Abstract
Gemtuzumab ozogamicin (GO) is a novel immunoconjugate therapy for acute myeloid leukemia (AML). P-glycoprotein (Pgp) confers resistance to GO and is associated with a worse clinical response. To address whether multidrug resistance protein (MRP) affects GO susceptibility, we characterized Pgp, MRP1, and MRP2 expression in CD33+ cell lines and CD33+ AML samples and analyzed the effect of the Pgp inhibitor cyclosporine (CSA) and the MRP inhibitor MK-571 on GO-induced cytotoxicity. MRP1, but not MRP2, expression correlated with MRP activity. MK-571 enhanced GO-induced cytotoxicity in Pgpnegative/MRP-positive NB4 and HL-60 cells. CSA, but not MK-571 alone, restored GO susceptibility in Pgp-positive/MRP-positive TF1 cells; however, MK-571 enhanced cytotoxicity in the presence of CSA. All patient samples exhibited MRP activity, and 17 of 23 exhibited Pgp activity. CSA increased GO-induced cytotoxicity in 12 Pgp-positive samples, whereas MK-571 alone was effective in only one sample with minimal Pgp activity. In 3 Pgp-positive/MRP-positive samples, MK-571 enhanced GO-induced cytotoxicity in the presence of CSA. Thus, MRP1 may attenuate susceptibility to GO. This effect was comparatively less than that for Pgp and required the inhibition of Pgp for detection in cells that coexpressed both transporters. Because MK-571 and CSA failed to affect cytotoxicity in a portion of Pgp-positive/MRP-positive AML samples, additional resistance mechanisms are likely important.
Introduction
Conventional induction chemotherapy induces complete remission (CR) in 50% to 80% of adults with de novo acute myeloid leukemia (AML). However, a significant proportion of responding patients have relapses and ultimately die of treatment-refractory disease.1 Thus, novel drugs and treatment strategies are major objectives of research, especially for the elderly, who respond poorly to standard therapy regimens and often experience dose-limiting toxicity from intensive chemotherapy.
A humanized anti-CD33 antibody conjugated to a calicheamicin derivative, gemtuzumab ozogamicin (CMA-676, Mylotarg; Wyeth-Ayerst Laboratories, Philadelphia, PA), was recently approved as a therapeutic option for relapsed AML in elderly patients not considered candidates for conventional salvage therapy. Phase 2 trials demonstrated that 2 doses of gemtuzumab ozogamicin (GO) given as monotherapy ultimately induced remission in 26% of adults with CD33+ relapsed AML.2,3 The mechanisms leading to resistance in the other 74% of the patients are so far incompletely understood; however, active drug efflux is likely important.4
Drug efflux mediated by members of the adenosine triphosphate–(ATP) binding cassette (ABC) superfamily of proteins has been implicated in AML resistance against conventional chemotherapeutic drugs (for reviews, see Klein et al5 and van der Kolk et al6 ). The best characterized and most intensively studied is permeabilityglycoprotein (Pgp or MDR1 or ABCB1). Pgp is expressed in many healthy tissues but is also found on blast cells of 19% to 75% of patients with de novo AML. Lower CR rates and decreased overall or disease-free survival rates have been noted among patients with Pgp-positive AML treated with conventional chemotherapy.6-9 Similarly, phase 2 trials with GO revealed that blast cell Pgp expression correlated with treatment failure.2,4 In vitro studies have further demonstrated that Pgp-expressing cell lines and patient AML blast cells are resistant to GO and that Pgp inhibitors can restore drug sensitivity in Pgp-expressing cells.10,11 Despite this evidence, the Pgp inhibitor cyclosporine (CSA) enhanced in vitro GO-induced cytotoxicity in only one third of Pgp-expressing AML blast cell samples,4 suggesting that other mechanisms play a role in GO resistance.
The multidrug resistance protein 1 (MRP1 or ABCC1), another widely expressed ABC transporter, extrudes conjugated and unconjugated organic anions and modulates the toxicities of chemotherapeutic agents in healthy tissues (for a review, see Leslie et al12 ). MRP1 is overexpressed (defined as a level surpassing that of healthy blood leukocytes) in 7% to 30% of patients with de novo AML (for a review, see Legrand et al13 ). Higher frequencies are found in younger patients, in patients with secondary AML, and among more immature CD34+ blast cell subpopulations.8,14,15 Although MRP1 overexpression confers a pattern of drug resistance similar to but not identical with that of Pgp in malignant cells in vitro, its clinical significance in AML is a matter of debate. Most studies to date observed no association between MRP1 protein expression and treatment outcome. Nevertheless, isolated reports have identified MRP1 expression or MRP activity alone, or the simultaneous activities of MRP and Pgp, as adverse prognostic factors.16-18 MRP2 (ABCC2) has more limited normal tissue distribution than MRP1 but transports a similar range of xenobiotic and chemotherapeutic agents.12 MRP2 is also expressed in a significant portion of patients with AML; however, the role of this transporter in drug resistance to AML is unkown.6,18 Limited information is available about a potential role of MRP1 in GO resistance. In a single study of 27 AML blast cell samples, the in vitro cytotoxic effect of GO did not correlate with the expression of MRP1 protein or MRP activity.11 However, concomitant Pgp activity was detected11 in most of the samples, thereby potentially confounding the interpretation of correlations between MRP expression or function and GO-induced cytotoxicity.
As an immunoconjugate, GO must be internalized and subsequently must undergo intracellular processing to release the toxic calicheamicin moiety. Because of this extensive trafficking, surface and intracellular membrane-associated transporter proteins, such as Pgp and MRP1, may have distinct roles in modulating GO susceptibility. The primary objectives of the present study were therefore to characterize a potential role of MRP activity in GO-induced cytotoxicity, to correlate this effect with MRP1 and MRP2 expression, and to differentiate this effect from that mediated by Pgp.
Patients, materials, and methods
Cell lines
The 2 human promyelocytic cell lines, HL-60 and NB4, were maintained in RPMI 1640 medium (Gibco Invitrogen, Carlsbad, CA) supplemented with 5% heat-inactivated bovine calf serum (BCS; HyClone, Logan, UT). The human erythroblastic TF1 cell line was maintained in RPMI 1640 medium with 25 mM HEPES (N-2-hydroxyethylpiperazine-N′2-ethanesulfonic acid) buffer (Gibco) supplemented with 10% heat-inactivated BCS, 1 mM minimum essential medium (MEM) sodium pyruvate (Gibco), and 4 ng/mL granulocyte macrophage–colony-stimulating factor (GM-CSF; Amgen, Thousand Oaks, CA). The anthracycline-resistant subline of HL-60 (HL-60/AR) and the vincristine-resistant subline of HL-60 (HL-60/VCR) (both kindly provided by Dr K. N. Bhalla, University of South Florida, Tampa) were grown in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum (FBS; HyClone), 1 mM MEM sodium pyruvate, and 0.1 mM MEM nonessential amino acids (Gibco). These cell lines were maintained in the presence of selective drugs (1 μM doxorubicin or 1.1 μM vincristine, respectively), which were removed at least 2 days before each experiment.
Patient AML blast cell samples
Marrow samples were taken from adult patients in untreated first relapse of AML (non-M3 subtypes) who participated in the phase 2 clinical trials with GO.2-4 Thawed aliquots of frozen samples of density-gradient isolated mononuclear cells containing leukemic blast cells were used for these studies. All patients signed informed consent, and the institutional review boards of the participating institutions approved all protocols.
Determination of multidrug resistance protein expression
A multiparameter cytofluorometric immunofluorescence staining method was used to characterize the membrane display of CD33 and the surface or intracellular expression of drug transporter proteins. Monoclonal antibodies included L4F319 and P67.6 (Becton Dickinson, San Jose, CA) to recognize CD33; 4E3.16 for Pgp (provided by R. Arceci, Johns Hopkins University, Baltimore, MD); MRPm68,14,16,20-22 for MRP1; and M2I-4 for MRP2 (both from Kamiya Biomedical, Seattle, WA). CD33 expression in cell lines was detected by staining with phycoerythrin (PE)–conjugated P67.6. For detection of the extracellular Pgp epitope, cells were stained with the 4E3.16 antibody followed by a secondary fluorescein isothiocyanate (FITC)–conjugated antibody, as previously described.4 For detection of the intracellular epitopes of MRP1 and MRP2, cells were first permeabilized (PermeaFix; Ortho Diagnostic Systems, Raritan, NJ) for 30 minutes at room temperature. Cells were then washed twice in ice-cold phosphate-buffered saline (PBS)/2% FBS and were incubated with the primary antibody for 20 minutes on ice, followed by a secondary FITC-conjugated antibody. For a staining negative control, parallel samples were incubated with an irrelevant isotype control antibody at the same protein concentration as the primary monoclonal antibody. To assess CD33 on AML blasts, samples were first incubated with the IgM anti-CD33 antibody L4F3 or the appropriate nonbinding isotype antibody for 20 minutes on ice, followed by staining with a secondary PE-conjugated antibody. To identify nonviable cells, all samples were also stained with propidium iodide (PI; Sigma, St Louis, MO). At least 10 000 events were acquired and analyzed on a FACScan flow cytometer using CellQuest software (Becton Dickinson). Data analyses were done on PI– cells (cell line samples) or CD33+/PI– cells (patient AML blast samples) with size and granularity properties consistent with those for leukemic cells. The staining intensity ratio was calculated by dividing the mean fluorescence intensity (MFI) of cells stained with a given specific antibody by the MFI of cells stained with the corresponding nonbinding isotype control antibody. A ratio greater than 1 indicated the presence of and a ratio less than 1 indicated the absence of the assessed antigen.
Determination of functional Pgp and MRP activity
Pgp function was determined by the efflux of the fluorescent dye 3,3′-diethyloxacarbocyanine iodide (DiOC2; Aldrich, St Louis, MO) in the presence or absence of the Pgp inhibitor CSA (Novartis Pharma AG, Basel, Switzerland), as previously described.4 The anionic dye 5-(and-6)-carboxy-2′,7′-dichlorofluorescein diacetate (CDCF; Molecular Probes, Eugene, OR) is a substrate for MRP1 and MRP2 after the lipophilic blocking groups are cleaved by nonspecific esterases.15,23 The efflux of CDCF in the presence or absence of the leukotriene D4 receptor antagonist MK-571 (Biomol Research Laboratories, Plymouth Meeting, PA) was used as a measure of functional MRP activity. MK-571 is a strong competitive inhibitor of MRP1, but it is less potent against MRP2.24 Dose-finding experiments with CDCF/MK-571 revealed that the sensitivity of this drug/inhibitor combination to detect functional MRP activity strongly depended on the dye concentrations. Thus, sensitivity was optimized when CDCF was used at 0.05 μM. For dye efflux assays, 6 × 105 cells were incubated for 30 minutes at 37°C in the dark in either a 10 ng/mL solution of DiOC2 or a 0.05 μM solution of CDCF in the presence or absence of CSA (2.5 μg/mL) or MK-571 (10 μM), respectively. Cells were then washed 3 times and resuspended in fresh dyeless medium with or without the inhibitor and were incubated for 90 minutes at 37°C to allow time for active efflux. Before analysis of dye fluorescence, cells were stained with PI and, for patient AML blast cell samples, with anti–CD33-PE or the respective isotype control antibody. All samples were analyzed on a FACScan flow cytometer, with gating on the PI– cell population (for cell lines) or CD33+/PI– cell population (for patient AML samples). Results are expressed as the ratio of the MFI in the presence of the inhibitor divided by the MFI in the absence of the inhibitor, also referred to as the inhibitor-modulating factor.15
Assays for GO-induced cytotoxicity
For experiments with cell lines, cells were taken during the log phase of growth and were distributed into 96-well round-bottom plates (Falcon; Becton Dickinson Labware, Franklin Lakes, NJ) in 240 μL culture medium (6.5 × 104 cells/well). Various concentrations of GO were added. After a 2-hour incubation at 37°C in 5% CO2 and air, the cells were washed and resuspended in 200 μL fresh complete medium without drug. Parallel cultures were incubated with GO in the presence of various concentrations of CSA or MK-571 or combinations of these inhibitors, before washing and resuspension in fresh medium containing the inhibitors but not containing GO. For experiments with patient AML blast cells, 5 × 104 cells/well were cultured in 240 μL RPMI 1640 medium with l-glutamine/2% FBS containing either 10 ng/mL GO or 10 ng/mL control immunoconjugate hCTM01 (calicheamicin conjugated to an anti-MUC1 antibody; provided by Wyeth-Ayerst)4 in the presence or absence of CSA (2.5 μg/mL), MK-571 (20 μM), or combinations thereof. After a 2-hour incubation, cells were washed and resuspended in the presence or absence of CSA (1 μg/mL), MK-571 (20 μM), or a combination of CSA and MK-571, in 200 μL Iscove modified Dulbecco medium (IMDM; Gibco) with 20% FBS and 100 ng/mL recombinant human cytokines (GM-CSF, stem cell factor, interleukin-3, or granulocyte colony-stimulating factor; all from Amgen), as previously described.4 Drug-induced cytotoxicity was determined after 3 days of culture using a cell viability assay or an annexin-V–based apoptosis assay. The 3-day time course was chosen based on our preliminary studies and on observations by others25 that apoptotic changes are minimal at 24 to 48 hours after exposure to GO but are significantly increased after 72 to 96 hours. To determine the number of viable cells after exposure to GO, a commercial assay was used that measures the conversion of the tetrazolium compound 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) to a colored formazan product (Promega, Madison, WI). With this method, the absorbance at 490 nm is directly proportional to the number of viable cells. For our assays, 20 μL MTS tetrazolium compound was added to 100 μL cell suspension and incubated for 4 hours at 37°C in 5% CO2 and air. Absorbance was then determined at 490 nm using a 96-well plate reader (μQuant; Bio-Tek Instruments, Winooski, VT). To assess apoptosis, cells were stained with annexin V (BD PharMingen, San Diego, CA) according to the manufacturer's instructions. All samples were also stained with PI and analyzed on the FACScan flow cytometer. Nonpermeable apoptotic cells were identified as annexin V+/PI–, whereas permeable necrotic cells were identified as PI+.
mRNA extraction and quantitative real-time reverse transcriptase–polymerase chain reaction
Total RNA was extracted and purified using a commercial kit according to the manufacturer's instructions (Absolutely RNA RT-PCR Miniprep Kit; Stratagene, La Jolla, CA). RNA was then quantitated, separated into aliquots, and stored at –80°C until further use. Quantitative reverse transcriptase–polymerase chain reaction (RT-PCR) for Pgp, MRP1, and MRP2 transcripts was performed using the TaqMan chemistry and the ABI Prism 7700 Sequence Detection System (Applied Biosystems, Branchburg, NJ). Transporter transcript levels within each sample were normalized for the expression of β2-microglobulin mRNA within that sample. The sequences of each primer set and the respective FAM/BHQ-1 florigenic probe (Synthegen, Houston, TX) are shown in Table 1. Transporter primer sets were designed to amplify cDNA across one intron-exon boundary, to avoid amplifying genomic DNA. Gel electrophoresis of the RT-PCR product obtained using ABI Prism 7700 was carried out to confirm amplification of a single product of the expected size. Duplicate RNA samples from cell lines or patient AML blast cells were used as templates with 500 nM each primer pair and 200 nM probe in TaqMan One-Step RT-PCR Master Mix supplemented with Multiscribe and RNase Inhibitor Mix (Applied Biosystems), in a total volume of 50 μL. Samples were reverse transcribed for 30 minutes at 50°C; this was followed by heat deactivation of the reverse transcriptase for 5 minutes at 95°C. The cDNA product was subsequently amplified for 40 cycles, each consisting of 30 seconds at 95°C, 30 seconds at 59.5°C, and 30 seconds at 72°C. Serial dilutions (1:10, from 1 μg to 100 pg) of RNA from the TF1 cell line were used to generate standard curves for each RT-PCR product and to calculate normalized transcript levels. Normalized transcript levels in TF1 cells were used to compare the relative expression levels of Pgp, MRP1, and MRP2 transcripts in all other cell lines and patient AML samples. The TF1 cell line was chosen for this purpose because Pgp, MRP1, and MRP2 are constitutively expressed in these cells. The Ct value (ie, the cycle number at which emitted fluorescence exceeds 10 × SD of baseline emissions as measured between cycles 3 and 15) was determined for each transporter and for the β2-microglobulin RT-PCR product for each sample. The relative amount of starting mRNA in the reaction sample was then calculated using Sequence Detection Systems software (version 1.9; Applied Biosystems). For each sample, mRNA levels of each multidrug resistance gene were divided by the β2-microglobulin mRNA level to obtain a normalized transcript level. To compare the relative transcript levels in the other cell lines and patient samples, the normalized ratios for Pgp, MRP1, or MRP2 (normalized for β2-microglobulin) in the TF1 RNA standard cells were arbitrarily set at 1.00.
Primer and probe combinations used in the quantitative RT-PCR
Target cDNA . | Primer/probe combinations . | . | |
|---|---|---|---|
| Pgp | Forward | 5′-CAGCTCCTGGAGCGGTTCTA | |
| Reverse | 5′-CGCTTTATTTCTTTGCCATCAA | ||
| Probe | 5′-CCCCTTGGCAGGGAAAGTGCTGC | ||
| PCR product size | 68 bp | ||
| MRP1 | Forward | 5′-TCATGGTGCCCGTCAATG | |
| Reverse | 5′-CGATTGTCTTTGCTCTTCATGTG | ||
| Probe | 5′-ATGGCGATGAAGACCAAGACGTATCAGGT | ||
| PCR product size | 79 bp | ||
| MRP2 | Forward | 5′-TGCAGCCTCCATAACCATGA | |
| Reverse | 5′-GGACTTCAGATGCCTGCCA | ||
| Probe | 5′-TCGAACACTTAGCCGCAGTTCTAGGTCCA | ||
| PCR product size | 88 bp | ||
| β2-microglobulin | Forward | 5′-CATTCGGGCCGAGATGTC | |
| Reverse | 5′-CTCCAGGCCAGAAAGAGAGAGTAG | ||
| Probe | 5′-CCGTGGCCTTAGCTGTGCTCGC | ||
| PCR product size | 70 bp | ||
Target cDNA . | Primer/probe combinations . | . | |
|---|---|---|---|
| Pgp | Forward | 5′-CAGCTCCTGGAGCGGTTCTA | |
| Reverse | 5′-CGCTTTATTTCTTTGCCATCAA | ||
| Probe | 5′-CCCCTTGGCAGGGAAAGTGCTGC | ||
| PCR product size | 68 bp | ||
| MRP1 | Forward | 5′-TCATGGTGCCCGTCAATG | |
| Reverse | 5′-CGATTGTCTTTGCTCTTCATGTG | ||
| Probe | 5′-ATGGCGATGAAGACCAAGACGTATCAGGT | ||
| PCR product size | 79 bp | ||
| MRP2 | Forward | 5′-TGCAGCCTCCATAACCATGA | |
| Reverse | 5′-GGACTTCAGATGCCTGCCA | ||
| Probe | 5′-TCGAACACTTAGCCGCAGTTCTAGGTCCA | ||
| PCR product size | 88 bp | ||
| β2-microglobulin | Forward | 5′-CATTCGGGCCGAGATGTC | |
| Reverse | 5′-CTCCAGGCCAGAAAGAGAGAGTAG | ||
| Probe | 5′-CCGTGGCCTTAGCTGTGCTCGC | ||
| PCR product size | 70 bp | ||
Statistical analysis
Results are presented as means ± SD from several independent experiments, with single measurements for data on antibody-staining ratios and inhibitor-modulating factors and for data on apoptosis/necrosis in patient samples. For data on apoptosis and cell viability in cell lines, results are presented as means ± SEM obtained from independent experiments with triplicate or quadruplicate wells. Parametric statistical tests were used throughout. Continuous variables between groups were compared using a 2-tailed unpaired Student t test or a one-sample t test, as appropriate. In addition, linear correlations (Pearson) were performed. Statistical calculations were performed using InStat version 3.05 software (GraphPad, San Diego, CA); P < .05 was considered significant.
Results
MRP and Pgp expression in human CD33+ cell lines
We first sought to characterize the expression of MRP1, MRP2, and Pgp in the 5 CD33+ hematopoietic cell lines—NB4 cells, HL-60 cells, an anthracycline-resistant HL-60 subline (HL-60/AR), a vincristine-resistant HL-60 subline (HL-60/VCR), and TF1 cells. All cell lines stained positively for MRP1 and MRP2 (Table 2). As anticipated, HL-60/AR cells highly overexpressed MRP1 protein.26 Accordingly, all cell lines showed functional MRP activity, as determined by CDCF efflux and an inhibitor-modulating factor for MK-571 of 1.1 or greater.15,27 The extent of efflux varied considerably with only minimal activity in NB4 cells, intermediate activity in HL-60 and HL-60/VCR cells, and high activity in TF1 and HL-60/AR cells (Table 2). Of note, the inhibitor-modulating factor of HL-60/AR cells likely underestimated the true MRP activity because those cells could not be loaded with CDCF to the same degree as the other cell lines. Whereas the high MRP activity in HL-60/AR cells correlated with the higher surface display of MRP1 protein, the high CDCF efflux activity of TF1 cells did not correlate with MRP1 and MRP2 immunostaining. To investigate this discordance further, Western blots were prepared using cell lysates and methods previously reported.28 Probing with MRPm6 yielded high background staining that prevented quantitation. Repeat blots were probed with QCRL-129 (Santa Cruz Biotechnology, Santa Cruz, CA), a purified anti-MRP1 antibody that performed similarly to MRPm6 in immunofluorescence assays (data not shown). This revealed relative MRP1 levels (normalized to μg total protein) of 0.30, 1.00, and 4.52 in NB4, TF1, and HL60/AR cells, respectively. Real-time RT-PCR assays revealed that HL-60/AR and TF1 cells expressed the highest relative levels of MRP1 mRNA compared with NB4, HL-60, and HL-60/VCR cells (Table 2). Relative mRNA levels for MRP2 varied less among the individual cell lines (Table 2). Furthermore, within each cell line, the normalized transcript levels of MRP2 were approximately 10- to 100-fold lower than the normalized levels of MRP1 mRNA (data not shown). Thus, MRP1 expression, reflected by relative mRNA and whole cell protein levels, but not MRP2 expression, appeared to correlate with CDCF efflux and inhibition by MK-571 in these cell lines.
Cell surface display, functional activity, and relative mRNA levels for Pgp and MRP in human CD33+ hematopoietic cell lines
. | Cell surface staining intensity ratio . | . | . | Dye efflux inhibitor-modulating factor . | . | Relative normalized mRNA level . | . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cell line . | Pgp . | MRP1 . | MRP2 . | Pgp . | MRP . | Pgp . | MRP1 . | MRP2 . | |||||
| HL-60 | 0.96 ± 0.03 (7) | 1.17 ± 0.04 (15) | 1.30 ± 0.05 (8) | 1.01 ± 0.00 (9) | 1.85 ± 0.19 (10) | < 0.001 | 0.15 | 0.98 | |||||
| NB4 | 0.96 ± 0.01 (5) | 1.16 ± 0.03 (5) | 1.19 ± 0.12 (2) | 1.01 ± 0.00 (3) | 1.23 ± 0.04 (3) | < 0.001 | 0.15 | 1.18 | |||||
| TF1 | 1.21 ± 0.11 (6) | 1.17 ± 0.04 (7) | 1.17 ± 0.03 (3) | 1.43 ± 0.19 (4) | 2.90 ± 0.74 (4) | 1.00 | 1.00 | 1.00 | |||||
| HL-60/AR | 0.95 ± 0.05 (6) | 1.43 ± 0.10 (6) | 1.23 ± 0.06 (3) | 1.02 ± 0.00 (4) | 2.39 ± 0.28 (4) | 0.006 | 3.30 | 0.53 | |||||
| HL-60/VCR | 1.75 ± 0.08 (4) | 1.17 ± 0.03 (5) | 1.26 ± 0.03 (2) | 4.51 ± 0.23 (3) | 1.80 ± 0.23 (4) | 38.8 | 0.10 | 0.31 | |||||
. | Cell surface staining intensity ratio . | . | . | Dye efflux inhibitor-modulating factor . | . | Relative normalized mRNA level . | . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cell line . | Pgp . | MRP1 . | MRP2 . | Pgp . | MRP . | Pgp . | MRP1 . | MRP2 . | |||||
| HL-60 | 0.96 ± 0.03 (7) | 1.17 ± 0.04 (15) | 1.30 ± 0.05 (8) | 1.01 ± 0.00 (9) | 1.85 ± 0.19 (10) | < 0.001 | 0.15 | 0.98 | |||||
| NB4 | 0.96 ± 0.01 (5) | 1.16 ± 0.03 (5) | 1.19 ± 0.12 (2) | 1.01 ± 0.00 (3) | 1.23 ± 0.04 (3) | < 0.001 | 0.15 | 1.18 | |||||
| TF1 | 1.21 ± 0.11 (6) | 1.17 ± 0.04 (7) | 1.17 ± 0.03 (3) | 1.43 ± 0.19 (4) | 2.90 ± 0.74 (4) | 1.00 | 1.00 | 1.00 | |||||
| HL-60/AR | 0.95 ± 0.05 (6) | 1.43 ± 0.10 (6) | 1.23 ± 0.06 (3) | 1.02 ± 0.00 (4) | 2.39 ± 0.28 (4) | 0.006 | 3.30 | 0.53 | |||||
| HL-60/VCR | 1.75 ± 0.08 (4) | 1.17 ± 0.03 (5) | 1.26 ± 0.03 (2) | 4.51 ± 0.23 (3) | 1.80 ± 0.23 (4) | 38.8 | 0.10 | 0.31 | |||||
Values represent mean ± SD of the cell surface staining intensity ratio (MFI of cells stained with specific antibodies divided by the MFI of cells stained with an isotype control antibody). Numbers in parentheses represent numbers of independent experiments.
Pgp activity was determined by the efflux of the fluorescent dye DiOC2 with or without the inhibitor CSA. MRP activity was determined by the efflux of CDCF with or without the inhibitor MK-571. Results are expressed as inhibitor-modulating factor (ratio of the MFI in the presence of the inhibitor divided by the MFI in the absence of the inhibitor).
Relative normalized mRNA levels were determined using real-time RT-PCR, and results of one representative experiment are shown. Results are expressed as the transcript levels of Pgp, MRP1, or MRP2 divided by the β2-microglobulin transcript level. Normalized ratios for TF1 transcript levels were arbitrarily set at 1 because that cell line constitutively expresses all 3 transporters. TF1 RNA was used to generate standard curves for each experiment (“Patients, materials, and methods”).
Pgp was detected on the surface of TF1 and HL-60/VCR cells26 by immunostaining, but not on HL-60, HL-60/AR, or NB4 cells, respectively (Table 2). Consistent with these findings, functional Pgp activity, as assessed by DiOC2 efflux and inhibition by CSA, was high in TF1 cells and very high in HL-60/VCR cells. Virtually no dye efflux was observed in HL-60 or NB4 cells. Real-time RT-PCR assays similarly detected no measurable levels of Pgp mRNA in NB4 or HL-60 cells. By comparison, minimal DiOC2 efflux activity and very low, but detectable, relative Pgp transcript levels were found in HL-60/AR cells, whereas 200-fold and 6500-fold higher relative levels of Pgp mRNA were found in TF1 and HL-60/VCR cells, respectively, correlating with the higher protein and activity levels in those cell lines (Table 2).
GO toxicity in human CD33+ cell lines: effect of inhibition of MRP and Pgp
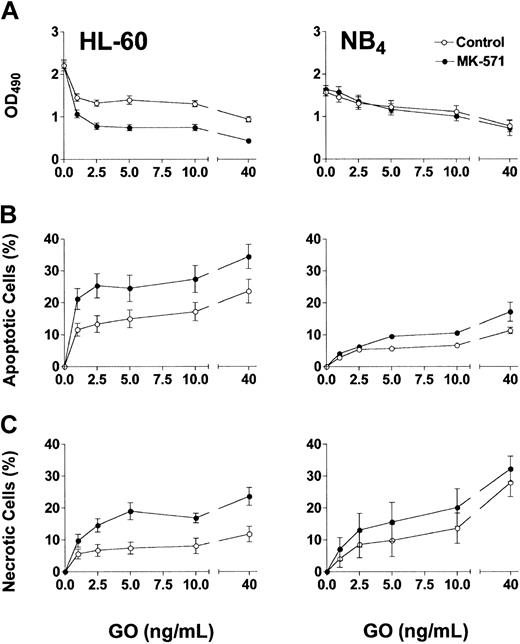
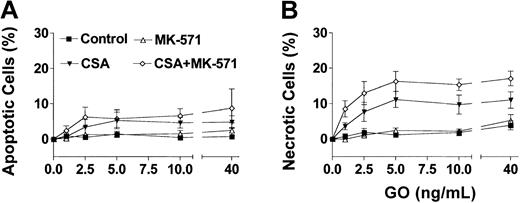
Based on the phenotypic characterizations for transporter expression, NB4 and HL-60 cells were used as models for Pgp-negative/MRP-positive cells, whereas the TF1 cells were used as the model for Pgp-positive/MRP-positive cells. Exposure of HL-60 and NB4 cells to GO for 2 hours, at clinically achievable concentrations (1-40 ng/mL),30 resulted in dose-dependent growth inhibition (Figure 1A) and induction of apoptosis and necrosis (Figure 1B-C). MK-571 enhanced the cytotoxic effect of GO in both cell lines; however, the effect on cell number at 72 hours was demonstrable only in HL-60 cells. The lesser effect of MK-571 in NB4 cells is consistent with the lower MRP activity in those cells compared with HL-60 cells. By comparison, Pgp-positive/MRP-positive TF1 cells were resistant to GO at concentrations up to 40 ng/mL (Figure 2). Drug susceptibility was restored by CSA but not by MK-571 alone (up to 10 μM). However, MK-571 further enhanced GO-induced necrotic cell death in the presence of CSA, suggesting that MRP activity contributes to GO resistance in that cell line (Figure 2). HL-60/AR and HL-60/VCR cells were completely resistant to GO up to 50 ng/mL. In contrast to the effects observed in the other cell lines, CSA (up to 2.5 μg/mL) and MK-571 (up to 30 μM), either alone or in combination, failed to enhance GO cytotoxicity in these drug-selected HL-60 sublines (data not shown), suggesting that the very high overexpression of Pgp or MRP1 prevented the effects of multidrug reversal agents or that other resistance mechanisms are active in these cells.26
Effect of MK-571 on GO-induced cytotoxicity in Pgp-negative/MRP-positive cell lines. Exposure of HL-60 and NB4 cells to GO for 2 hours resulted in dose-dependent cytotoxicity, as reflected by decreased viable cell numbers measured by the MTS assay (A), and increased percentages of apoptotic (annexin V+/PI–) and necrotic (PI+) cells (B-C). The MRP inhibitor MK-571 (5 μM) increased GO-induced apoptosis and necrosis in both cell lines at 72 hours and further decreased HL-60 cell numbers compared with controls. Results are shown as mean ± SEM from 4 to 7 individual experiments performed in quadruplicate (A) or triplicate (B-C) wells.
Effect of MK-571 on GO-induced cytotoxicity in Pgp-negative/MRP-positive cell lines. Exposure of HL-60 and NB4 cells to GO for 2 hours resulted in dose-dependent cytotoxicity, as reflected by decreased viable cell numbers measured by the MTS assay (A), and increased percentages of apoptotic (annexin V+/PI–) and necrotic (PI+) cells (B-C). The MRP inhibitor MK-571 (5 μM) increased GO-induced apoptosis and necrosis in both cell lines at 72 hours and further decreased HL-60 cell numbers compared with controls. Results are shown as mean ± SEM from 4 to 7 individual experiments performed in quadruplicate (A) or triplicate (B-C) wells.
Effect of multidrug resistance reversal agents on GO-induced cytotoxicity in Pgp-positive/MRP-positive TF1 cells. Exposure of TF1 cells to GO for 2 hours did not change the frequency of apoptotic (annexin V+/PI–) cells (A) or necrotic (PI+) cells (B) after 72 hours. CSA alone (1.25 μg/mL) enhanced GO-induced apoptosis and cell death, whereas MK-571 (10 μM) alone was ineffective. The combination of CSA and MK-571, however, significantly increased the percentage of necrotic cells compared with the effects of CSA alone. Results are shown as mean ± SEM from 3 individual experiments performed in triplicate wells.
Effect of multidrug resistance reversal agents on GO-induced cytotoxicity in Pgp-positive/MRP-positive TF1 cells. Exposure of TF1 cells to GO for 2 hours did not change the frequency of apoptotic (annexin V+/PI–) cells (A) or necrotic (PI+) cells (B) after 72 hours. CSA alone (1.25 μg/mL) enhanced GO-induced apoptosis and cell death, whereas MK-571 (10 μM) alone was ineffective. The combination of CSA and MK-571, however, significantly increased the percentage of necrotic cells compared with the effects of CSA alone. Results are shown as mean ± SEM from 3 individual experiments performed in triplicate wells.
Effect of MK-571 on GO-induced cytotoxicity in patient AML blast cells
We next addressed whether MK-571 could enhance GO-induced cytotoxicity in blast cells taken from patients with AML in first relapse. Twenty-three samples were analyzed for expression and functional activity of Pgp and MRP and for GO-induced cytotoxicity in the presence or absence of multidrug reversal agents. CDCF efflux was found to a variable degree in all samples, with an inhibitor-modulating factor for MK-571 ranging from 1.10 to 1.41. Of note, the patient blast cell samples did not take up the CDCF dye as well as the cell lines, potentially leading to an underestimation of MRP efflux activity. MRP proteins were detected in all samples; staining fluorescence ratios for MRP1 ranged from 1.02 to 1.23, and for MRP2 they ranged from 1.10 to 1.41. Adequate RNA was available for analysis of MRP1 and MRP2 transcripts in 11 samples. Relative levels of MRP1 mRNA, normalized to amounts of β2-microglobulin, ranged from 0.01 to 0.78 compared with the level of 1.00 arbitrarily established for the TF1 RNA standard. Relative levels of MRP1 mRNA significantly correlated with MRP activity in these samples (r = 0.62; P < .05), whereas the amount of MRP1 protein detected by immunofluorescence did not (r = –0.116; P = .66). Because of limited sample availability, Western blot assays could not be carried out. MRP2 mRNA was also detectable in all 11 samples. Relative transcript levels, normalized to β2-microglobulin, ranged from 0.04 to 1.39 (compared with the level of 1.00 arbitrarily established for the TF1 RNA standard). In comparison with MRP1, neither the amount of MRP2 protein detected by immunofluorescence (r = 0.168; P = .51) nor the relative level of MRP2 mRNA (r = 0.037; P = .91) correlated with the MRP activity in these samples. Thus, similar to the observations in cell lines, MRP1 but not MRP2 mRNA expression correlated with functional dye efflux associated with MRP activity.
Pgp activity was observed in 17 of 23 patient samples. DiOC2 inhibitor-modulating factor values for CSA ranged from 1.02 to 3.15 (mean, 1.42). In these samples, there was a significant correlation between the staining intensity ratio for Pgp and Pgp activity (r = 0.639; P < .008). For the 13 samples with adequate RNA, a highly significant correlation was also observed between Pgp activity and the relative levels of Pgp mRNA normalized to the amounts of β2-microglobulin (r = 0.95; P < .0001). Among these samples, the relative normalized mRNA levels ranged from 0.01 to 0.36 compared with the level of 1.00 arbitrarily established for the TF1 RNA standard. Of note, these levels were all higher than the low, but detectable relative Pgp mRNA level found in HL-60/AR cells. The level of MRP coexpression among the Pgp-positive samples did not differ from the level in Pgp-negative samples (mean inhibitor-modulating factor values for MK-571, 1.20 ± 0.09 vs 1.23 ± 0.10; P = .56), suggesting that, at least in this small cohort, Pgp and MRP were independently expressed resistance factors.
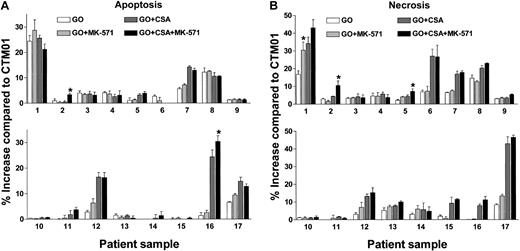
CSA enhanced GO-induced cytotoxicity in 12 of 17 Pgp-positive samples (Figure 3). The percentage of apoptotic cells was increased by the addition of CSA in 5 samples (Figure 3A; patients 5, 7, 12, 16, 17; range, 2.1%-23.0%; mean increase, 11.1% ± 7.8%), whereas CSA increased necrotic cells in 12 samples (Figure 3B; patients 1, 2, 5-8, 11-13, 15-17; range, 1.5%-34.6%; mean increase, 10.1% ± 9.7%). The ability of CSA to enhance GO-induced cytotoxicity was not strongly associated with the relative level of Pgp functional activity (Figure 3). The mean DiOC2 inhibitor-modulating factor value for CSA-responsive samples was 1.52 ± 0.73 compared with 1.19 ± 0.26 for nonresponsive samples (P = .35). In contrast to the frequent effects of CSA alone, GO-induced cytotoxicity was increased by the addition of MK-571 alone (20 μM) in only one patient sample (Figure 3B, sample 1). This sample coexpressed minimal Pgp activity (CSA inhibitor-modulating factor, 1.02) but was responsive to CSA. Because we were concerned that an effect caused by MK-571 might be masked by the coexpression of Pgp, we studied the effects of combinations of CSA and MK-571 with GO. In 3 of the other 11 Pgp-positive/MRP-positive samples that showed increased GO cytotoxicity with CSA alone, the addition of MK-571 further enhanced the percentages of apoptotic or necrotic cells (Figure 3, samples 2, 5, 16). Reproducible MK-571 enhancement was seen on repeated assays of responsive samples (data not shown). There were no differences in the MRP activities of the MK-571–responsive samples (mean inhibitor-modulating factor level, 1.26 ± 0.08) compared with the nonresponsive samples (mean inhibitor-modulating factor level, 1.20 ± 0.10; P = .39). No MK-571 responses were seen among the 6 Pgp-negative AML samples (data not shown).
Effect of multidrug resistance reversal agents on GO-induced cytotoxicity in Pgp-positive/MRP-positive AML blast cell samples. The 17 patient samples are ordered (1-17) from the lowest to the highest DiOC2 inhibitor-modulating factor values for CSA (range, 1.02-3.15). The effect of GO alone or GO in combination with the MRP inhibitor MK-571, the Pgp inhibitor CSA, or MK-571+CSA are depicted as the percentage increases in apoptosis (A) or necrosis (B) compared with patient samples treated with the control immunoconjugate CTM01. GO-induced cytotoxicity (apoptosis, necrosis, or both) was significantly enhanced by MK-571 alone in sample 1, whereas MK-571 further enhanced the effect of CSA on GO-induced cytotoxicity in samples 2, 5, and 16 (*P < .05). These effects were confirmed in a second independent experiment. CSA alone caused significant enhancement (P < .05) in GO-induced cytotoxicity in samples 1, 2, 5-8, 11-13, and 15-17. Values are shown as mean ± SD of triplicate assays.
Effect of multidrug resistance reversal agents on GO-induced cytotoxicity in Pgp-positive/MRP-positive AML blast cell samples. The 17 patient samples are ordered (1-17) from the lowest to the highest DiOC2 inhibitor-modulating factor values for CSA (range, 1.02-3.15). The effect of GO alone or GO in combination with the MRP inhibitor MK-571, the Pgp inhibitor CSA, or MK-571+CSA are depicted as the percentage increases in apoptosis (A) or necrosis (B) compared with patient samples treated with the control immunoconjugate CTM01. GO-induced cytotoxicity (apoptosis, necrosis, or both) was significantly enhanced by MK-571 alone in sample 1, whereas MK-571 further enhanced the effect of CSA on GO-induced cytotoxicity in samples 2, 5, and 16 (*P < .05). These effects were confirmed in a second independent experiment. CSA alone caused significant enhancement (P < .05) in GO-induced cytotoxicity in samples 1, 2, 5-8, 11-13, and 15-17. Values are shown as mean ± SD of triplicate assays.
Discussion
In the present study, the specific MRP-inhibitor MK-571 enhanced GO-induced cytotoxicity in 3 of 5 MRP-expressing CD33+ hematopoietic cell lines. However, MK-571 alone increased cytotoxicity only in HL-60 and NB4 cells, which do not concomitantly express Pgp. By comparison, MK-571 enhanced cytotoxicity in the Pgppositive/MRP-positive TF1 cells only when combined with the Pgp inhibitor CSA. Likewise, GO-induced toxicity was enhanced to a variable degree in a small proportion of blast cell samples from patients with relapsed AML. In only one case was MK-571 alone effective; in 3 cases, GO-induced cytotoxicity was enhanced by MK-571 only in combination with CSA. These data indicate that MRP may contribute to GO resistance but that the inhibition of Pgp function may be required to reveal an effect of MRP on GO susceptibility.
All relapsed AML blast samples assessed in this study showed some level of MRP activity, as indicated by MK-571–sensitive efflux of CDCF. The differences of CDCF efflux between individual samples were small. Overall, the inhibitor-modulating factor values for MK-571 were roughly comparable to those observed in NB4 cells, and none reached the level found in parental HL-60 cells. These observations support the hypothesis of others27 that the MRP activity in most clinical AML cases may represent “basal” MRP activity rather than “overexpression” of this transporter protein. Despite this relatively low level of MRP activity, an effect of MK-571 on GO-induced cytotoxicity could be demonstrated in the NB4 and HL-60 cell lines and in some patient cell samples. Thus, basal MRP expression may reduce GO susceptibility, presumably through efflux of the calicheamicin moiety of the conjugate. This finding would be consistent with the notion that even very low levels of MRP can affect the cells' sensitivity to conventional chemotherapeutic drugs and/or protect against a toxin.6,12
To date, only one study has addressed a potential role of MRP in GO resistance.11 In that study, low MRP activity was found in almost all of the 27 AML patient samples studied, but neither MRP1 protein expression nor MRP activity correlated with the in vitro cytotoxic effect of GO. In addition, the MRP inhibitor probenecid had no effect on GO-induced cytotoxicity. Importantly, most of the samples from that study, similar to our findings, also expressed Pgp activity.11 It is therefore possible that effects of MRP, including the inability of probenecid alone to increase GO-induced cytotoxicity, were confounded by unblocked Pgp function.
Our observations concur with recent studies demonstrating that Pgp function mediates GO resistance in patient blast cell samples and in cell lines.4,10,11 Theoretically, Pgp extrudes the calicheamicin moiety that dissociates from the immunoconjugate after internalization. Indeed, Pgp was implicated as a drug resistance factor in early studies of calicheamicin derivatives,31 and these compounds are similar in size and structure to other known Pgp substrates.32 However, systematic studies to track intracellular calicheamicin are hindered by the limitations that calicheamicins do not autofluoresce and labeling is difficult. We detected Pgp activity in 17 of 23 of our relapsed AML cell samples. However, in many samples, the Pgp activity was relatively low (mean inhibitor-modulating factor level of 1.42). CSA significantly enhanced in vitro GO-induced cytotoxicity in roughly 70% of the samples, including some with low Pgp activity.
Our results suggest that MRP function plays a comparatively minor role in GO resistance compared with Pgp. First, MK-571 alone enhanced GO-induced cytotoxicity in only one patient blast cell sample, one which had minimal Pgp activity. Second, the relative increases in percentages of apoptotic or necrotic cells in the presence of CSA were greater than the increases in the presence of MK-571. Third, an effect attributed to MK-571 always required concomitant CSA in cells that coexpressed more than minimal Pgp activity. We hypothesize that, in those coexpressing cells, unopposed efflux by Pgp likely prevented intracellular drug accumulation and therefore masked a minor effect of MK-571 alone. A predominant role of Pgp over MRP is further suggested by our recent observations that repeated exposure of parental HL-60 cells to incremental concentrations of GO induces functional Pgp activity without a change in basal MRP activity (R.B.W., unpublished observations, June 2003). Together, these findings emphasize the importance of Pgp in GO resistance and the rationale for treatment trials combining GO with Pgp inhibitors.
The functional assays used for these studies were chosen because they are among the most specific available for an assessment of MRP and Pgp. Unfortunately, specific fluorescent substrates and inhibitors have not yet been developed for unique members of the MRP family, such as MRP1 and MRP2. CDCF is known to be a substrate for MRP1 and MRP2, but not for Pgp.33-35 MK-571 is a competitive inhibitor of transport mediated by MRP1 and, to a lesser degree, MRP2,24,36 but it does not affect Pgp activity.37,38 Our experiments confirmed these findings; MK-571 did not block DiOC2 efflux in TF1 cells, HL-60/VCR cells, or Pgp-positive blast cell samples. By comparison, DiOC2 is pumped out of the cell by Pgp, but the dye is not a substrate for MRP.39 Our studies observed no DiOC2 efflux in cell lines that expressed MRP but not Pgp. Thus, these dye/inhibitor combinations served as valid surrogates for MRP and Pgp transporter activities in cells expressing both proteins.
Because MRP1 and MRP2 have been found in AML blasts,6,18 we wanted to understand the potential role of each transporter in GO resistance. The different levels of MRP activity among the CD33+ cell lines correlated with whole cell MRP1 protein levels and were partially reflected by cell surface display of MRP1, whereas they were not associated with MRP2 surface immunostaining. Neither MRP1 nor MRP2 immunofluorescence staining correlated with CDCF efflux in the patient AML blast cell samples, likely because of the low levels in this group and the relative insensitivity of the assay. By comparison, a relatively good correlation was found between the functional MRP activity and normalized mRNA levels for MRP1 among the cell lines and the 11 patient AML blast cell samples tested. MRP2 mRNA levels, on the other hand, showed no correlation with MRP activity in either the CD33+ hematopoietic cell lines or the patient AML samples. A similar association between MRP1 mRNA levels and functional MRP dye efflux activity, but no correlation with MRP2 transcript levels, has been observed in AML patient samples by others.18 These data therefore suggest that quantitative levels of MRP1 mRNA may accurately assess the relative functional activity of MRP and that MRP2 does not contribute significantly to MRP activity in AML blasts. Although the level of Pgp protein detected by immunofluorescence correlated with Pgp activity in the patient AML blast cell samples, we observed an even better correlation between the relative level of Pgp mRNA and Pgp activity. This finding could be explained by the recent observation that intracellular compartmentalized Pgp may be functionally active but not detected by surface immunostaining.40 Thus, real-time quantitative mRNA assays appear to be valid and useful for the estimation of relative MRP and Pgp functional activities, especially in patients with low-level expression.
CSA was used in the present study because this drug has been used clinically as a Pgp reversal agent.9,41,42 Of note, previous studies have shown that CSA may also weakly inhibit MRP1- and MRP2-mediated transport.43-45 Indeed, we observed that CSA partially inhibited CDCF efflux in all cell lines (inhibitor modulating factor level, ranges, 1.13 ± 0.03 [NB4 cells]-1.57 ± 0.15 [HL-60/AR cells]) and in patient AML blast cell samples (inhibitor modulating factor level, 1.06-1.29 [P < .0001]). Although CSA inhibits MRP function to a lesser extent than MK-571, it is possible that some of the effect of CSA we observed in in vitro cytotoxicity assays was caused, at least in part, by the inhibition of MRP function. On the other hand, the fact that MK-571 had an additive effect with CSA in enhancing GO-induced cytotoxicity in some patient samples and in the TF1 cell line suggests that CSA does not sufficiently block MRP activity to fully restore GO susceptibility.
Because MK-571 and CSA failed to enhance GO-induced cytotoxicity in roughly 30% of the Pgp-positive/MRP-positive patient cell samples, alternative mechanisms of resistance were likely operative. Hypothetically, a variety of mechanisms may contribute to diminished GO activity—alterations in the process of GO internalization, resulting in decreased GO uptake; differences in intracellular trafficking,46 resulting in diminished release of the active calicheamicin moiety; additional drug efflux systems, such as other ABC transporter proteins; and dysregulation of downstream factors that either increase cell capability to repair DNA or prevent cascades leading to cell death. Additional studies will be required to distinguish alternative mechanisms that may be important for GO resistance and may also be relevant for other antibody-based treatments. Knowledge of such mechanisms will facilitate the development of new agents that avoid resistance or clinical interventions that reverse or bypass these barriers.
Prepublished online as Blood First Edition Paper, April 10, 2003; DOI 10.1182/blood-2003-02-0396.
Supported in part by research funding from the Leukemia and Lymphoma Society (7040-03) and the National Cancer Institute (CA92316). I.D.B. is a recipient of a FM Kirby/American Cancer Society Clinical Research Professorship. R.B.W. is a recipient of a fellowship from the Swiss Foundation for Medical-Biological Grants (1098).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Steven J. Staats for performing Western blot assays and Deborah E. Banker for helpful discussions.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal