cells and supports the notion that during ES cell differentiation newly generated CD45+ precursors rapidly lose their T-cell potential as they are induced to commit to other hematopoietic lineages within the OP9 stromal environment. It is interesting to speculate that the difficulties in isolating CD45+ progenitors with T-cell potential from ES/OP9 cocultures amount to the same difficulties that have persisted in isolating HSCs from ES cells differentiated in vitro4,34 : if true HSCs are generated, they may rapidly differentiate to restricted multipotent progenitors. The characterization of the Flk1+CD45– precursors generated in ES/OP9 cocultures not only helps to further define ES cell–derived T-cell progenitors but will also aid in identifying the inducible factors governing T-cell–lineage commitment and/or maintenance of HSC-like multipotency.
Two key findings have contributed to the ability to reproducibly generate T cells from ES cells in vitro. First, Flk1+ prehematopoietic precursors were nondeleterious and responsive to the differentiation cues provided by the thymic microenvironment. Second, ES cell–derived Flk1+ progenitors, as opposed to Flk1– cells, exhibited T-cell potential at a much higher incidence than ES cell–derived CD45+ hematopoietic progenitors, which suggests that the Flk1+CD45– population is enriched for uncommitted hematopoietic precursors. Furthermore, the improved frequency of reconstitution achieved with RTOCs supports existing evidence that ES cell–derived progenitors might be limited in their ability to home or migrate into sites of hematopoiesis.35 In this regard, Arroyo et al31,32 demonstrated that while CD49d, although expressed by fetal cells, is entirely dispensable for the entry of fetal precursors into the thymus, it is however required for postnatal homing to the thymus. This is in contrast to CD29, which Potocnik et al showed to be required for both fetal and adult homing to sites of hematopoiesis.30,36 Interestingly, although we found CD29 was ubiquitously expressed at high levels on day 5 ES/OP9 coculture cells, CD49d expression was absent on Flk1+ cells. While the molecular interaction responsible for the apparent migratory block suffered by ES cell–derived Flk1+ progenitors remains to be elucidated, our initial findings suggest that the lack of CD49d expression may be in part responsible for this defect. Also of note, recent work by Prockop et al37 implicated CD49d and its ligands in the migration of developing thymocytes within the thymus. Thus, the lack of CD49d expression by Flk1+ cells might contribute to the low yields of T cells in our system.
Taken together, these findings clearly demonstrate that ES cells have the potential to differentiate into mature T cells in vitro and provide new insights into their requirements for this lineage fate decision. Furthermore, they extend the potential of ES cells as a model system for the study of hematopoiesis to include T-cell differentiation and lineage commitment, the only remaining hematopoietic lineage for which their in vitro potential had not yet been shown. In conjunction with the recent derivation of human ES cell lines,38 and establishment of mammalian ES cell lines after nuclear transfer from somatic cells,39,40 the model system we have established represents a fundamentally important first step toward the development of human T-cell reconstitution therapies from a genetically defined source of stem cells.
Prepublished online as Blood First Edition Paper, May 8, 2003; DOI 10.1182/blood-2003-01-0224.
Supported by the National Cancer Institute of Canada with funds from the Canadian Cancer Society. S.K.C. was supported by a studentship from the Lady Tata Memorial Fund (United Kingdom). R.F. de P. is supported by a Doctoral Research Award (DRA) from the Canadian Institute of Health Research (CIHR). J.R.C. was supported by a DRA from the Medical Research Council. J.C.Z.-P. is supported by an Investigator Award from the CIHR.
R.F. de P. and S.K.C. contributed equally to this study.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Drs Alison Michie and Ross La Motte-Mohs for critical reading of the manuscript.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal