Abstract
The mechanisms of interaction between anti-CD40 monoclonal antibody (mAb) therapy and external beam irradiation were investigated in 2 syngeneic B-cell lymphoma models. We have established doses of anti-CD40 mAb and irradiation which, although ineffective when given singly, were capable of providing long-term protection when used in combination. Furthermore, such treatment was not only critically dependent on the dose of mAb and irradiation but also on tumor load, with greater efficacy only occurring at higher tumor burden. Using blocking antibody, the potency of treatment was shown to be totally dependent on CD8+ T cells, with protective levels of CD8+ cells occurring only in mice receiving the combination of anti-CD40 and irradiation. Interestingly, the ratio of T cells (CD8+) to tumor cells in mice receiving combination treatment was between 10 and 15 times that seen in animals given anti-CD40 or irradiation alone. In vivo tracking experiments revealed a 2-phase decrease in tumor burden, the first resulting directly from the external irradiation and the second, occurring 5 days later, concomitant with the rise in tumor-specific CD8+ cells. We suggest that the external irradiation induced an initial kill of lymphoma cells, probably by apoptosis, which releases tumor antigens and slows the progression of the malignancy to allow generation of a curative cytotoxic T lymphocyte (CTL) response promoted by the anti-CD40 mAb. Combining irradiation with immunomodulatory mAb as described here appears to provide a powerful new approach to the management of cancer.
Introduction
Lymphomas generally exhibit exquisite radiosensitivity, and for many years irradiation has played an integral role in the treatment of a range of B-cell malignancies.1,2 Localized irradiation remains the only potentially curative option for early-stage “low-grade” non-Hodgkin lymphomas (NHLs), and the use of total nodal irradiation in NHL has led to 10-year disease-free survival times, which are comparable to cytotoxic drugs.1 Total body irradiation (TBI) has the additional theoretical advantage over chemotherapy of eradicating tumor in sanctuary sites.3
Integrating monoclonal antibody (mAb) with irradiation appears a promising strategy, given the radiosensitivity of lymphoma and the potency of a number of mAbs when used as single agents against B-cell antigens. In view of the lack of overlapping toxicity and the different mechanisms of action, mAbs are increasingly being successfully combined with chemotherapy and radiotherapy in the clinic.4,5
While the optimal dose and fractionation schedule of TBI and the relative merits of mAb-targeted radiation versus TBI remain the subject of ongoing investigation,6 it is becoming increasingly clear that mAb specificity is critical to improving therapeutic outcomes when mAb is combined with irradiation. Our group and other workers have previously shown that some signaling mAbs have additive antitumor activity when combined with irradiation.7,8 A number of different antibody-mediated effector mechanisms including antibody-dependent cell cytotoxicity (ADCC), complement-dependent cytotoxicity (CDC), and cell surface signaling are likely to operate and may act in concert with the irradiation to enhance antitumor activity. Recent data suggest that some signaling mAbs that are able to regulate antigen presentation and cytotoxic T-cell (CD8+) responses may be effective as anticancer therapies.9,10
One such immunomodulatory mAb is anti-CD40, which has shown therapeutic activity in a range of preclinical models.10,11 CD40 is a member of the family of tumor necrosis factor (TNF) receptor molecules and has a broad pattern of expression, including B cells and a variety of antigen-presenting cells (APCs).12 CD40 ligation triggers a range of cellular functions including the activation of APCs, “licensing” them to present antigen to cytotoxic T cells.13 These effects on the generation of CD8+ T cells have made anti-CD40 treatment particularly attractive for tumor immunotherapy in the appropriate immunization context. With large amounts of anti-CD40 mAb and optimal tumor burden, activation of tumor-specific T cells and durable responses in tumor-bearing hosts have been seen for a range of both CD40+ and CD40– syngeneic B-cell lymphomas.10,11 However, in each of these models it was clear that the immune system can be overwhelmed by a rapidly growing tumor in such a way that mice succumb to the malignancy before the anti-CD40 can promote an effective T-cell response. Combining anti-CD40 with an effective cancer treatment such as irradiation appears a promising strategy whereby the host immune system is simultaneously stimulated while debulking tumor and liberating antigens that might be recognized by responding T cells.
In this study we have investigated the efficacy and feasibility of combining TBI with anti-CD40 treatment. We have demonstrated for the first time that there is a dose-dependent additive effect between irradiation and anti-CD40 mAb, which affords long-term tumor protection via a CD8+ T-cell–dependent pathway. Moreover, we have shown that there is a clear radiation threshold for this therapeutic activity, and with increasing doses of irradiation T-cell activation is impaired and long-term tumor protection is lost. We believe these observations have important implications for a number of different immunotherapy strategies for cancer.
Materials and methods
Mice
Tumor cells
All cell cultures were maintained in supplemented Dulbecco modified eagle medium (DMEM) (Life Technologies, Paisley, United Kingdom) containing glutamine (2 mM), pyruvate (1 mM), penicillin and streptomycin (100 IU/mL), amphotericin B (Fungizone; Bristol-Meyers Squibb, Chester, United Kingdom; 2 μg/mL), and 5% to 10% fetal calf serum (FCS; Myoclone; Life Technologies), except πBCL1, which was maintained in supplemented RPMI (Life Technologies), plus 50 μmol/L 2-mercaptoethanol (2-ME) (BDH, Poole, United Kingdom) and 15% FCS.
Monoclonal antibodies
Monoclonal antibodies used in this study were Mc106A5 (rat anti-BCL1 Id)9 ; Mc39-12 (rat antimouse Fcμ)9 ; Mc39-16 (rat anti-A31 Id)9 ; 3/23 (rat antimouse CD40)17 ; 1D3 (rat antimouse CD19)18 ; TI2-3 (rat antimouse major histocompatibility complex [MHC] II; Tenovus Research Laboratory); HB79 (rat antimouse MHC I Kd and Dd, American Type Culture Collection, Manassas, VA); NIMR6 (rat antimouse CD22)19 ; YTA1.3.2 (rat antimouse CD4)20 ; and YTS169 (rat antimouse CD8).20 Hybridoma cells were maintained in stationary culture using 5% supplemented DMEM (as above). To purify the immunoglobulin G (IgG) mAb, supernatants were concentrated by membrane filtration (Amicon, Beverly, MA), precipitated with saturated ammonium sulfate, and dialyzed and fractionated on protein G (Pharmacia, Piscataway, NJ).
Immunotherapy
Groups of age-matched mice were injected intravenously with 106 A31 (CBA/H) or BCL1 (BALB/c) lymphoma cells on day 0. Total body irradiation (TBI) was conducted using a modified 225 kV x-ray unit (Gulmay, Chertsey, United Kingdom). TBI was given on day 10 at a dose rate of 1.15 Gy/min. In all cases animals were fed acidified water (pH 2.5; 1N HCl) supplemented with neomycin sulfate (2 g/L) (Sigma Aldrich, Poole, United Kingdom), starting 1 week prior to TBI and continuing for 4 weeks afterward. Animals were housed in a sterile maximizer unit (Techniplast, Kettering, United Kingdom). Mice were treated with intravenous mAb 4 hours after irradiation.
Both tumors develop primarily in the spleen, with a terminal leukemic overspill. Survival was monitored daily, and the results were analyzed using the χ2 test of Peto.21 Animal immunotherapy was approved by the local ethical committee of Southampton University and was performed under a United Kingdom Home Office license.
Flow cytometry
Mouse splenocytes were analyzed by direct immunofluoresence staining using a FACSCalibur (Becton Dickinson, Mountain View, CA). Briefly, splenocytes at 106/mL (100-μL sample) were incubated with fluorescein isothiocyanate (FITC)– or phycoerythrin (PE)–labeled mAb (50 μg/mL) for 20 minutes on ice, then washed with phosphate-buffered saline (PBS) containing 1% bovine serum albumin, and immediately analyzed.
In vivo tracking
To measure tumor growth kinetics following treatment and chart the progress of any immunologic response, groups of mice were inoculated with 106 tumor cells intravenously on day 0 and treated on day 10 with TBI, mAb (1 mg intravenously), or a combination of the two. Mice were housed under sterile conditions (as before). Mice were killed at sequential time points after treatment, spleens removed, homogenized, washed once by centrifugation, and resuspended at 106/mL in supplemented RPMI.
Tumor cells were detected by dual-fluorescence staining using FITC–Mc39-16 (rat anti-A31 idiotype)10 versus PE-ID3 (rat antimouse CD19). Cells were stained with mAb (50 μg/mL) on ice for 20 minutes, washed, and analyzed on a FACSCalibur (Becton Dickinson) as before.
Measurement of in vitro apoptosis and clonogenic survival
Measurement of in vitro apoptosis was conducted according to the method of Nicoletti et al.23 Essentially, culture plates (24-well multiplates; Life Technologies) were coated with mAb (5 μg/mL) for 2 hours at 37°C prior to addition of cells (πBCL1 lymphoma; 2 × 105/mL). Cells were then immediately treated with 5 Gy external beam irradiation (dose rate 1.15 Gy/min as before) or left untreated. Controls were maintained under similar conditions to test samples while irradiation was conducted, reducing the risk of experimental differences due to variations in environmental parameters. Samples (approximately 5 × 105 cells) were harvested 72 hours after treatment, washed once in PBS, and resuspended in hypotonic fluorochrome solution (50 μg/mL propidium iodide [PI], 0.1% [wt/vol] sodium citrate, 0.1% [vol/vol] Triton X-100) and then stored overnight at 4°C to allow staining of DNA. PI-stained nuclei were analyzed using a FACSCalibur as previously described.7
Clonogenic survival was determined using a serial dilution assay according to the method of Olive et al.24 Briefly, πBCL1 lymphoma cells were seeded into individual wells of a 96-well plate over a range of cell densities (density: 10 wells per cell density; 8 cell densities per dose) and treated with 0 to 5 Gy of irradiation as before. Cells were plated in 70% fresh medium and 30% conditioned medium. Control (anti-A31 Id) or anti-CD40 mAb was added at a final concentration of 5 μg/mL. After 14 days the fraction of wells containing more than 25 surviving cells was determined using a light microscope. The cell number that resulted in 30% empty wells was calculated and used to generate a clonogenic survival curve. Experiments were repeated at least 3 times.
Cytotoxicity assay
To determine T-cell specificity, BALB/c mice were inoculated with tumor (106 cells, intravenously) and treated with 5 Gy TBI and anti-CD40 (100 μg) on day 15, as before. Splenocytes were harvested from mice 5 days after treatment and levels of cytotoxic activity against target lymphoma cells were assessed using a standard chromium 51Cr-release assay.9 Briefly, 51Cr-labeled targets (A31 or πBCL1) were prepared from spleens of terminal animals (A31) or cultured cells (πBCL1) and resuspended at 105/mL in supplemented RPMI. The effectors were splenocytes taken from animals 5 days after treatment with TBI, anti-CD40, or a combination of the two. For the assay, 50 μL aliquots of target cells (5 × 103) were seeded in wells of a 96-well U-bottomed plate (Life Technologies) and 100 μL aliquots of effector cells were then added at various CD8+ T cells to target (E/T) ratios. Plates were centrifuged at 200g for 5 minutes at room temperature, incubated for 4 hours at 37°C, and recentrifuged at 500g for 5 minutes before 100 μL supernatant was harvested from each well to estimate the released 51Cr. Determinants were performed in triplicate and maximal release of 51Cr obtained using target cells to which 150 μLof 1% Nonidet P-40 had been added. The percentage of specific 51Cr release was calculated using the formula: ([sample release – background release]/[maximum release – background release]) × 100. Controls included effector and target cells incubated with a chemically engineered bispecific F(ab′)2 antibody with specificities for murine CD3 and MHC II, capable of retargeting any T cell against any B-cell target. Details of this reagent and its activity have been described previously.25 Finally, to show that killing activity was dependent on CD8+ T cells, blocking monoclonal antibody against MHC I (HB79, anti-Kd and Dd, American Type Culture Collection) was included at a final concentration of 25 μg/mL.
Adoptive transfer of CD8+ T cells from treated mice
Mice were inoculated with tumor and treated with 5 Gy TBI and anti-CD40 as before. Just prior to the peak of the CD8 response (typically day 4 after treatment), spleens were removed and the remaining tumor was depleted using FITC-labeled anti-CD19 mAb, followed by anti-FITC MACS beads and LS columns (Miltenyi Biotec, Bergisch Gladbach, Germany) as described in the manufacturer's instructions. Splenocytes from immunized mice were then mixed with 104 fresh lymphoma cells at a ratio of 100:1 CD8+ T cells to tumor cells and given by intraperitoneal injection to naive mice. Control animals received tumor alone or tumor plus normal splenocytes. Survival was recorded daily.
Results
Combination TBI and anti-CD40 therapy results in long-term survival in a radiation dose–dependent manner
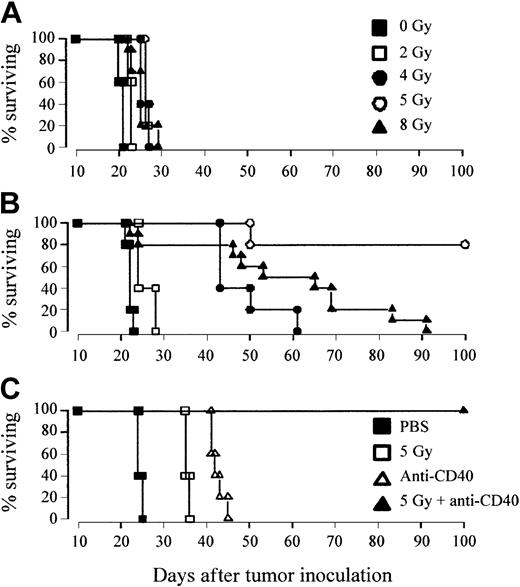
Therapy was assessed in 2 B-cell lymphoma models, A31 and BCL1, which both express similar levels of the CD40 antigen (approximately 4 × 104 molecules per cell).9 Previous work in these B-cell lymphoma models has shown that anti-CD40 mAb was highly effective at promoting T-cell responses but that once the tumors had reached a critical size the immune system became overwhelmed before immunity could be established. Flow cytometry estimates suggested that treatment with anti-CD40 mAb could promote immunity in mice while the splenic lymphoma load remained below 10%. Building on these observations, we first tested the relative efficacy of a combination of anti-CD40 and TBI in comparison with anti-CD40 or irradiation alone. Mice received 106 tumor cells, intravenously on day 0, and were treated on day 10 with either TBI (1 to 8 Gy), anti-CD40 mAb (1 mg intravenously), or a combination of the two, whereby the mAb was given 4 hours after irradiation. Figure 1A illustrates that treatment of the A31 lymphoma with TBI alone results in only a modest increase in survival of just a few days over that seen in control animals (PBS treated). Likewise, treatment with anti-CD40 mAb alone provided minimal improvement in survival (Figure 1B). However, when anti-CD40 and TBI were used in combination, there was a clear radiation dose–dependent increase in survival with 2 Gy providing around 6 days of advantage over controls, 4 Gy around 35 days, and 5 Gy consistently affording long-term protection (more than 100 days) to more than 80% of animals (Figure 1B). A similar radiation dose response was also observed in combination with another anti-CD40 mAb, 1C10,26 (data not shown). Interestingly, as the dose of radiation was increased further to 8 Gy there was a significant decrease in therapeutic efficacy compared with the 5 Gy plus anti-CD40–treated cohort (P < .02), with no long-term survivors (Figure 1B). Similar results were obtained in a second lymphoma model, BCL1 (Figure 1C), where long-term survival was obtained using as little as 100 μg anti-CD40 plus TBI. This tumor model is more sensitive to anti-CD40 treatment than the A31 model and, although some tumor protection is observed, no long-term survival is seen when treating with 100 μg mAb alone. Long-term survival was, however, seen with anti-CD40 mAb when the dose was increased 10-fold higher (1 mg) (data not shown).
Combination but not single-agent treatment results in long-term protection against lymphoma. (A-B) Groups of 5 age-matched CBA mice were inoculated with 106 A31 tumor cells intravenously on day 0 and were treated with either (A) external beam TBI (single dose of 2 to 8 Gy at a rate of 1.15 Gy/min) plus PBS or (B) TBI plus anti-CD40 monoclonal antibody (1 mg, intravenously, giving the mAb 4 hours after irradiation). Treatment was as indicated. (C) Groups of 5 age-matched BALB/c mice were inoculated with 106 BCL1 lymphoma cells on day 0 and were treated with 5 Gy TBI with or without anti-CD40 (100 μg, intravenously) on day 15. All animals were fed acidified water substituted with neomycin sulfate starting 3 days prior to irradiation and continuing for a further 2 weeks. Survival was recorded daily. There is a clear radiation dose–dependent increase in survival treating with combined TBI and anti-CD40, with 5 Gy plus mAb, but not single treatment regimens, providing long-term survival against both lymphoma lines. Similar results were obtained in at least 2 separate experiments.
Combination but not single-agent treatment results in long-term protection against lymphoma. (A-B) Groups of 5 age-matched CBA mice were inoculated with 106 A31 tumor cells intravenously on day 0 and were treated with either (A) external beam TBI (single dose of 2 to 8 Gy at a rate of 1.15 Gy/min) plus PBS or (B) TBI plus anti-CD40 monoclonal antibody (1 mg, intravenously, giving the mAb 4 hours after irradiation). Treatment was as indicated. (C) Groups of 5 age-matched BALB/c mice were inoculated with 106 BCL1 lymphoma cells on day 0 and were treated with 5 Gy TBI with or without anti-CD40 (100 μg, intravenously) on day 15. All animals were fed acidified water substituted with neomycin sulfate starting 3 days prior to irradiation and continuing for a further 2 weeks. Survival was recorded daily. There is a clear radiation dose–dependent increase in survival treating with combined TBI and anti-CD40, with 5 Gy plus mAb, but not single treatment regimens, providing long-term survival against both lymphoma lines. Similar results were obtained in at least 2 separate experiments.
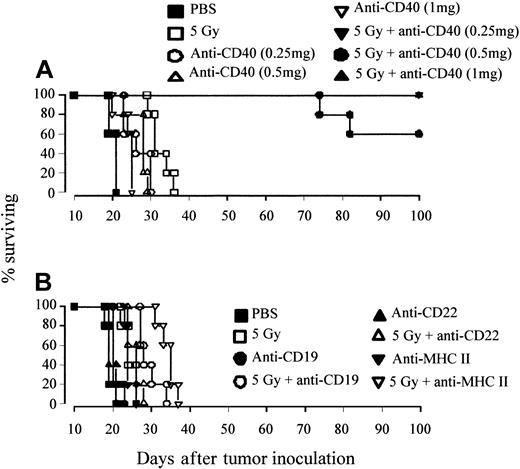
We further assessed the effect of mAb dose on tumor protection by using the A31 tumor model, which was considerably more resistant to the effects of anti-CD40 mAb alone. Mice were inoculated with tumor as before and treated on day 10 with 5 Gy TBI plus a range of anti-CD40 doses. Figure 2A shows that the A31 lymphoma is still highly sensitive to combination treatment with doses of mAb as low as 0.25 mg, with more than 60% of animals becoming long-term protected at all doses. Clearing of tumor was not seen with mAb directed to other B-cell antigens, such as CD19, MHC II, and CD22 (Figure 2B). Thus, long-term eradication of tumor appears to be dependent on the inherent activity of the anti-CD40 mAb.
Long-term protection can be achieved with anti-CD40 but not other mAb specificities. (A) Groups of 5 age-matched CBA mice were inoculated with 106 A31 tumor cells intravenously on day 0 and were treated with 5 Gy TBI (as before), together with 0.25, 0.5, or 1 mg anti-CD40 mAb intravenously. Control animals received PBS. Treatment was as indicated. (B) Groups of 5 CBA mice were inoculated with A31 as before and treated with combinations of 5 Gy TBI with or without mAb to a panel of B-cell markers (CD19, CD22, MHC II as indicated; all 1 mg intravenously). All animals were fed acidified water substituted with neomycin sulfate as before. Survival was recorded daily. When given in combination with TBI, long-term protection can be achieved with anti-CD40 mAb (but not other mAb specificities) at doses that are nontherapeutic when given as a single agent. Similar data were obtained in at least 2 independent experiments.
Long-term protection can be achieved with anti-CD40 but not other mAb specificities. (A) Groups of 5 age-matched CBA mice were inoculated with 106 A31 tumor cells intravenously on day 0 and were treated with 5 Gy TBI (as before), together with 0.25, 0.5, or 1 mg anti-CD40 mAb intravenously. Control animals received PBS. Treatment was as indicated. (B) Groups of 5 CBA mice were inoculated with A31 as before and treated with combinations of 5 Gy TBI with or without mAb to a panel of B-cell markers (CD19, CD22, MHC II as indicated; all 1 mg intravenously). All animals were fed acidified water substituted with neomycin sulfate as before. Survival was recorded daily. When given in combination with TBI, long-term protection can be achieved with anti-CD40 mAb (but not other mAb specificities) at doses that are nontherapeutic when given as a single agent. Similar data were obtained in at least 2 independent experiments.
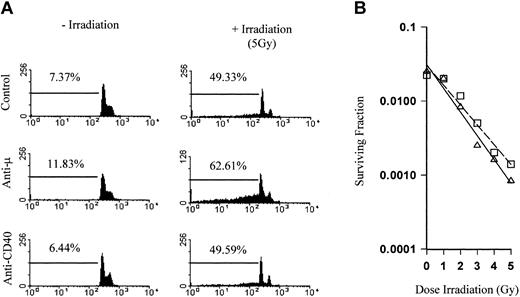
We next investigated the mechanisms behind the tumor protection and whether the combination of irradiation and the anti-CD40 mAb were acting directly on the tumor cells, resulting in an additive or synergistic increase in tumor cell kill. In vitro apoptosis and clonogenic survival assays were therefore performed. The πBCL1 lymphoma cells, a variant of the BCL1 tumor, were irradiated and then cultured in the presence of mAb. The levels of cell death were determined by flow cytometry using PI staining to measure DNA fragmentation. Figure 3A shows the DNA profile of cells treated with control mAb, anti-CD40, or anti-Fcμ for 72 hours. The percentage of DNA in the sub-G1/G0 phase of the cell cycle is shown gated and represents fragmented DNA present in apoptotic cells.23 Anti-CD40 had no effect on the DNA profile compared with controls, while as expected anti-Fcμ induced cell death (12%). When cells were treated with 5 Gy external beam irradiation, there was a marked increase in DNA fragmentation (49%). In the presence of anti-Fcμ this increased still further (63%), indicating an additive effect between the mAb and irradiation as we have previously described.7 However, in stark contrast there was no increase in apoptosis over controls when irradiation was combined with anti-CD40 mAb. Likewise, there was no significant difference in clonogenic survival between control and anti-CD40–treated cells (Figure 3B).
Radiation does not sensitize lymphoma to direct anti-CD40 cytotoxicity. (A) The πBCL1 lymphoma cells either left untreated or treated with 5 Gy external beam irradiation were cultured for 72 hours in 24-well plates coated with mAb (5 μg/mL) with specificity as indicated. Cells were then harvested, stained with PI to detect DNA content, and analyzed by flow cytometry. Gated regions indicate the percentage of sub-G1 fragmented DNA, indicative of apoptosis. There was a clear additive effect between irradiation and mAb to the B-cell receptor (BCR) but not between irradiation and anti-CD40 mAb. (B) Clonogenic survival of πBCL1 treated with external beam radiation (0 to 5 Gy) in the presence of control (□) or anti-CD40 mAb (as before; ▵). While there was a clear difference in survival over the range of radiation doses, addition of anti-CD40 mAb did not significantly affect clonogenic survival.
Radiation does not sensitize lymphoma to direct anti-CD40 cytotoxicity. (A) The πBCL1 lymphoma cells either left untreated or treated with 5 Gy external beam irradiation were cultured for 72 hours in 24-well plates coated with mAb (5 μg/mL) with specificity as indicated. Cells were then harvested, stained with PI to detect DNA content, and analyzed by flow cytometry. Gated regions indicate the percentage of sub-G1 fragmented DNA, indicative of apoptosis. There was a clear additive effect between irradiation and mAb to the B-cell receptor (BCR) but not between irradiation and anti-CD40 mAb. (B) Clonogenic survival of πBCL1 treated with external beam radiation (0 to 5 Gy) in the presence of control (□) or anti-CD40 mAb (as before; ▵). While there was a clear difference in survival over the range of radiation doses, addition of anti-CD40 mAb did not significantly affect clonogenic survival.
Long-term clearance of tumor requires a critical tumor antigen load
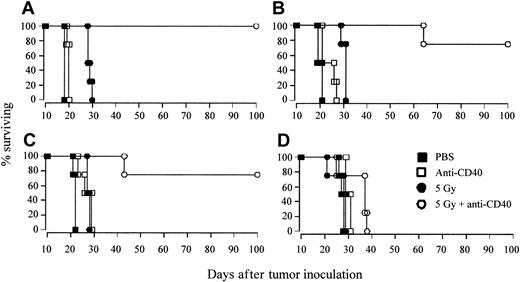
Following on from these in vitro observations, we decided to address the issue of a potential additive effect between the radiation and the mAb through direct cytotoxicity in an in vivo setting. If treatment with anti-CD40 had the effect of sensitizing lymphoma cells to irradiation, we might expect such an interaction to be independent of tumor load and to be potentially more effective with smaller tumor burdens. Mice were therefore inoculated with between 106 and 103 tumor cells intravenously on day 0 and were then treated as before on day 10 with anti-CD40 mAb in combination with 5 Gy TBI. The results shown in Figure 4 reveal a critical threshold for the number of tumor cells required for the combination therapy to deliver long-term tumor protection. With the lowest tumor burden of 103 (Figure 4D), lasting tumor immunity was not seen and only when the inoculum was increased to above 104 tumor cells was long-term tumor protection seen (Figure 4A-C). These results suggest that there is not a direct process of sensitization occurring in vivo, but instead indicate that a critical tumor load is required in order to achieve long-term protection, pointing instead to there being an immunologic component to the therapy.
A critical dose of tumor antigens is required for long-term eradication of tumor. Groups of 4 CBA mice were inoculated with (A) 106, (B) 105, (C) 104, or (D) 103 A31 lymphoma cells, intravenously on day 0, and were treated on day 10 with either 5 Gy TBI, anti-CD40 mAb (1 mg intravenously), or a combination of the two, as before. Controls received PBS. Survival was recorded daily. Length of protection correlated to tumor burden, with long-term survival completely lost at an inoculating dose of 103 lymphoma cells. Shown is 1 of 2 similar experiments.
A critical dose of tumor antigens is required for long-term eradication of tumor. Groups of 4 CBA mice were inoculated with (A) 106, (B) 105, (C) 104, or (D) 103 A31 lymphoma cells, intravenously on day 0, and were treated on day 10 with either 5 Gy TBI, anti-CD40 mAb (1 mg intravenously), or a combination of the two, as before. Controls received PBS. Survival was recorded daily. Length of protection correlated to tumor burden, with long-term survival completely lost at an inoculating dose of 103 lymphoma cells. Shown is 1 of 2 similar experiments.
Kinetics of the antitumor response following combination TBI and anti-CD40
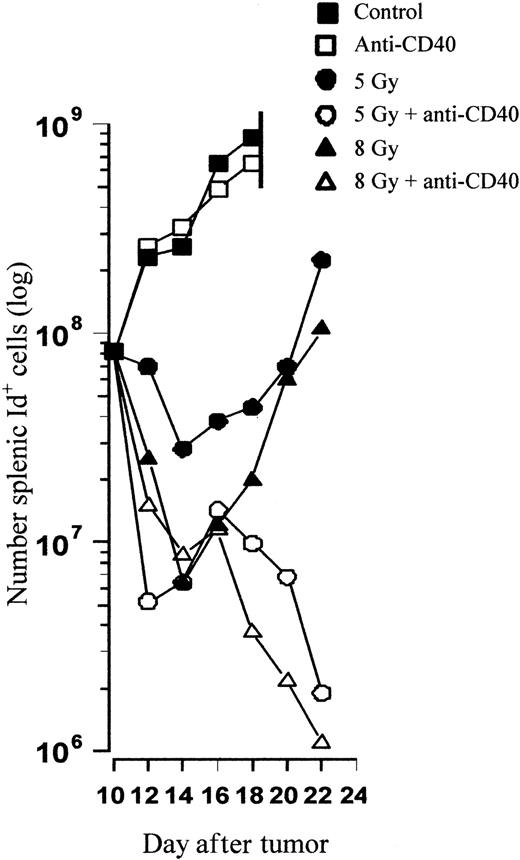
To try to identify the mechanism of tumor clearance in vivo, tracking experiments were performed to monitor the kinetics of tumor growth and assess the nature of the possible immune response. Mice were inoculated with 106 tumor cells intravenously on day 0 and treated as before on day 10 with anti-CD40 mAb in combination with TBI. Animals were subsequently killed at sequential time points after the treatments and the number of splenic tumor cells was determined using flow cytometry. Figure 5 shows that the kinetics of tumor growth in control and anti-CD40–treated animals is similar and that they become terminally ill due to massive splenic tumor just 8 days after treatment. TBI results in a radiation dose–dependent decrease in tumor cell number, and 8 Gy clears a whole log more tumor than 5 Gy alone. However, tumor regrowth occurs rapidly after TBI when used alone, and similar amounts of tumor to those found before treatment are reached 10 to 12 days after treatment for both 5 and 8 Gy. In stark contrast, with the addition of anti-CD40 mAb the combination treatment results in an initial and then much more pronounced and sustained second decrease in the number of tumor cells. By day 12 with the combination therapy, less than 1% of the initial tumor burden present prior to treatment remains. Interestingly, there appears to be little difference in the profile of tumor eradication between mice receiving anti-CD40 and 5 or 8 Gy of TBI, although there are major differences in overall survival.
In vivo tracking reveals delayed tumor growth followed by regression after combination therapy. CBA mice were inoculated with 106 tumor cells and treated on day 10 with TBI and anti-CD40 mAb (1 mg intravenously) according to the standard protocol. Control animals received PBS. At sequential time points, 2 mice per group were killed and the number of splenic tumor cells was calculated by 2-color flow cytometry with PE-labeled anti-CD19 and FITC-labeled anti-idiotype (see “Materials and methods”). Shown is the mean number of tumor cells present in the spleen at each time point. TBI results in a radiation dose–dependent decrease in tumor burden, with less than 1% of the tumor volume present prior to treatment (day 10) remaining 12 days after combination but not single-agent treatment. Similar results were obtained in at least 2 separate experiments.
In vivo tracking reveals delayed tumor growth followed by regression after combination therapy. CBA mice were inoculated with 106 tumor cells and treated on day 10 with TBI and anti-CD40 mAb (1 mg intravenously) according to the standard protocol. Control animals received PBS. At sequential time points, 2 mice per group were killed and the number of splenic tumor cells was calculated by 2-color flow cytometry with PE-labeled anti-CD19 and FITC-labeled anti-idiotype (see “Materials and methods”). Shown is the mean number of tumor cells present in the spleen at each time point. TBI results in a radiation dose–dependent decrease in tumor burden, with less than 1% of the tumor volume present prior to treatment (day 10) remaining 12 days after combination but not single-agent treatment. Similar results were obtained in at least 2 separate experiments.
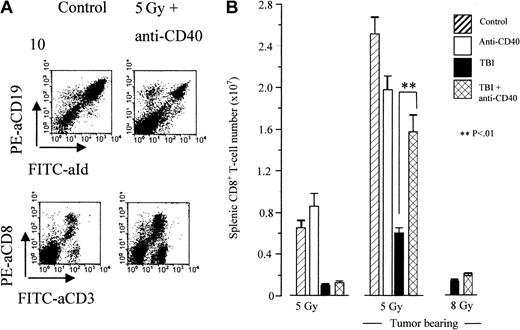
Despite similarities in tumor eradication, there is a profound difference in the number of splenic cytotoxic CD8+ T cells. Figure 6 shows the number of CD8+ cells present in the spleens of animals 5 days after treatment. Mice treated with 5 Gy TBI plus anti-CD40 show a dramatic increase in the number of CD8+ T cells with a rapid expansion that peaks from day 5 to 7, which is highly significant compared with mice given TBI alone (P < .01). Importantly, this increase coincides with the observed decrease in tumor burden and does not occur in nontumor-bearing mice receiving similar treatment, indicating that the response is dependent on tumor. Moreover, at combinations of 8 Gy irradiation this T-cell response does not occur. Neither does it occur at lower doses such as 2 Gy (data not shown). Importantly, absolute lymphocyte numbers were greatly increased in tumor-bearing animals, a phenomenon that has been observed before,10 presumably as a result of the considerably larger spleen size. Furthermore, mice treated with combinations of irradiation and mAb of other specificities, such as anti–MHC II, develop tumor at a similar rate to controls and show no expansion of CD8+ T cells (not shown), which again points directly to the response being specific to anti-CD40 mAb.
Combination therapy results in expansion of CD8 T cells in tumor-bearing animals. Tumor-bearing (106 A31 cells, intravenously, day 0) CBA mice were treated on day 10 with TBI and anti-CD40 mAb (1 mg intravenously) according to the standard protocol. Naive (nontumor-bearing) mice were treated at the equivalent time point. Animals were killed 5 days after treatment, spleens removed, and the presence of tumor and CD8 T cells determined by 2-color flow cytometry using FITC-labeled anti-Id versus PE-labeled anti-CD19 and using FITC-labeled anti-CD3 versus PE-labeled anti-CD8, respectively (A). The actual number of CD8 T cells present in the spleen following each treatment was then calculated (B). Shown is the mean for triplicate animals. Error bars represent SEM. Similar results were obtained in at least 3 separate experiments. A 10-fold expansion of CD8 T cells in tumor-bearing mice over naive animals was observed following combination therapy with 5 Gy TBI. This expansion did not occur at higher (8 Gy) or lower doses (2 Gy; data not shown).
Combination therapy results in expansion of CD8 T cells in tumor-bearing animals. Tumor-bearing (106 A31 cells, intravenously, day 0) CBA mice were treated on day 10 with TBI and anti-CD40 mAb (1 mg intravenously) according to the standard protocol. Naive (nontumor-bearing) mice were treated at the equivalent time point. Animals were killed 5 days after treatment, spleens removed, and the presence of tumor and CD8 T cells determined by 2-color flow cytometry using FITC-labeled anti-Id versus PE-labeled anti-CD19 and using FITC-labeled anti-CD3 versus PE-labeled anti-CD8, respectively (A). The actual number of CD8 T cells present in the spleen following each treatment was then calculated (B). Shown is the mean for triplicate animals. Error bars represent SEM. Similar results were obtained in at least 3 separate experiments. A 10-fold expansion of CD8 T cells in tumor-bearing mice over naive animals was observed following combination therapy with 5 Gy TBI. This expansion did not occur at higher (8 Gy) or lower doses (2 Gy; data not shown).
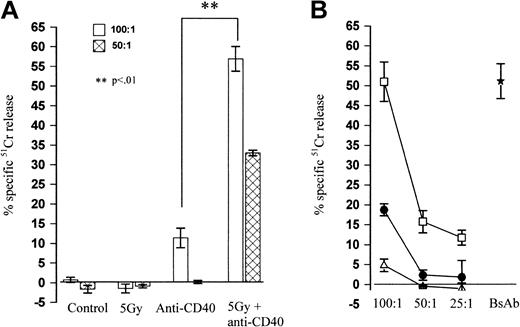
Despite the rapid expansion of CD8+ cells following treatment with 5 Gy TBI plus anti-CD40, the total number of CD8+ cells was still less than in control or anti-CD40 alone–treated groups. However, only the combined irradiation and anti-CD40 treatment groups provided long-term tumor clearance. This apparent paradox can perhaps be explained by looking at splenic CD8+ T-cell activity. Splenocytes from the combination treatment group show a highly significant (P < .01) increase in cytotoxic activity against syngeneic lymphoma cells in vitro (Figure 7A), compared with splenocytes from anti-CD40–treated, control, or irradiated mice, which show little or no activity. Cytotoxicity was specific in that splenocytes killed only syngeneic lymphoma cells and was strictly CD8+ T-cell dependent, because killing was blocked by mAb to MHC I (Figure 7B). To ensure that the A31 lymphoma was susceptible to killing, splenocytes were incubated with a bispecific monoclonal antibody (anti–MHC II × anti-CD3)25 that retargeted effector T cells through CD3 against B-cell targets through MHC II (Figure 7B, bispecific antibody [BsAb]). Together, these data indicate that the CD8+ population in the TBI plus anti-CD40–treated mice is expanding as part of a specific response against the lymphoma.
Splenocytes from combination therapy kill lymphoma cells. BALB/c mice received BCL1 lymphoma (106 intravenously, day 0) and were treated on day 15 with anti-CD40 (100 μg intravenously) and 5 Gy TBI as before. Five days later splenocytes were harvested and used as effectors against 51Cr-labeled targets. (A) Cytotoxic activity of splenocytes from control, 5 Gy–irradiated, anti-CD40, or combination therapy–treated mice against πBCL1 lymphoma cells. The effector-target (E/T) ratio used was as indicated. Only splenocytes from mice given combination therapy were able to kill lymphoma cells. (B) Cytotoxic activity of slenocytes from therapy mice against πBCL1 in the presence (•) or absence (□) of blocking anti–MHC I mAb (25 μg/mL) or against A31 lymphoma cells (▵). As a positive control bispecific (anti–MHC II × anti-CD3)25 monoclonal antibody (⋆) was included (10 μg/mL) to confirm target cell sensitivity (E/T 100:1; A31 targets). The E/T ratio was as shown. For both figures each point is the mean and SD of triplicate samples. Shown is 1 of 2 separate experiments.
Splenocytes from combination therapy kill lymphoma cells. BALB/c mice received BCL1 lymphoma (106 intravenously, day 0) and were treated on day 15 with anti-CD40 (100 μg intravenously) and 5 Gy TBI as before. Five days later splenocytes were harvested and used as effectors against 51Cr-labeled targets. (A) Cytotoxic activity of splenocytes from control, 5 Gy–irradiated, anti-CD40, or combination therapy–treated mice against πBCL1 lymphoma cells. The effector-target (E/T) ratio used was as indicated. Only splenocytes from mice given combination therapy were able to kill lymphoma cells. (B) Cytotoxic activity of slenocytes from therapy mice against πBCL1 in the presence (•) or absence (□) of blocking anti–MHC I mAb (25 μg/mL) or against A31 lymphoma cells (▵). As a positive control bispecific (anti–MHC II × anti-CD3)25 monoclonal antibody (⋆) was included (10 μg/mL) to confirm target cell sensitivity (E/T 100:1; A31 targets). The E/T ratio was as shown. For both figures each point is the mean and SD of triplicate samples. Shown is 1 of 2 separate experiments.
Combination anti-CD40 and TBI leads to CD8+ T-cell protective immunity
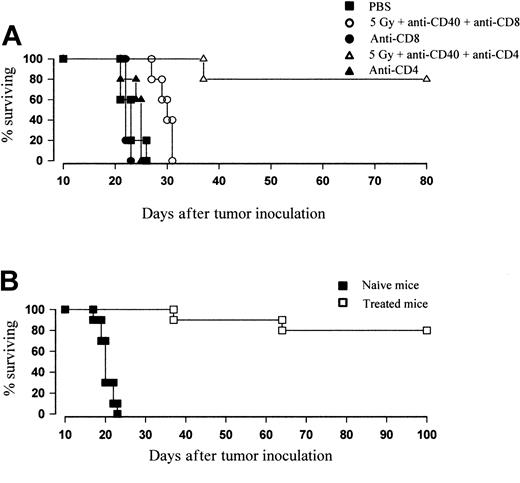
To confirm the importance of CD8+ T cells to in vivo protection, therapy was conducted in the presence of T-cell–depleting mAb. Mice received anti-CD8 mAb (YTS169, 0.5 mg/d) or anti-CD4 mAb (YTA1.3.2, 1 mg/d) 1 day prior to treatment and subsequently every 3 days to a total of 4 inoculations. Figure 8A shows that the therapeutic efficacy of TBI and anti-CD40 is lost in mice depleted of CD8+ but not CD4+ T cells. These data strongly suggest that the combination therapy is able to eradicate lymphoma through the generation of a CD8+ T-cell response. Furthermore, mice previously inoculated with lymphoma that had become long-term disease-free survivors with combination TBI and anti-CD40 were reinoculated with fresh tumor (106 cells intravenously). Whereas control animals developed tumor as usual, 80% of previously treated mice were able to reject tumor and remained disease free for over another 100 days (Figure 8B). The rechallenge experiments suggest that the response generated by anti-CD40 and irradiation is able to provide antitumor immunity for the long-term survivors.
Depletion of CD8 T cells by mAb completely abrogates the therapeutic effect of combination therapy. (A) Groups of 5 age-matched BALB/c mice were inoculated with 106 BCL1 tumor cells intravenously on day 0 and were treated with 5 Gy TBI and anti-CD40 mAb (100 μg intravenously, day 15) as before. To deplete CD8+ or CD4+ T cells, mice received anti-CD8 (YTS169; 0.5 mg) or anti-CD4 (YTA1.3.2; 1 mg) mAb, respectively, intraperitoneally 1 day prior to treatment and again 2, 5, and 8 days after treatment. Treatment was as indicated. Survival was recorded daily. Depletion of CD4 T cells did not affect the degree of protection over controls, with 80% remaining disease free over day 80. Depletion of CD8 T cells caused the therapeutic effect to be completely abrogated. Similar results were obtained in at least 3 separate experiments. (B) Naive or 5 Gy plus anti-CD40–treated mice (day 35 to 40 after treatment) were injected with 106 fresh BCL1 cells intravenously. While naive animals succumbed to disease, previously “cured” animals were resistant to lymphoma challenge and remained disease free (more than 100 days).
Depletion of CD8 T cells by mAb completely abrogates the therapeutic effect of combination therapy. (A) Groups of 5 age-matched BALB/c mice were inoculated with 106 BCL1 tumor cells intravenously on day 0 and were treated with 5 Gy TBI and anti-CD40 mAb (100 μg intravenously, day 15) as before. To deplete CD8+ or CD4+ T cells, mice received anti-CD8 (YTS169; 0.5 mg) or anti-CD4 (YTA1.3.2; 1 mg) mAb, respectively, intraperitoneally 1 day prior to treatment and again 2, 5, and 8 days after treatment. Treatment was as indicated. Survival was recorded daily. Depletion of CD4 T cells did not affect the degree of protection over controls, with 80% remaining disease free over day 80. Depletion of CD8 T cells caused the therapeutic effect to be completely abrogated. Similar results were obtained in at least 3 separate experiments. (B) Naive or 5 Gy plus anti-CD40–treated mice (day 35 to 40 after treatment) were injected with 106 fresh BCL1 cells intravenously. While naive animals succumbed to disease, previously “cured” animals were resistant to lymphoma challenge and remained disease free (more than 100 days).
Protection can be conferred to naive recipients following adoptive transfer of T cells
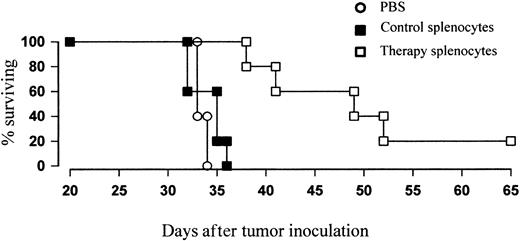
Finally, to confirm that the combination therapy results in protective immunity, we performed adoptive transfer studies. Splenocytes were therefore harvested from treated mice just prior to the peak of the primary response and were transferred in to naive recipients together with fresh lymphoma cells at an effector-target ratio of 100:1. While cohorts receiving tumor plus splenocytes from naive, untreated controls had a median survival time of around 35 days (Figure 9), similar to that of animals given PBS, those given tumor plus splenocytes from treated animals showed a significant increase in protection (P < .01), with a median survival of around 50 days. Thus, it appears that adoptive transfer of T cells from mice given combination therapy is able to delay the rate of lymphoma growth in naive recipients.
Adoptive transfer of T cells from treated mice can protect naive recipients against lymphoma growth. BALB/c mice were inoculated with tumor and treated with 5 Gy TBI and anti-CD40 mAb (100 μg intravenously) as before. Four days after treatment just prior to the peak of the primary immune response, splenocytes were harvested, CD19+ cells depleted by MACS, and remaining T cells coinjected into naive recipients together with fresh lymphoma cells at an E/T ratio of 100:1 (CD8+ cells/T). Controls received either PBS or tumor plus CD19-depleted splenocytes from untreated mice. Survival was recorded daily. Mice that received adoptively transferred “peak” T cells showed a significant enhancement in protection over controls (P < .01).
Adoptive transfer of T cells from treated mice can protect naive recipients against lymphoma growth. BALB/c mice were inoculated with tumor and treated with 5 Gy TBI and anti-CD40 mAb (100 μg intravenously) as before. Four days after treatment just prior to the peak of the primary immune response, splenocytes were harvested, CD19+ cells depleted by MACS, and remaining T cells coinjected into naive recipients together with fresh lymphoma cells at an E/T ratio of 100:1 (CD8+ cells/T). Controls received either PBS or tumor plus CD19-depleted splenocytes from untreated mice. Survival was recorded daily. Mice that received adoptively transferred “peak” T cells showed a significant enhancement in protection over controls (P < .01).
Discussion
Integrating mAb and irradiation is emerging as an important new approach for lymphoma therapy.7,8 We have demonstrated for the first time in 2 different tumor model systems that irradiation and anti-CD40 can work in concert to eradicate lymphoma and produce long-term survival when anti-CD40 treatment or irradiation alone is ineffective in extending survival. The combination of anti-CD40 and irradiation is able to generate a T-cell immunity that provides long-term protection consistently in more than 80% of treated animals. The tumor protection seen is critically dependent on the radiation dose. The CD8+ T-cell response is essential to provide long-term tumor protection, and as the radiation dose increases, the therapeutic efficacy of the combination of anti-CD40 and irradiation decreases secondary to the immunosuppressive effects of irradiation on the CD8+ T-cell response.
These data suggest that TBI, which is primarily thought to be immunosuppressant, when used at a modest level in combination with anti-CD40 mAb can eradicate tumor but still allow stimulation of host immunity. TBI is known to lead to chronic lymphocytopenia, and the immunosuppressive effects are used as part of the conditioning regimens prior to bone marrow transplantation.27 We have observed similar lymphocytopenia in our model system, with a dose-dependent reduction of lymphocyte numbers. CD8+ T cells appear to be among the most sensitive lymphocyte populations, and after 5 Gy TBI the CD8+ population is severely reduced (up to 75% compared with controls) for more than 50 days after irradiation (data not shown). Despite this depletion, it is still possible to generate a potent CD8+ T-cell response following the same dose of irradiation in the presence of anti-CD40 mAb and tumor.
In this study we have shown that radiation-induced tumor apoptosis alone is inadequate in generating long-term tumor clearance in animals and that an anti-CD40–driven CD8+ T-cell activation is required to achieve long-term survival. Treatment with TBI alone, even at a higher single dose of 8 Gy, was unable to enhance survival by more than a few days, and even when supralethal doses of irradiation are followed by bone marrow transplantation all animals developed tumor after immune reconstitution (data not shown). In addition, the lack of T-cell expansion we observed in vivo after higher doses of TBI plus anti-CD40 appears to account for the decrease in therapeutic efficacy.
Anti-CD40 has been shown to protect mice against tumor in a range of lymphoma models.10,11 However, tumor protection was found to be dependent not only on large tumor burdens but also on large doses of anti-CD40 mAb, indicating the necessity of sufficient tumor antigen for the successful eradication of tumor. Delivering large doses of anti-CD40 mAb to patients may potentially induce autoimmune disorders.28,29 We have shown that it is possible to use much smaller doses of anti-CD40 mAb with irradiation to produce long-term tumor protection. Lower doses of mAb may be sufficient to activate CD8+ cells against tumor antigen liberated by irradiation-induced apoptosis but may be insufficient to induce autoimmunity triggered, for example, by breaking tolerance to self-antigen.
We have made a number of important observations that all indicate that the tumor eradication seen here is not due to the direct additive cytotoxic effects of irradiation and anti-CD40 on tumor cells but is instead due to the generation of an active immune response. This contrasts to previously published work from our laboratory and others that has demonstrated a significant increase in the tumor cell kill with mAb such as anti-idiotype (anti-Id)7 and anti-CD2030 when combined with irradiation. This additive effect is believed to be related to the combined cytotoxic effect of signaling mAb and irradiation. Although others have reported that anti-CD40 signaling can induce apoptosis of both normal31 and malignant B cells,32 we did not see such an effect in our lymphoma models even when using secondary cross-linking mAb (data not shown). While there is a clear radiation dose–dependent increase in apoptosis of the πBCL1 lymphoma line in vitro that is enhanced in the presence of mAb to the B-cell receptor such as anti-μ and anti-Id,7 there is no additive effect between radiation and anti-CD40 mAb. Neither does culturing lymphoma in the presence of anti-CD40 mAb affect clonogenic survival. This suggests that ligation of CD40 is not sensitizing the tumor to radiation-induced cell death (or vice versa). Similar results were obtained using a second in vitro cell line BCL1-3B3 (data not shown).
In addition, as the number of tumor cells inoculated was increased, a threshold response was seen and, above 104 tumor cells, long-term disease-free survival was achieved in most animals after irradiation with anti-CD40 mAb. This argues against irradiation simply acting as a means of tumor debulking, allowing the stimulation of APCs after anti-CD40 mAb to clear residual disease. We believe instead that irradiation-induced apoptosis liberates a critical tumor antigen load that works in concert with anti-CD40–activated APCs to provide long-term tumor protection.
Stimulation of APCs via CD40 is critical for their activation and also to the effector function of CD8+ T cells, bypassing cognate T-cell help.13,33,34 Irradiation is well known to induce large amounts of apoptosis in lymphoma cells,35 and this type of cell death is conducive to antigen release. Moreover, evidence is emerging that suggests exogenous antigen can be taken up from dying cells by APCs and presented to CD8+ T cells through the process of “cross-priming.”36 Cross-priming appears to be most efficient when antigen is acquired from apoptotic cells by professional APCs, such as dendritic cells.37 Thus, the balance of irradiation-induced tumor antigen release versus immunosuppression may determine the degree of tumor protection. With lower doses of irradiation, the level of tumor cell death or antigen release may be insufficient to supply enough antigen to prime the immune response, despite activation of APCs by anti-CD40 mAb. Above a certain threshold, increasing doses of irradiation may generate larger amounts of apoptosis but impair the host immune response. This effect at higher doses of irradiation could either be secondary to a direct impairment of T-cell survival and function or by switching DCs from being immunogenic to tolerogenic.38 At the optimal radiation dose it is clear that a protective T-cell response is being generated. Splenocytes harvested from combination therapy–treated animals show tumor-specific activity in vitro that can be blocked by mAb to MHC I, indicating that activity is CD8+ T-cell mediated. Splenocytes from control animals or those given either irradiation or anti-CD40 mAb alone showed little or no activity. The fact that a substantial expansion of tumor-reactive CD8+ T cells is observed only in lymphoma-bearing animals given combination therapy further indicates that the tumor itself is important in T-cell proliferation. It was further confirmed that these T cells are responsible for protection by in vivo depletion of CD8 T cells with mAb. Here, the response was completely abrogated, consistent with observations in the immunotherapy model.10 It is possible that other cytotoxic cellular populations such as natural killer (NK) cells may also be depleted by such an approach. However, it has previously been shown that NK cells do not play an essential part in anti-CD40 immunotherapy,11 and while it seems unlikely that they have a role here, this could be addressed by performing combination therapy in NK-depleted mice, for example, using anti-ASGM1 antiserum.
Further evidence supporting CD8+ T cells to be the main immune effector mechanism is provided by adoptive transfer experiments. Here, T cells taken just prior to the peak of the primary therapeutic response are able to delay tumor growth and enhance survival of naive recipients. While no long-term protection was observed, this is most likely due to the fact that the T cells are taken directly ex vivo and are not restimulated in vitro. Successful eradication of tumor has been shown to be highly dependent on the dose and timing of transfer, and it is possible that coadministration of interleukin-2 (IL-2) or other adjuvant may improve efficacy by altering the kinetics of the response.39,40
In conclusion, we believe that long-term tumor protection is dependent on the balance between radiation-induced tumor apoptosis and anti-CD40 activation of APCs to cross-present tumor antigen to CD8+ T cells. Our data suggest that combining anti-CD40 mAb and radiation therapy may be a promising approach in the clinic for treatment of B-cell lymphomas. Further work is required to increase our understanding of the other stimuli that may act in concert with the APC stimulatory pathway in the host immune response to tumor rejection and the importance of recognition and trafficking of tumor antigen in this response.
Prepublished online as Blood First Edition Paper, April 24, 2003; DOI 10.1182/blood-2002-12-3717.
Supported by Cancer Research UK.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank all the members of the Tenovus Research Laboratory who provided expert technical support. In particular, we thank Sam Martin, Maureen Power, and Dr Ruth French for invaluable technical assistance and thank colleagues in the Cancer Sciences division for helpful comments about the manuscript.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal