Abstract
Factor V (FV) deficiency, also known as parahemophilia, is a rare bleeding disorder. Herein we investigate the first reported missense mutation associated with FV deficiency, Ala221Val, assigned as FV New Brunswick. To elucidate the molecular pathology associated with the Ala221Val substitution, the mutation was recreated in a recombinant system together with 3 FV mutants (Ala221Gly, Glu275Gln, and Cys220Ala/Cys301Ala) designed to help explain the Ala221Val phenotype. The expression pattern was analyzed by pulse-chase experiments and an FV-specific enzyme-linked immunosorbent assay (ELISA), the results suggesting the Ala221Val mutation not to interfere with the synthesis or secretion. The functional properties of the recombinant FV New Brunswick were evaluated in both plasma clotting and purified systems. The Ala221Val mutation did not affect the factor Xa (FXa) cofactor function; nor did it interfere with the activated protein C (APC)–mediated down-regulation of activated FV (FVa) activity. However, FV New Brunswick demonstrated reduced stability at 37°C due to an increased rate of dissociation of light and heavy chains of FVa. In conclusion, this in vitro study of FV New Brunswick suggests the Ala221Val mutation not to impair synthesis and expression of procoagulant activity, indicating overall proper folding of the mutant molecule. Rather, the Ala221Val substitution appears to interfere with the stability of the activated FVa mutant, the reduced stability possibly explaining the deficiency symptoms associated with the mutation.
Introduction
At sites of vascular injury, rapid generation of thrombin is crucial for the formation of the fibrin clot. Factor V (FV), a protein that is essential for thrombin generation, circulates in plasma as a high molecular weight (330 kDa) single-chain precursor. It is structurally and functionally homologous to factor VIII (FVIII), the 2 proteins sharing the domain structure A1-A2-B-A3-C1-C2.1 Either thrombin or factor Xa (FXa) activates FV. Activation is associated with removal of the B domain from the remaining part of FV that constitutes activated FV (FVa). FVa is composed of a 105 kDa heavy chain (A1-A2) and a 71/74 kDa light chain (A3-C1-C2), the 2 chains being held together by noncovalent calcium-dependent bonds.2-6 FVa is a cofactor to the serine protease FXa, which, in the presence of calcium ions and an appropriate phospholipid (PL) membrane surface, enhances the activation of prothrombin to thrombin by several orders of magnitude.7-9 The FXa-FVa-PL complex is referred to as the prothrombinase complex. Activated FVIII (FVIIIa) has a homologous function to FVa, serving as a cofactor in the tenase complex to the enzyme factor IXa (FIXa) in the activation of factor X (FX).9
Deficiency of FV, called parahemophilia, was first discovered by Owren in 1947.10 FV deficiency follows an autosomal recessive inheritance pattern. Unlike FVIII deficiency (hemophilia A), it is a rare bleeding disorder, with an estimated frequency of 1 in 1 million. The phenotypic expression of parahemophilia varies— heterozygotes often being asymptomatic, while homozygotes show mild, moderate, or severe bleeding symptoms.11,12 FVIII deficiencies, being more common with an occurrence of 1 in 10 000, have been an object for careful studies,13 whereas the less frequent FV deficiencies have not been extensively studied. The elucidation of the molecular bases of FV deficiency has been hampered for a long time by the large size of the FV gene (around 100 kilobase [kb]) and by the low prevalence of the disorder.
To date, around 20 mutations associated with FV deficiency have been reported.11,14 Different types of gene defects are known to result in the absence of functional FV such as nonsense and frameshift mutations causing nonfunctional mRNA, consensus changes affecting RNA processing, and missense mutations. There are only 5 missense mutations reported to be associated with FV deficiency, and the pathology of most of them are not characterized at the molecular level.11,15 On the other hand, multiple natural mutations causing quantitative and qualitative deficiencies of the homologous FVIII have been characterized at the protein level.13 Studies of these mutations have been helpful for the understanding of structural features essential for the function and regulation of FVIII. Identifying the basis of structural pathology of FV will therefore help to obtain insight into the relationship between the sequence, structure, and function of the FV molecule.
In 1994, the first missense mutation associated with FV deficiency was reported, designated as FV New Brunswick.16 The mutation involves an Ala221Val substitution in the A1 domain. Plasma from the patient with the Ala221Val substitution was reported to have reduced FV antigen and activity levels. The authors further suggested that the mutation could interfere with binding of FXa to FVa. The binding site for FXa on FVa appears to be similar to the one of FIXa on FVIIIa,17 and the region of FVa Ala221 seems to be located outside the binding site for FXa, and the homologous region on FVIIIa also does not seem involved in FIXa binding. Yet, a putative binding site for FXa on FVa at position 221 could confer specificity to this latter interaction and possibly underline a new binding surface on FVa. The aim of the present study was to elucidate the potential roles of the Ala221Val FV mutation. Therefore, the Ala221Val variant was created together with other FV variants that could help explain the phenotype of the disorder. These constructs were designed after structural analysis of a theoretical model for the 3 A domains of FVa. The recombinant proteins were expressed in a eukaryotic system,18 and the secretion pattern and the function of the mutant molecules were then evaluated. Observed effects of the Ala221Val mutation were then investigated in the context of a 3-dimensional (3D) model of FV.
Materials and methods
Materials
Bln1 was purchased from Boehringer Mannheim (Mannheim, Germany) and Bsu36I from New England BioLabs (Beverly, MA). Pwo polymerase and T4 DNA ligase were purchased from Boehringer Mannheim. Double-stranded DNA sequencing kit was from Perkin Elmer (Shelton, CT). Lipofectamine 2000 reagent was purchased from Life Technologies (Bethesda, MD). Cell culture media (Optimem Glutamax) were from Invitrogen (Carlsbad, CA). Benzamidine was purchased from Acros Organics (Geel, Belgium). Phe-Pro-Arg-chloromethylketone (PPACK) was obtained from Calbiochem (San Diego, CA). Monoclonal HV-1 reacting with the C2 domain of FV was from Sigma (St Louis, MO). A polyclonal antibody (A299) against FV was from Dako (Glostrup, Denmark). Polyclonal antihuman FV antibody (8806) was raised by our group and showed epitopes to both the heavy and light chains as well as to the B domain. Egg extracts (more than 99% pure) of phosphatidylcholine (PC) and phosphatidylethanolamine (PE) and brain extracts (more than 99% pure) of phosphatidylserine (PS) were purchased from Avanti Polar Lipids (Alabaster, AL). The chromogenic substrate d-Phe-(pipelocyl)-Arg-pNA (S-2238) was a kind gift from Chromogenix (Milan, Italy).
Buffers
Cell lysis buffer was 10 mM Tris (tris(hydroxymethyl)aminomethane) (pH 7.5), 150 mM NaCl, 2 mM EDTA (ethylenediaminetetraacetic acid), 1% Nonidet P-40, 0.1% sodium dodecyl sulfate (SDS), 2 μM PPACK, 10 mM benzamidine, 50 μg/mL soy bean trypsin inhibitor. Immunoprecipitate washing buffer was 10 mM Tris (pH 7.5), 150 mM NaCl, 2 mM EDTA, 0.5% Nonidet P-40 (NP-40), 0.1% SDS. The buffer used during the activation of FV (HNBSACa) was 25 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), 150 mM NaCl, 5 mM CaCl2 (pH 7.5) containing 0.5% bovine serum albumin (BSA). The prothrombinase assay buffer (HNO) was 25 mM HEPES, 150 mM NaCl, 2 mM CaCl2 (pH 7.5) containing 0.05% ovalbumin. The EDTA buffer was 50 mM HEPES, 100 mM NaCl, 1% polyethylene glycol (PEG), and 20 mM EDTA (pH 7.5).
Proteins
Human α-thrombin was from Haematologic Technologies (Essex Junction, VT). Human FXa, human protein S, and human prothrombin were obtained from Kordia (Leiden, the Netherlands). Recombinant human activated protein C (APC) was prepared as described.19 BSA, ovalbumin, and soy bean trypsin inhibitor were purchased from Sigma.
Phospholipid vesicle preparation
The phospholipid vesicles were prepared as described.20
Site-directed mutagenesis
Mutations were introduced into the expression vector pMT2 containing the full-length cDNA of human FV using the QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA). For each mutant, 2 complementary oligonucleotides containing the appropriate mutations were used. The mutations were then isolated by restriction enzymes and used to replace corresponding fragments in the template. For each mutant the sense primers used for mutagenesis and the restriction enzymes used for digestion are shown in Table 1. The sequences of the fragments were confirmed by DNA sequencing.
Sense primers used for mutagenesis and restriction enzymes employed for isolating the mutations and replacing the corresponding fragment of the template
Mutant . | Primer . |
|---|---|
| Ala221Val | 5′-ACA GTT TGT GTC CAT GAC CAC ATC AGCG-3′ |
| Ala221Gly | 5′-ACA GTT TGT GGC CAT GAC CAC ATC AGC-3′ |
| Glu275Gln | 5′-ACT GTG GGC CCA CAG GGA AAG TGG-3′ |
| Cys220Ala | 5′-GAT ATA ACA GTT GCT GCC CAT GAC CAC-3′ |
| Cys301Ala | 5′-CAT TGA CAT TAA AAA CGC CCC AAA GAA AAC CAG G-3′ |
Mutant . | Primer . |
|---|---|
| Ala221Val | 5′-ACA GTT TGT GTC CAT GAC CAC ATC AGCG-3′ |
| Ala221Gly | 5′-ACA GTT TGT GGC CAT GAC CAC ATC AGC-3′ |
| Glu275Gln | 5′-ACT GTG GGC CCA CAG GGA AAG TGG-3′ |
| Cys220Ala | 5′-GAT ATA ACA GTT GCT GCC CAT GAC CAC-3′ |
| Cys301Ala | 5′-CAT TGA CAT TAA AAA CGC CCC AAA GAA AAC CAG G-3′ |
Changes introduced are underlined and in bold; antisense counterpart primers are not shown. The size of each cleaved fragment that was isolated and ligated into a template lacking the corresponding fragment is shown in parentheses. Each mutant was cleaved by the enzymes Bln1 and Bsu361, yielding a fragment of 2 kb.
Expression of recombinant FV (rFV)
Expression plasmids containing the various FV cDNA constructs were transfected into COS 1 cells using both the diethylaminoethyl (DEAE)–dextran method as described18 and the Lipofectamine 2000 (Life Technologies) method as described by the manufacturer. Proteins were collected 72 hours after transfection in serum-free media (Optimem Glutamax). Pulse-labeling experiment was performed 24 hours after transfection by radioactive labeling with [35S]methionine and [35S]cysteine, as previously described with some modifications.21 In brief, cell extracts were prepared by lysis in a cell lysis buffer supplemented with inhibitors. Radioactive FV in the cell lysates and media were precleared with protein A–Sepharose (Pharmacia, Uppsala, Sweden) for 2 hours at 4°C and immunoprecipitated overnight at 4°C using a polyclonal anti-FV antibody, 8806 (20 μg). The immune complexes were precipitated and washed with immunoprecipitate washing buffer and eluted by boiling. Proteins were separated by electrophoresis on a 7.5% SDS–polyacrylamide gel electrophoresis (SDS-PAGE).22 The gels were then exposed in a cassette and finally scanned using a Phosphorimager (Molecular Dynamics, Eugene, OR).
Quantification of rFV
The media were concentrated approximately 40-fold using Vivaspin 100 000 MWCO (Vivascience, Hannover, Germany) on the day of harvest and the FV concentrations quantified using both prothrombinase assay and enzyme-linked immunosorbent assay (ELISA), as described.23,24 To rule out that the cell culture medium interfered with the ELISA and prothrombinase assay, plasma-purified FV was diluted in mock medium or in buffer and measured in the 2 assay systems. No differences were detectable, indicating that the medium did not interfere with the assays.
Prothrombinase-based FVa assay
FVa cofactor activity was measured by determining the rate of FXa-catalyzed thrombin generation. Typically, if not specifically noted, the conditions for the prothrombinase assay were 50 μM small unilamellar phospholipid vesicles (PS/PC, 10:90, mol/mol), FXa (5 nM), and prothrombin (0.5 μM). Prothrombin, FXa, and phospholipid vesicles were preincubated at 37°C, and the thrombin generation was started by addition of preheated FVa. The reactions were stopped after 1 minute by 1:40 dilution in ice-cold EDTA buffer. The generated thrombin was quantified using the chromogenic substrate S-2238.
Factor V clotting assay
The assay, which is based on the prothrombin time, has been reported before.25 Shortly, in the 1-stage clotting assay, the recombinants were added to FV-depleted plasma, and coagulation was triggered by addition of calcium-containing rabbit brain thromboplastin reagent, Simplastin Excel (Biomerieux, Marcy l'Etoile, France). A 2-stage clotting assay was used to assess the activity of the recombinant FV (rFV) variants after thrombin activation, as described.26 The FV activity was calculated from standard curves based on human pooled plasma, assuming the plasma FV concentration in plasma to be 1 U/mL.
Determination of apparent Kd of FXa for FVa using the prothrombinase assay
The prothrombinase assay was used to determine the apparent dissociation constant (Kd) for the binding of FXa to the thrombin-activated FV variants, essentially as described before.20 Shortly, FVa was activated by thrombin and diluted to 50 pM and preincubated for 4 minutes with increasing concentrations of FXa (0.1 to 50 000 pM) and phospolipid vesicles (50 μM 10:90 PS/PC). Thrombin generation was started by the addition of 0.5 μM preheated prothrombin and allowed to proceed for 1 minute before being stopped by dilution with ice-cold EDTA buffer.
Determination of Km for prothrombin activation by FXa using the prothrombinase assay
The Michaelis-Menten constant (Km) for prothrombin activation by the different prothrombinase complexes was determined by varying the prothrombin concentrations. FVa (100 pM) was preincubated for 4 minutes with increasing concentrations of prothrombin (1 to 2500 nM) and 25 μM phospholipipid vesicles (5:95, PS/PC). Thrombin generation was started by addition of preheated FXa (5 nM final concentration) and allowed to proceed for 1 minute.
Inactivation of FVa by activated protein C
To study the inactivation of FVa, FV was activated with thrombin (0.5 U/mL) for 10 minutes at 37°C in HNBSACa buffer. After activation of FV (0.8 nM final FVa concentration in the experiment), phospholipid vesicles (PS/PE/PC, 10:20:70, wt/wt/wt, at final concentration of 25 μM) and protein S (when included) at a final concentration of 100 nM were added. A subsample was drawn from the mixture and diluted 1:5 in ice-cold HNBSACa buffer. APC was subsequently added to a final concentration of either 0.05 nM or 0.1 nM. At different time points, samples were drawn from the inactivation mixture and diluted 1:5 in ice-cold HNBSACa to stop the reaction. The FVa activities in the diluted samples were then measured in the prothrombinase assay for remaining FVa activity.
Western blot analysis of recombinant FV before and after thrombin activation
The recombinant FV variants were incubated with 2 U/mL thrombin for 10 minutes at 37°C and subjected to 6% SDS–polyacrylamide gel electrophoresis (SDS-PAGE) under reducing conditions.22 The proteins were transferred to polyvinylidene fluoride (PVDF) membranes and detected using a polyclonal antibody against FV (A299). To develop the Western blots, the Vectastain Elite ABC kit (Vector Laboratories, Burlington, ON, Canada) was used according to the manufacturer's instructions.
Determination of the stability of the FV variants
The recombinant FV variants (0.5 nM) were incubated at 37°C both before and after thrombin activation (0.5 U/mL, for 10 minutes.). At intervals, aliquots were drawn and the remaining FVa activity determined in the prothrombinase assay. The FV aliquots that were drawn from the nonactivated incubation mixture were activated with thrombin (0.5 U/mL, for 10 minutes at 37°C) prior to the determination of residual FVa activity. The stability of the heavy-light chain complexes of the activated FV variants was analyzed using a combination of chain-specific immunoprecipitation and Western blotting. In this analysis, the aliquots (100 μL) from the stability test were first incubated with a biotinylated monoclonal antibody against FV (HV-1) (10 μL, 30 μg/mL) for 30 minutes at 4°C. The immune complexes were collected using streptavidin-coated magnetic beads (Dynabeads, Dynal, Oslo, Norway). After wash in HNBSACa buffer, FVa was eluted by denaturation using SDS-PAGE sample preparation buffer. The samples were then reduced and the supernatant subjected to 10% SDS-PAGE. After transfer of the separated proteins to polyvinylidene fluoride (PVDF) membrane, the blot was detected with a monoclonal antibody against the heavy chain of FVa (HV-5146) and developed using the Vectastain Elite ABC kit.
Results
Mutagenesis strategy
The FV cDNA corresponding to the New Brunswick variant (Ala221Val) was constructed together with 3 other FV variants, Ala221Gly, Glu275Gln, and Cys220Ala/Cys301Ala. Based on the computer model of the A domains of FVa27 and due to the moderate resolution in this region of the structure, the Ala221Val mutation has been suggested to induce different reactions. It could either disturb a putative partially buried salt bridge between Lys304 and Glu275 or interfere with the nearby disulfide bridge located at Cys220-Cys301.27 We therefore created an FV variant lacking the disulfide bridge (Cys220Ala/Cys301Ala) and another FV variant with the putative salt bridge removed (Glu275Gln). Analysis of the FV 3D model suggested that the Ala221Val mutation could be tolerated, because sufficient room was noticed in this area of the molecule. However, exact side chain positioning in the 3D structure is not trivial, and we decided to also replace Ala221 by a glycine to see if substitution with a less bulky amino acid than valine could lead to the generation of a normal FV mutant molecule.
Expression and characterization of the recombinant FV variants
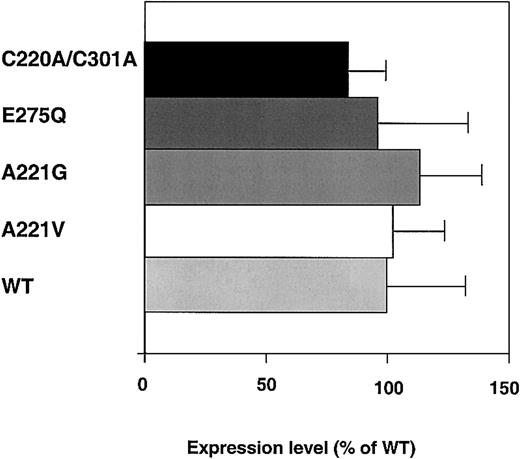
The expression levels, as determined by ELISA, were around 200 ng/mL for all of the FV variants (Figure 1). To determine if the Ala221Val mutation was secreted less efficiently and accumulated intracellularly, its secretion pattern was analyzed by pulse-chase. The pulse-chase experiments indicated that the expression efficiency of the Ala221Val mutation was similar to that of wild-type (WT) FV (Figure 2). The secretion patterns of the other 3 FV variants were also investigated by pulse-chase but did not differ from that of WT FV (data not shown). Consequently, the data from the ELISA measurements and the pulse-chase experiment suggested the New Brunswick mutation not to interfere with the expression. Also, in general, such observations suggest proper folding of the molecules.
Expression levels of the recombinant FV variants. COS 1 cells expressing the WT, Ala221Val, Ala221Gly, Glu275Gln, and Cys220Ala/Cys301Ala FV variants were transiently transfected, and the expression levels of FV in the culture media after 72 hours were measured by ELISA. The histograms and bars represent the means ± SEMs (n = 6). The mean value of WT FV was 200 ng/μL and is assigned as 100% in the figure.
Expression levels of the recombinant FV variants. COS 1 cells expressing the WT, Ala221Val, Ala221Gly, Glu275Gln, and Cys220Ala/Cys301Ala FV variants were transiently transfected, and the expression levels of FV in the culture media after 72 hours were measured by ELISA. The histograms and bars represent the means ± SEMs (n = 6). The mean value of WT FV was 200 ng/μL and is assigned as 100% in the figure.
SDS-PAGE analysis of the expression of WT FV and the Ala221Val FV variant. COS 1 cells expressing WT FV or Ala221Val:FV were depleted for Cys/Met and then radiolabeled with [35S]Met/Cys for 30 minutes. After increasing time the radiolabeled FV in cell lysates and media were immunoprecipitated and electrophoresed on 7.5% SDS–polyacrylamide gels under reducing conditions (A). The radioactivity of the FV bands was analyzed with a Phosphorimager. To quantify the secretion profiles of WT (▪) and Ala221Val (□), the radioactivity of the FV-specific bands from cell lysate (B) and medium (C) was measured. The amount of radioactive FV in the cell lysate at 0 hours was assigned to be 100% for each FV variant. Each data point represents the mean of 3 independent experiments. Error bars represent ± SDs.
SDS-PAGE analysis of the expression of WT FV and the Ala221Val FV variant. COS 1 cells expressing WT FV or Ala221Val:FV were depleted for Cys/Met and then radiolabeled with [35S]Met/Cys for 30 minutes. After increasing time the radiolabeled FV in cell lysates and media were immunoprecipitated and electrophoresed on 7.5% SDS–polyacrylamide gels under reducing conditions (A). The radioactivity of the FV bands was analyzed with a Phosphorimager. To quantify the secretion profiles of WT (▪) and Ala221Val (□), the radioactivity of the FV-specific bands from cell lysate (B) and medium (C) was measured. The amount of radioactive FV in the cell lysate at 0 hours was assigned to be 100% for each FV variant. Each data point represents the mean of 3 independent experiments. Error bars represent ± SDs.
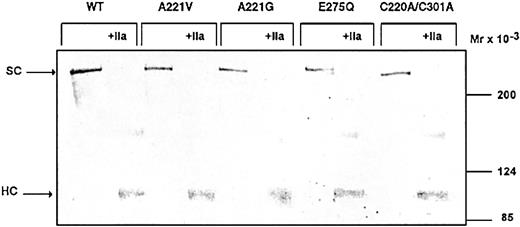
To further characterize the recombinant FV variants, they were analyzed by Western blotting before and after activation by thrombin (Figure 3). All of the FV variants contained a 330 kDa band, which corresponds to the intact form of FV. When the FV variants were activated by thrombin, the single-chain FV disappeared and a band of approximately 105 kDa appeared, corresponding to the heavy chain of FVa.
Characterization of the FV variants by Western blot. The recombinant FV variants (1 μg/mL) in conditioned medium were analyzed both before and after 30 minutes of incubation with 2 U/mL thrombin (37°C) on Western blots (6% SDS-PAGE under reducing conditions). FV was detected using a polyclonal antibody (A299). Vectastain Elite ABC kit was used to develop the Western blots.
Characterization of the FV variants by Western blot. The recombinant FV variants (1 μg/mL) in conditioned medium were analyzed both before and after 30 minutes of incubation with 2 U/mL thrombin (37°C) on Western blots (6% SDS-PAGE under reducing conditions). FV was detected using a polyclonal antibody (A299). Vectastain Elite ABC kit was used to develop the Western blots.
Assessment of the activity of recombinant FV New Brunswick
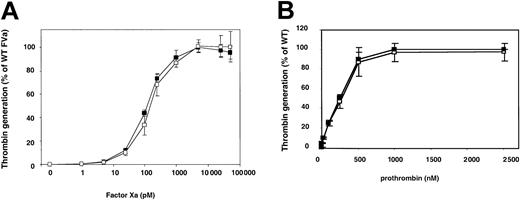
FV specific activity was measured by a clotting-based assay, based on prothrombin time (Table 2). The activity estimated for WT FV was around 300 U/mg, which is comparable to levels of activity previously reported.28,29 The activity of the Ala221Val mutant was similar to that of WT, both before and after thrombin activation. To further elucidate if the Ala221Val mutation was associated with reduced FXa cofactor activity, the ability of the thrombin-activated Ala221Val mutant to support prothrombin activation was tested at increasing concentrations of FXa (Figure 4). The Ala221Val mutant appeared to interact with FXa equally as well as WT FVa, suggesting that the mutation did not interfere with the FXa affinity. Both WT and Ala221Val had an apparent Kd of around 200 pM, which is in agreement with results on record for WT FVa.30-32 The rate of thrombin generation at increasing concentrations of prothrombin was investigated to determine the Km, thus estimating the interaction between prothrombin and the Ala221Val mutant (Figure 4B). To ensure that the prothrombin interacted specifically with the FVa-FXa complex rather than with the membrane surface, suboptimal phospholipid vesicles (5:95 PS/PC, final concentration 25 μM) and high concentrations of FVa (100 pM) were used. Control experiments indicated that prothrombin interacted specifically with the FVa-FXa complex.17 Both the WT FVa and the Ala221Val mutant exhibited similar catalytic constant (kcat) values and Km for prothrombin (Table 3).
Activity values determined by both 1-stage and 2-stage clotting assays
Mutant . | 1-stage, % of WT . | 1-stage, U/mg . | 2-stage, of WT . | 2-stage, U/mg . | Activation ratio . |
|---|---|---|---|---|---|
| WT | 100 | 315 | 100 | 4750 | 15 |
| Ala221Val | 99.3 | 313 | 99.7 | 4740 | 15 |
| Ala221Gly | 89.0 | 280 | 80.3 | 3810 | 14 |
| Glu275Gln | 97.2 | 306 | 94.1 | 4470 | 15 |
| Cys220Ala/Cys301Ala | 97.9 | 308 | 89.0 | 4230 | 14 |
Mutant . | 1-stage, % of WT . | 1-stage, U/mg . | 2-stage, of WT . | 2-stage, U/mg . | Activation ratio . |
|---|---|---|---|---|---|
| WT | 100 | 315 | 100 | 4750 | 15 |
| Ala221Val | 99.3 | 313 | 99.7 | 4740 | 15 |
| Ala221Gly | 89.0 | 280 | 80.3 | 3810 | 14 |
| Glu275Gln | 97.2 | 306 | 94.1 | 4470 | 15 |
| Cys220Ala/Cys301Ala | 97.9 | 308 | 89.0 | 4230 | 14 |
Factor V activity before and after thrombin activation was measured in a clotting assay, based on the prothrombin time. Normal plasma pool was used as standard, assuming a plasma concentration of 1 U/mL.
Titrating FXa and prothrombin in the prothrombinase assay. Wild-type FV and the Ala221Val FV variant were incubated with thrombin (0.5 NIH units/mL) for 10 minutes at 37°C. (A) FVa was diluted to 50 pM (final concentration) and incubated for 4 minutes with FXa (1 to 50 000 pM) and 10:90 PS/PC phospholipid vesicles (50 μM) at 37°C. Thrombin generation was started by the addition of prothrombin (0.5 μM). (B) FVa was diluted to 100 pM (final concentration) and incubated for 4 minutes with prothrombin (PT) (1 to 2500 pM) and 10:90 PS/PC phospholipid vesicles (50 μM) at 37°C. Thrombin generation was started by the addition of FXa (5 nM). The reactions were stopped after 1 minute by dilution with ice-cold EDTA buffer. The generated thrombin was determined with the chromogenic substrate S-2238. The activity was expressed as percentage of maximum activity generated in the presence of WT FVa. WT FVa (▪) and Ala221Val (□). Each data point represents the mean of 3 independent experiments performed in duplicate. Error bars represent ± SDs.
Titrating FXa and prothrombin in the prothrombinase assay. Wild-type FV and the Ala221Val FV variant were incubated with thrombin (0.5 NIH units/mL) for 10 minutes at 37°C. (A) FVa was diluted to 50 pM (final concentration) and incubated for 4 minutes with FXa (1 to 50 000 pM) and 10:90 PS/PC phospholipid vesicles (50 μM) at 37°C. Thrombin generation was started by the addition of prothrombin (0.5 μM). (B) FVa was diluted to 100 pM (final concentration) and incubated for 4 minutes with prothrombin (PT) (1 to 2500 pM) and 10:90 PS/PC phospholipid vesicles (50 μM) at 37°C. Thrombin generation was started by the addition of FXa (5 nM). The reactions were stopped after 1 minute by dilution with ice-cold EDTA buffer. The generated thrombin was determined with the chromogenic substrate S-2238. The activity was expressed as percentage of maximum activity generated in the presence of WT FVa. WT FVa (▪) and Ala221Val (□). Each data point represents the mean of 3 independent experiments performed in duplicate. Error bars represent ± SDs.
Apparent Kd for binding of FXa to FV mutants estimated in the prothrombinase assay
FV variant . | Kd, pM . | kcat, S-1 . | Km, nM . |
|---|---|---|---|
| WT FVa | 200 | 165 | 250 |
| Ala221Val | 200 | 160 | 250 |
FV variant . | Kd, pM . | kcat, S-1 . | Km, nM . |
|---|---|---|---|
| WT FVa | 200 | 165 | 250 |
| Ala221Val | 200 | 160 | 250 |
The apparent Kd's for the interaction between FXa and the different FV mutants were calculated by fitting the data shown in Figure 4A to an equation describing a single-site binding isotherm via nonlinear least squares regression analysis. Km values were calculated from data of Figure 4B fitted to the Michaelis-Menten equation. The kcat values were calculated from the absolute rate of maximal thrombin observed in the experiment presented in Figure 4B.
The APC-mediated inactivation of activated Ala221Val and WT was followed over time and analyzed in the prothrombinase assay. The inactivation by APC was performed both in the presence and in the absence of protein S. Inactivation of WT FVa by APC is known to be a biphasic reaction. The rapid cleavage at Arg506, yielding a partially active intermediate, is followed by the slower cleavage atArg306, which results in complete loss of FXa cofactor activity.33 The inactivation of the FVa by APC was examined at 2 different conditions. In the first experimental setup, the low APC concentration (0.05 nM) combined with the absence of protein S allowed investigation of the 506 cleavage, whereas little of the kinetically less favored 306 cleavage occurred under these conditions (Figure 5A). The residual activity was measured with the prothrombinase assay using low FXa concentrations (0.05 nM) to minimize the contribution of procoagulant activity from the 506-cleaved FVa intermediate. APC-mediated inactivation in the presence of protein S was also performed and followed for a longer time (20 minutes) to enable both the 306 and the 506 cleavage to occur. The residual activity was then determined using high FXa concentrations (5 nM) (Figure 5B). At this FXa concentration, FVa cleaved at Arg506 expresses around 40% to 50% remaining activity, whereas Arg306-cleaved FVa is inactive. Therefore, these conditions maximize the functional difference between the Arg506- and Arg306-cleaved FVa. The inactivation curves of WT and Ala221Val were indistinguishable, both in the presence and in the absence of protein S, suggesting the Ala221Val mutation not to affect the APC-mediated inactivation.
APC-mediated inactivation of the Ala221Val FVa variant. Recombinant FV variants (0.8 nM) were incubated with thrombin (0.5 U/mL) for 10 minutes in 37°C. In panel A, 0.05 nM APC was added together with 10:20:70 PS/PE/PC phospholipids at a final concentration of 25 μM, and the FVa degradation was followed for 5 minutes. The subsamples withdrawn at different time points were stopped by 5-fold dilution in ice-cold HNBSACa buffer. The residual activity was measured with the prothrombinase assay using suboptimal FXa concentrations (0.05 nM). In panel B, 0.1 nM APC was added together with 100 nM protein S and 10:20:70 wt/wt/wt PS/PE/PC phospholipids (final concentration of 25 μM). The FVa degradation was followed for 20 minutes. The subsamples were stopped as in panel A. The residual activity was determined with the prothrombinase assay using saturated FXa concentrations (5 nM). The remaining activity was expressed as percentage of the activity generated by each FVa variant in the absence of APC. WT FVa (▪) and Ala221Val (□). Each data point represents the mean of 3 independent experiments performed in duplicate. Error bars represent ±SDs.
APC-mediated inactivation of the Ala221Val FVa variant. Recombinant FV variants (0.8 nM) were incubated with thrombin (0.5 U/mL) for 10 minutes in 37°C. In panel A, 0.05 nM APC was added together with 10:20:70 PS/PE/PC phospholipids at a final concentration of 25 μM, and the FVa degradation was followed for 5 minutes. The subsamples withdrawn at different time points were stopped by 5-fold dilution in ice-cold HNBSACa buffer. The residual activity was measured with the prothrombinase assay using suboptimal FXa concentrations (0.05 nM). In panel B, 0.1 nM APC was added together with 100 nM protein S and 10:20:70 wt/wt/wt PS/PE/PC phospholipids (final concentration of 25 μM). The FVa degradation was followed for 20 minutes. The subsamples were stopped as in panel A. The residual activity was determined with the prothrombinase assay using saturated FXa concentrations (5 nM). The remaining activity was expressed as percentage of the activity generated by each FVa variant in the absence of APC. WT FVa (▪) and Ala221Val (□). Each data point represents the mean of 3 independent experiments performed in duplicate. Error bars represent ±SDs.
Stability of the recombinant FV variants
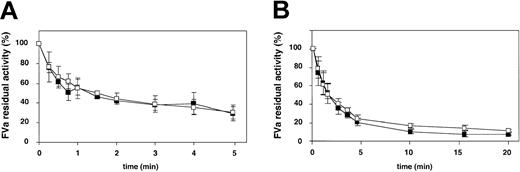
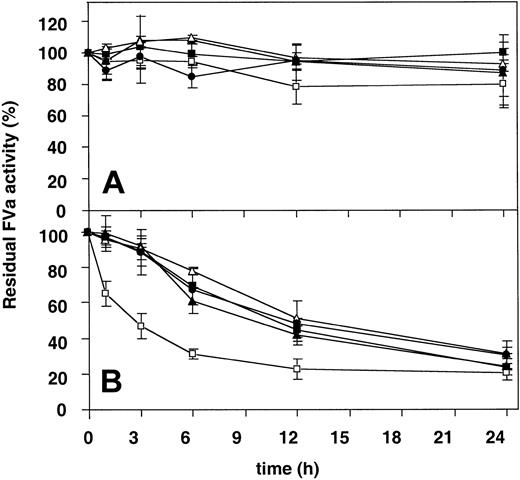
The stability of the different FV variants at 37°C before and after activation were followed over time (Figure 6). The intact, nonactivated forms of the FV variants were all very stable and had after 24 hours at 37°C not lost more than 20% of their initial activities. On the other hand, when the thrombin-activated forms of the FV variants were examined, it was apparent that the Ala221Val mutant exhibited significantly lower stability than the other FVa variants. The FV New Brunswick mutant had already after 3 hours lost half of its initial cofactor activity, while the other FVa variants had in comparison residual FVa activities of around 90%. Control experiments using plasma-purified FVa diluted in either buffer or mock medium demonstrated that the medium did not interfere with the stability at 37°C.
Determination of the functional stability over time at 37°C of the recombinant FV variants. (A) Recombinant FV variants (0.5 nM) were incubated at 37°C over time. Subsamples were withdrawn at different time points and activated with thrombin (0.5 U/mL) for 10 minutes, and the remaining procoagulant activity was then determined with the prothrombinase assay. (B) The recombinant FV variants (0.5 nM) were activated with thrombin (0.5 U/mL) for 10 minutes and then left on 37°C. Subsamples were then withdrawn at different time points, and the remaining activity was determined as in panel A. WT FVa (▪), Ala221Val (□), Ala221Gly (▴), Glu275Gln (▵), and Cys220Ala/Cys301Ala (•). Each data point represents the mean of 3 independent experiments performed in duplicate. Error bars represent ± SDs.
Determination of the functional stability over time at 37°C of the recombinant FV variants. (A) Recombinant FV variants (0.5 nM) were incubated at 37°C over time. Subsamples were withdrawn at different time points and activated with thrombin (0.5 U/mL) for 10 minutes, and the remaining procoagulant activity was then determined with the prothrombinase assay. (B) The recombinant FV variants (0.5 nM) were activated with thrombin (0.5 U/mL) for 10 minutes and then left on 37°C. Subsamples were then withdrawn at different time points, and the remaining activity was determined as in panel A. WT FVa (▪), Ala221Val (□), Ala221Gly (▴), Glu275Gln (▵), and Cys220Ala/Cys301Ala (•). Each data point represents the mean of 3 independent experiments performed in duplicate. Error bars represent ± SDs.
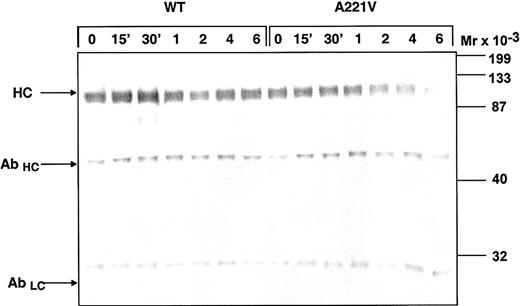
To assess if the impaired stability was due to increased rate of dissociation of the light and heavy chain of FVa, subsamples of the WT and Ala221Val FVa were drawn at intervals and subjected to immunoprecipitation with a biotinylated antibody against the light chain of FVa (HV-1). The FVa that bound to the antibody was collected using streptavidin-coated magnetic beads. FVa was eluted by boiling under denaturing conditions and analyzed by Western blotting using an antibody against the heavy chain (HV-5146; Figure 7). The monoclonal HV-1 antibody that also eluted from the beads was also visualized in this Western blot system and could thereby function as an internal control, ensuring that equal amounts of sample had been loaded on the gel. This system demonstrated that increased rate of dissociation of heavy and light chains of the Ala221Val FVa mutant explained the lower stability. On the Western blot, the heavy chain of the Ala221Val FVa variant, as compared with that of WT FVa, appeared fainter after 2 hours and had almost totally disappeared after 6 hours.
Estimation of the dissociation of the light and heavy chain of the Ala221Val FV variant. The recombinant FV variants (0.5 nM) were activated with thrombin (0.5 U/mL) for 10 minutes and then left on 37°C. Subsamples were then withdrawn at different time points and immunoprecipitated with a biotinylated monoclonal antibody against the light chain (HV-1). For precipitation streptavidin-coated magnetic beads were used. FVa was eluted by boiling and analyzed on 10% SDS-PAGE under reducing conditions. The Western blots were detected with a monoclonal antibody against the heavy chain (AHV 5146). Vectastain Elite ABC kit was used to develop the Western blots. HC denotes the heavy chain of FVa, while AbHC and AbLC denote the heavy and light chain of the HV-1 antibody.
Estimation of the dissociation of the light and heavy chain of the Ala221Val FV variant. The recombinant FV variants (0.5 nM) were activated with thrombin (0.5 U/mL) for 10 minutes and then left on 37°C. Subsamples were then withdrawn at different time points and immunoprecipitated with a biotinylated monoclonal antibody against the light chain (HV-1). For precipitation streptavidin-coated magnetic beads were used. FVa was eluted by boiling and analyzed on 10% SDS-PAGE under reducing conditions. The Western blots were detected with a monoclonal antibody against the heavy chain (AHV 5146). Vectastain Elite ABC kit was used to develop the Western blots. HC denotes the heavy chain of FVa, while AbHC and AbLC denote the heavy and light chain of the HV-1 antibody.
Discussion
In this investigation, we have studied the biostructural pathology of the FV New Brunswick Ala221Val using a combination of recombinant protein expression, functional assays, and computer-based structural analysis. The 2 originally described patients with FV New Brunswick were pseudohomozygous for the Ala221Val mutation (ie, the patients carried a null allele in addition to the Ala221Val allele) and were reported to have histories of mucus membrane bleeding and menorrhagia. A discrepancy in FVa cofactor activity (26% proband and 27% C2 sibling) and antigen level (39% proband and 50% C2 sibling)16 suggested the mutation mainly to result in a slight functional defect. However, it is apparent that the functional defect was more pronounced after purification of the mutant FV from the C2 sibling with only 17% FVa activity compared with a normal purified FV. This suggests that the FV New Brunswick had decreased stability and that it lost activity during the purification procedure.
To characterize the FV New Brunswick variant, we introduced the Ala221Val mutation in the FV cDNA and expressed the FV variant in eukaryotic cells. In addition, 3 other FV mutations (Ala221Gly, Glu275Gln, and Cys220Ala/Cys301Ala) that could help explain the FV New Brunswick phenotype were created. Pulse-chase analysis and FV quantification by ELISA and a prothrombinase-based assay were used to determine whether any of the FV mutations impaired synthesis or secretion. However, this was not found to be the case, suggesting the mutations to be well tolerated and not to induce major folding problems.
Because the original report suggested the FV Ala221Val variant to express reduced procoagulant activity, the recombinant FV Ala221Val variant was investigated in a clotting-based assay as well as in a prothrombinase assay. In both types of assays, the activity of FV Ala221Val was similar to that of WT FV, both before and after thrombin activation. The thrombin-activated FV Ala221Val variant also yielded a normal dose-response curve to FXa and prothrombin in a prothrombinase-based assay, suggesting the FXa- and prothrombin-binding sites in FVa to be unaffected by the Ala221Val mutation.
Another explanation for a reduced FVa activity could be enhanced sensitivity to degradation by APC. We therefore analyzed the APC-mediated inactivation of the recombinant Ala221Val FVa in parallel with that of WT FVa. The results suggested that there were no significant differences between the Ala221Val FVa variant and WT FVa in APC-mediated cleavages at either Arg306 or Arg506.
Impaired stability of FV/FVa due to enhanced rate of dissociation of the heavy and light chains of FVa is another possible mechanism contributing to the FV deficiency. Therefore, we investigated the stability at 37°C of both intact and thrombin-activated FV. The intact FV variants were all quite stable and, after 24 hours, the residual activities were around 80% to 100%. In contrast, after thrombin incubation, the Ala221Val FVa variant demonstrated reduced stability, with a 50% loss of activity already after 3 hours. A combination of light chain–specific immunoprecipitation and heavy chain–specific Western blotting demonstrated the lability of FVa Ala221Val to be due to enhanced rate of dissociation of the heavy and light chains. These results suggest that the strength of the interaction between the A1-A2 segment and the A3-C1-C2 domains was affected by the Ala221Val mutation. Even though we could not demonstrate any consequence of this in the stability of intact FV in vitro, it is still possible that this affects the stability of FV New Brunswick in vivo. In this context it is noteworthy that the FV New Brunswick from the C2 sibling of the original family lost activity during purification.
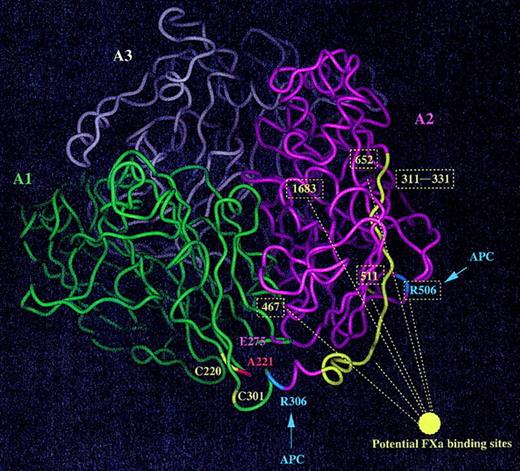
The recently created molecular models of FVa and FVIIIa based on the experimentally determined structure of ceruloplasmin provide a useful foundation for theoretical analysis of the potential structural effects of the Ala221Val mutation in FV.7,34 Alanine 221 is conserved in ceruloplasmin but replaced with a histidine in FVIII, suggesting that a relatively large residue could be tolerated at position 221. From the computer model of FVa we hypothesized that the structural/functional problems (if any) of the Ala-to-Val substitution could either be disturbances of a putative salt bridge between Lys304 and Glu275 or of the closely located disulfide bridge involving Cys220-Cys301 (Figure 8).27 Surprisingly, protein synthesis and function were unaffected in the 2 recombinant variants, Cys220Ala/Cys301Ala and Glu275Gln. This suggests that the salt bridge between Glu275 and Lys304 is either not crucial for the stability of the protein or that the 2 side chains do not interact closely. Similarly, the Cys220-Cys301 disulfide bridge does not seem to play a major role for the folding or stability of FV. Furthermore, these data suggest that these residues are not directly involved in FXa or prothrombin binding, indicating that other mechanisms result in the originally observed functional defects of the FV Ala221Val mutant.
Ribbon diagram of the A domains of factor Va. The 3 A domains of FV are shown (green, A1; magenta, A2; and white, A3) while the 2 cleavage sites for APC visible in this predicted structure are highlighted in blue (Arg306 and Arg505). Ala221 is in red, Glu275 (possibly involved in a salt bridge although the orientation of the side chain is not very well defined) is in magenta, and 2 cysteine residues forming a disulfide bond (Cys220-Cys301) are in yellow. Possible binding sites for FXa are shown in yellow (the ribbon is colored yellow and the residue numbers boxed).
Ribbon diagram of the A domains of factor Va. The 3 A domains of FV are shown (green, A1; magenta, A2; and white, A3) while the 2 cleavage sites for APC visible in this predicted structure are highlighted in blue (Arg306 and Arg505). Ala221 is in red, Glu275 (possibly involved in a salt bridge although the orientation of the side chain is not very well defined) is in magenta, and 2 cysteine residues forming a disulfide bond (Cys220-Cys301) are in yellow. Possible binding sites for FXa are shown in yellow (the ribbon is colored yellow and the residue numbers boxed).
Another explanation for the Ala221Val phenotype could be that the valine has not enough room to be accommodated in this region of FV and induces some local structural changes that could propagate toward the interdomain interfaces. Investigation of the static theoretical model of FVa suggested that the valine could possibly be accommodated in this region of FVa, but because the present experimental data showed increased light-heavy chain dissociation, we aimed at trying to understand this reaction in a more dynamic 3D structure of FVa. Molecular dynamic simulations on both WT and the Ala221Val mutant were performed, and we noticed that the mutation could induce structural changes at relatively large distance, toward the A3-A2 domain interface, possibly affecting the stability of the molecule (data not shown). The role of the B domain is not known in this respect, but it is possible that this large glycosylated segment stabilizes, somewhat, the FV interdomain interfaces. Taken together, these data suggest that subtle structural adjustments are required to fully accommodate a valine at position 221. This also indicates that the local packing of the side chains in this area of FV should be relatively tight. This assumption is strengthened by the observation that the replacement of Ala by a Gly was structurally tolerated. Thus, a short amino acid like alanine or glycine seems to be required at this position in FVa, while a bulkier amino acid (eg, valine) induces structural changes that likely propagate up to the domain interface regions. According to the present study, functional defects of the FVa Ala221Val mutant seem to be due to destabilization of the light chain heavy chain and subsequent reduced cofactor activity in prothrombin generation rather than residue 221 being a direct recognition site for FXa or prothrombin. When mapping some possible binding sites for FXa at the surface of the FVa A domains,17,35-37 we observe that the Ala221 area is likely to be outside the potential regions directly involved in FXa binding (Figure 8). Regions of FVa involved in prothrombin binding are not well defined, and the area of Ala221 does seem to be involved in this reaction.
In conclusion, our data suggest that the Ala221Val mutation is associated with reduced stability, resulting in enhanced rate of dissociation of the light and heavy chains of the mutant FVa. In contrast to the previous report on FV New Brunswick,16 the procoagulant activity and the binding of FXa were not directly affected by the mutation. The data indicate that the Ala221Val mutation does not impair the expression of FV and most likely does not affect the folding of the molecule. The reduced stability of the recombinant Ala221Val FV variant upon activation could possibly explain the FV deficiency associated with the Ala221Val mutation. Finally, our investigation emphasizes the benefit of using functional analysis in combination with computer-based structural investigations to understand sequence-structure-function relationship.
Prepublished online as Blood First Edition Paper, April 24, 2003; DOI 10.1182/blood-2003-01-0116.
Supported by grants from the Swedish Medical Research Council (grant 07143), a Senior Investigator's Award from the Foundation for Strategic Research, the Albert Påhlsson's Trust, and research funds from the University Hospital, Malmö, Sweden.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We warmly thank Ann-Louise Tholander for expert technical assistance.

![Figure 2. SDS-PAGE analysis of the expression of WT FV and the Ala221Val FV variant. COS 1 cells expressing WT FV or Ala221Val:FV were depleted for Cys/Met and then radiolabeled with [35S]Met/Cys for 30 minutes. After increasing time the radiolabeled FV in cell lysates and media were immunoprecipitated and electrophoresed on 7.5% SDS–polyacrylamide gels under reducing conditions (A). The radioactivity of the FV bands was analyzed with a Phosphorimager. To quantify the secretion profiles of WT (▪) and Ala221Val (□), the radioactivity of the FV-specific bands from cell lysate (B) and medium (C) was measured. The amount of radioactive FV in the cell lysate at 0 hours was assigned to be 100% for each FV variant. Each data point represents the mean of 3 independent experiments. Error bars represent ± SDs.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/4/10.1182_blood-2003-01-0116/6/m_h81634754002.jpeg?Expires=1765895618&Signature=DFgeroMcKp27KipgZBvK8yZlhXrgHIcwHxeufFrTt5i7Snfdri0y2g4DA-Fw4Y4p2ZV0wJF-gHBNN8rUBbogMVUX4Uz5gRQvPZou8NakPYk5gfqsMZ41dKTkVULqDzKzoS-AvMQ7EOXPKcSRKTbyGuaJuQ0sRrpp1Q3Mp0IEj0aRCenH6vKDVZEtuqbc7MpENk89Gayz1-ZG3fLUm7oCiyZx1ejwcJ3hDkdCPMX0P4-939BIYpAHgR6hwoU9~7WoLmiHzkWM2Il22wJg6g9QIDLhifcdxU6bcGEtegNViC2nx893kxalUQ~nhmTjdE2t1tBRY8jwk8IqM2iRunOoTg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal