Abstract
The aorta-gonads-mesonephros (AGM) region of the mouse embryo has been assigned as the origin of definitive hematopoiesis. The transcription factor GATA-2 has specific but unclarified roles in early hematopoiesis. To elucidate the expression profile of GATA-2, we prepared transgenic mouse lines containing the green fluorescent protein (GFP) gene driven by GATA-2 gene regulatory elements. We also prepared a mouse line in which GFP reporter sequences were inserted into the endogenous GATA-2 gene. Both mouse mutants expressed GFP in the early hematopoietic tissues. The CD45 antigen, a marker of hematopoietic cells, was expressed in a small fraction of transgene (TG)–derived GFP+ cells. The remaining TG-GFP+/CD45- cells were adherent to plastic and produced CD45+ hematopoietic cells abundantly when cultured in vitro. Exogenous expression of GATA-2 in TG-GFP+/CD45- cells from the AGM region inhibited their differentiation into CD45+ cells. Loss of GATA-2 function through the disruption of the GATA-2 locus enhanced the earlier emergence of CD45+ cells in the yolk sac of the 9.5-day conceptus. These results demonstrated that GATA-2 is expressed in the precursor of hematopoietic cells and works as a gatekeeper to preserve their immaturity. A reduction of GATA-2 expression or activity is required for the differentiation of precursors to hematopoietic cells.
Introduction
In mammals, the dorsal aorta and its neighboring tissues, the para-aortic splanchno-pleura (P-Sp) at embryonic day (E) 9.5 and the aorta-gonads-mesonephros (AGM) region at E10.5 and E11.5, have been assigned as the origin of definitive (adult-type) hematopoietic cells.1-3 In order to distinguish the early development of definitive hematopoietic cells in the P-Sp and AGM from their differentiation into mature hematopoietic cells, their development is referred to as “hematogenesis.”4,5 The hematogenic precursor cells4 have also been designated hemangioblasts,6-8 lymphohematopoietic endothelial cells,9 or hemogenic endothelial cells,10 as they have the potential to differentiate into endothelial cells or they indeed have the characteristics of endothelial cells.
Several marker molecules are available for the analysis of murine early hematogenesis. CD45 is a tyrosine phosphatase expressed in all hematopoietic lineages, except mature erythrocytes and platelets, and is also expressed in hematopoietic stem cells in the AGM region.5,9 CD34 and PCLP1 are ligands for L-selectin and markers of immature hematopoietic cells and hematogenic precursors.8,11 Fetal liver kinase-1 (Flk-1), one of the vascular endothelial growth factor (VEGF) receptors, is an essential molecule for both angiogenesis and hematogenesis12 and is the earliest marker for hemangioblasts.9,13 Transcription factors Runx1 (also known as phosphatidylethanolamine-binding protein 2αB [PEBP2αB], Cbf2, or acute myeloid leukemia 1 [AML-1]5,14,15 ), SCL/tal-1,16,17 and c-Myb18 are also known to be expressed in the AGM region.
Transcription factor GATA-2 is indispensable for hematopoiesis, especially for definitive hematopoiesis.19,20 We previously described GATA-2 expression in the P-Sp and AGM regions.21 In amphibia and teleost fish, GATA-2 is expressed in the dorsal lateral plate mesoderm, or intermediate cell mass, which are the evolutionary equivalents of the P-Sp and AGM region of mammals, and GATA-2 is considered an early marker of the cells with the fate to differentiate into hematopoietic cells.22,23 These observations suggest that the role of GATA-2 in hematogenesis and hematopoiesis may be conserved among species. The Flk-124,25 and SCL16,26 genes contain indispensable GATA-factor binding sites in their regulatory regions, implying that these are the likely target genes of GATA-2. However, the exact role GATA-2 plays in hematopoietic development remains to be elucidated.
Currently, available data suggest that the other hematopoietic GATA factors may also be involved in early hematopoiesis.27-29 GATA-3 is expressed in mesenchymal cells in the AGM region and this observation suggests a contribution of GATA-3 to hematopoietic cell development.30 A GATA-3 contribution to hematogenesis, however, remains to be proven in knockout mice.29 Similarly, while GATA-1 is indispensable for the later stages of erythroid differentiation,27,28 GATA-1's contribution to the early development of the definitive hematopoietic lineage has not been examined closely.
To elucidate the function of GATA-2 in hematopoietic tissues, several overexpression experiments have been carried out.31-33 A GATA-2–estrogen-receptor fusion protein inhibits the maturation of erythroid cells, when introduced into chicken bone marrow erythroid progenitor cells.31 Retroviral expression of GATA-2 in multipotent FDCP-mix cells and in mouse bone marrow cells resulted in the repression of cell proliferation, the induction of myeloid cell differentiation, and the conversion of the differentiation program from the erythroid to the myeloid lineage.32,33 In contrast, transgenic expression of GATA-2 driven by the hematopoietic regulatory domain of the GATA-1 locus rescues the anemia caused by disruption of the GATA-1 gene.34 These mice, expressing GATA-2 where GATA-1 is normally expressed, survive and are fertile.34
Thus, in one situation GATA-2 antagonizes GATA-1, whereas in another GATA-2 can replace GATA-1. Since vertebrate GATA factors contain the well-conserved double zinc finger motifs,35 the zinc finger–mediated function of other GATA factors could be replaced or augmented by the artificial overexpression of GATA-2. Therefore, we think that it is important to analyze GATA-2 function in a context where the GATA-2 is expressed and functioning physiologically.
In order to examine the functional contribution of GATA-2 to early definitive hematopoiesis, we exploited in this study the transcriptional regulatory region of the GATA-2 gene. To distinguish and isolate cells expressing GATA-2, we generated transgenic mice with a construct containing a 9–kilobase pair (kbp) GATA-2 gene fragment linked to the green fluorescent protein (GFP) reporter gene. We also generated knock-in (KI) mice in which the GFP reporter gene was inserted into the GATA-2 locus. Importantly, CD45- hematogenic precursor cells in the AGM region of these transgenic and KI mice were found to express GFP. After differentiation into hematopoietic cells, their fluorescence was lost and GATA-2 expression decreased. Constitutive expression of GATA-2 from a retroviral vector in AGM cells inhibited the production of CD45+ cells from the GFP+/CD45- cells. The loss of GATA-2 expression in homozygous KI mice stimulated the production of CD45+ cells in the early stage of yolk sac–definitive hematopoiesis. These results demonstrate an inhibitory function for GATA-2 in the differentiation of hematogenic precursor cells and suggest that GATA-2 may act to preserve the pool of immature cells.
Materials and methods
Transgenic mouse lines
The genomic fragment containing 8993 base pairs (bp) of the mouse GATA-2 gene, extending from the upstream XhoI site to the translation start site in the second exon, was ligated to the EGFP (enhanced GFP) gene (Clontech, Palo Alto, CA) and injected into fertilized murine eggs with standard procedure.36 Of the 4 transgenic mouse lines with this construct (3.1ISIGIIGFP), 3 lines expressed the transgene (TG)–derived GFP gene in embryonic hematopoietic tissues, and 2 of these lines (no. 685 and no. 798) with bright fluorescence were used in the experiments. KI mice were generated by knock-in knockout strategy inserting the GFP coding sequences into the translation start site of the GATA-2 locus. The neomycin cassette was eliminated using the Cre-loxP system. Detailed analysis of the KI mice will be reported elsewhere (N.S., N.M., and M.Y., manuscript in preparation).
Immunologic analysis
Murine tissues used for immunohistochemistry were fixed in 1% formaldehyde, 0.2% glutaraldehyde, and 0.06% NP40 and embedded in OCT compound (Sakura Finetek, Tokyo, Japan). Sections (7 μm) were incubated with rabbit anti-GFP (Clontech) and anti–GATA-2 (RC.1.1.137 ), and CD45 antibody (BD PharMingen, San Jose, CA). Specific antibody binding was visualized using horseradish peroxidase (HRP)–conjugated antirabbit immunoglobulin or HRP-conjugated antirat immunoglobulin. Color detection was performed using diaminobenzidine as a chromogen. AGM regions of E11.5 embryos were fixed in 3% paraformaldehyde, 0.01% sodium azide, and 0.02% Triton X-100 and then stained with fluorescein isothiocyanate (FITC)–conjugated rat anti–GATA-2 antibody or FITC-conjugated rat immunoglobulin isotype control (BD PharMingen).
The preparation of a single-cell suspension, the staining procedure, fluorescence-activated cell sorter (FACS) analysis, and cell sorting were carried out as previously described.21 Monoclonal antibodies to Flk-138 and PCLP18 have already been described elsewhere. Phycoerythrin (PE)–conjugated CD45, PE–c-kit, biotin-conjugated CD34, biotin-conjugated CD31 antibodies, and streptavidin-allophycocyanin, were purchased from BD PharMingen. Immunoblotting analysis with RC1.1.1 antibody, polyclonal anti–GATA-2 antibody (C-20), anti-GFP, and anti–β-actin antibodies (the latter 3 are from Santa Cruz Biotechnology, Santa Cruz, CA) was performed using Lumi-Light Western Blotting Substrate (Roche Diagnostics, GmbH Mannheim, Germany) according to the instructions provided by the manufacturer.
RT-PCR analysis
Cell sorting, RNA isolation, and reverse transcriptase–polymerase chain reaction (RT-PCR) were performed as described previously.39 Cycling conditions were as follows: denaturation at 94°C for 20 seconds, annealing at 58°C for 20 seconds, and extension at 72°C for 1 minute for 45 cycles. The results were compared with the control experiments without reverse transcriptase.
Primers used (and the resultant product sizes) were as follows. Hypoxanthine-guanine phosphoribosyltransferase (HPRT): sense 5′-GCAAACTTTGCTTTCCCTGG-3′, antisense 5′-CAACTTGCGCTCATCTTAGGC-3′ (band size 244 bp); GATA-2: sense 5′-TGCAACACACCACCCGATACC-3′, antisense 5′-CAATTTGCACAACAGGTGCCC-3′ (336 bp); SCL: sense 5′-TAGCCTTAGCCAGCCGCTCG-3′, antisense 5′-GCGGAGGATCTCATTCTTGC-3′ (277 bp); PEBP2β: sense 5′-GAGTGCGAGATTAAGTACACGGG-3′, antisense 5′-TCGCTCCTCATC AAACTCCAGGCA-3′ (324 bp); RUNX1: (AML-1, PEBP2αB) sense 5′-CCTGCTTGGGTGTGAGGCCC-3′, antisense 5′-GCCTCGCTCATCTTGCCGGG-3′ (292 bp); c-Myb: sense 5′-AAGCTTGTCCAGAAATATGGTCCGAAG-3′, antisense 5′-GGCTGCCGCAGCCGGCTGAGGGAC-3′ (525 bp); Ets-1: sense 5′-AGAGCCAGTCGTGGTAAACTCG-3′, antisense 5′-GCACATAGTCCTTGAAGGTGCC-3′ (208 bp).
Cell culture
Sorted or nonsorted cells were cultured in alpha modified minimum essential media (MEM) medium containing 15% fetal calf serum (FCS; JRH Biosciences, Lenexa, KS), leukemia inhibitory factor (LIF; R&D Systems, Minneapolis, MN), stem cell factor (SCF; PeproTech, Rocky Hill, NJ), human basic fibroblast growth factor (b-FGF; GIBCO BRL, Grand Island, NY), and oncostatin M (OSM; R&D Systems), as described previously.40 In the coculture system with OP-9 stromal cells, the condition without cytokine addition was used.13 After 4 and 7 days of incubation, hematopoietic colonies were counted. The frequency of hematopoietic colony-forming cells was estimated from the results of a series of cultures with 100, 1000, and 10 000 cells per well.
Retroviral overexpression of the GATA-2 gene
Mouse GATA-2 cDNA was inserted into the BglII and EcoRI sites of the MSCV2.2-IRES-EGFP (murine stem cell virus 2.2–internal ribosomal entry site–EGFP) virus (kindly provided by Dr Akihiro Kume, Center for Molecular Medicine, Jichi Medical School).41 Various GATA-2 mutants and fusion proteins were constructed by PCR. Transcriptional activities of the VP16 activation domain42 GATA-2 zinc finger fusion protein (VP-F) and engrailed repressor domain43 GATA-2 zinc finger fusion protein (Eng-F) were confirmed based on their Luciferase reporter activity. Viral supernatant was harvested as described previously.41 The primary AGM cultures of E10.5 embryos were infected on days 0 to 2. LO cells8 were also infected and used to confirm the expression of the virally introduced genes.
Results
Establishment of transgenic mouse lines expressing GFP in early hematopoietic tissues
The distal IS exons of the mouse and human GATA-2 genes are used specifically in hematopoietic and neural tissues, whereas the proximal IG exons are expressed in a variety of tissues, including hematopoietic tissues.39,44 Three transgenic mouse lines containing the 7.0ISGFP construct, which covers the 7-kbp 5′ flanking region of the IS exon (Figure 1A), were found to express GFP in hematopoietic and neural tissues.21 Meanwhile, we found that several segments from the region extending from exon IS to the second exon, which are missing in the 7.0ISGFP, are highly homologous between species.44 This observation suggests the existence of additional transcription regulatory element(s) in this region, so we tested this possibility by generating 3.1ISIGIIGFP transgenic mouse lines, which contain the approximately 9-kbp GATA-2 gene fragment extending from the upstream XhoI site to the translation start site in the second exon (Figure 1A).
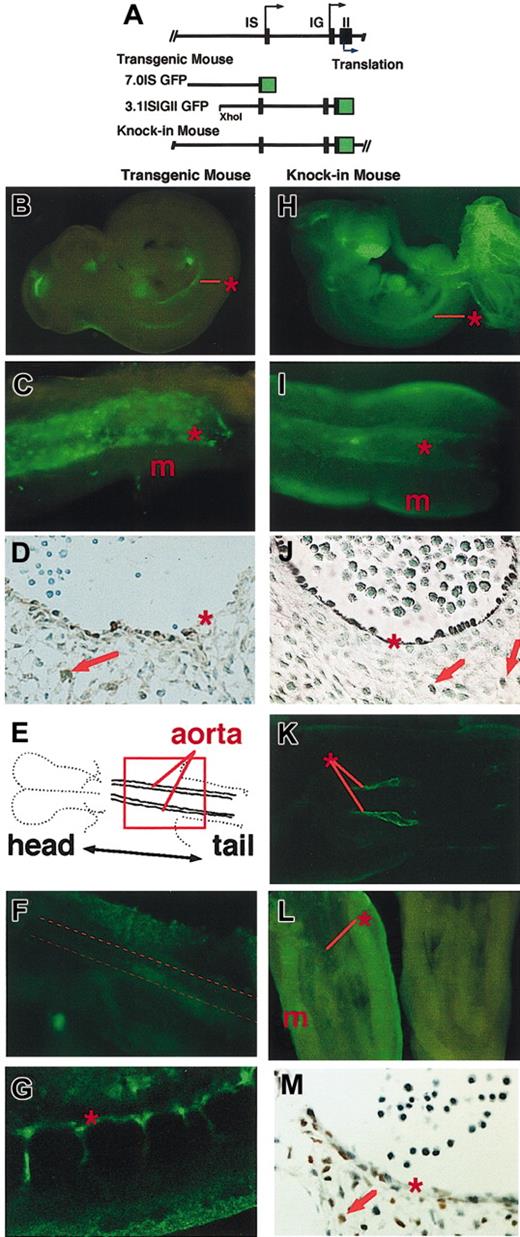
GFP is expressed in transgenic and knock-in mouse embryos. (A) Structure of 7.0IS GFP and 3.1ISIGII GFP transgenic constructs. Top line represents the structure of the mouse GATA-2 gene. Black arrow and blue arrow indicate the transcription and translation initiation sites, respectively. The map of the murine GATA-2 gene, modified to express EGFP in knock-in mouse, is also shown. (B-C) TG-GFP expression profile of the 3.1ISIGII GFP transgenic mouse E10.5 embryo (line no. 685). Panel C shows a ventral view of the AGM region. Asterisk and m indicate the positions of dorsal aorta and mesonephros, respectively. (D) Immunostaining with anti-GFP antibody confirming the TG-GFP expression (brown) in the lining of the aortic wall and in the cells ventral to the dorsal aorta (red arrow) of an E11.5 embryo (line no. 685). (E) Outline of the planar orientation of E8.0 embryo.17 (F) Confocal microscopic image of the region corresponding to the red square in panel E of line no. 798 transgenic embryo. Broken line in panel F indicates the position of the dorsal aorta. (G) Sagittal image of E9.0 embryo (line no. 798). (H-I) KI-GFP expression in the E10.5 KI-heterozygous embryo. Panel I shows the AGM region of the KI heterozygous embryo. Asterisk and m indicate the position of the dorsal aorta and mesonephros, respectively. (J) Anti-GFP antibody staining of the transverse section of the aorta of E11.5 embryo. (K) KI-GFP fluorescence along the dorsal aorta of E8.0 KI-heterozygous embryo. (L) Immunostaining of E11.5 AGM region with FITC anti–GATA-2 antibody (left), and with control rat antibody (right). (M) Immunohistochemical staining with anti–GATA-2 antibody showing endogenous GATA-2 expression in the lining of the dorsal aorta and in neighboring mesenchymal cells of an E11.5 embryo. See “Materials and methods.” Original magnification for panels D, J, and M is × 100.
GFP is expressed in transgenic and knock-in mouse embryos. (A) Structure of 7.0IS GFP and 3.1ISIGII GFP transgenic constructs. Top line represents the structure of the mouse GATA-2 gene. Black arrow and blue arrow indicate the transcription and translation initiation sites, respectively. The map of the murine GATA-2 gene, modified to express EGFP in knock-in mouse, is also shown. (B-C) TG-GFP expression profile of the 3.1ISIGII GFP transgenic mouse E10.5 embryo (line no. 685). Panel C shows a ventral view of the AGM region. Asterisk and m indicate the positions of dorsal aorta and mesonephros, respectively. (D) Immunostaining with anti-GFP antibody confirming the TG-GFP expression (brown) in the lining of the aortic wall and in the cells ventral to the dorsal aorta (red arrow) of an E11.5 embryo (line no. 685). (E) Outline of the planar orientation of E8.0 embryo.17 (F) Confocal microscopic image of the region corresponding to the red square in panel E of line no. 798 transgenic embryo. Broken line in panel F indicates the position of the dorsal aorta. (G) Sagittal image of E9.0 embryo (line no. 798). (H-I) KI-GFP expression in the E10.5 KI-heterozygous embryo. Panel I shows the AGM region of the KI heterozygous embryo. Asterisk and m indicate the position of the dorsal aorta and mesonephros, respectively. (J) Anti-GFP antibody staining of the transverse section of the aorta of E11.5 embryo. (K) KI-GFP fluorescence along the dorsal aorta of E8.0 KI-heterozygous embryo. (L) Immunostaining of E11.5 AGM region with FITC anti–GATA-2 antibody (left), and with control rat antibody (right). (M) Immunohistochemical staining with anti–GATA-2 antibody showing endogenous GATA-2 expression in the lining of the dorsal aorta and in neighboring mesenchymal cells of an E11.5 embryo. See “Materials and methods.” Original magnification for panels D, J, and M is × 100.
We analyzed the expression profiles of GFP in 2 lines of 3.1ISIGIIGFP transgenic mice (no. 685 and no. 798). Even though the 3.1ISIGIIGFP construct exploits both IS and IG exons for GFP reporter gene expression as discussed in the next paragraph, transgenic embryos showed significantly overlapping expression to those containing the 7.0ISGFP construct.21 These results suggest that the homologous segments in the intergenic and first intron regions did not add much to the activity controlling GATA-2 expression in embryonic hematopoietic tissues.
In E10.5 transgenic mouse embryos containing 3.1ISIGIIGFP (line no. 685), GFP expression was observed in the central nervous system, fetal liver, and the aortic wall (Figure 1B, asterisk). Macroscopic observation of the E11.5 AGM region showed TG-GFP expression in the lining of the aortic wall (Figure 1C, asterisk) and in neighboring mesenchymal cells. The expression pattern of GFP in line no. 798 embryos was outwardly identical with that of no. 685 embryos (data not shown). Immunohistochemical staining with anti-GFP antibody confirmed TG-GFP expression in the lining cells of the aortic wall and neighboring mesenchymal cells of E11.5 embryos (asterisk and arrow, respectively, Figure 1D).
In E8.0 transgenic embryos, TG-GFP fluorescence was detected in the lateral plate, extra-embryonic mesoderm, and yolk sac (data not shown) but not along the dorsal aorta (Figure 1F, dotted line). The earliest appearance of TG-GFP fluorescence in the large-vessel–forming cells was in E9.0 embryos (Figure 1G) and correlated well with the potential to produce hematopoietic cells.45
Knock-in mouse containing GFP reporter inserted into GATA-2 locus
We also generated a GFP KI mutant mouse in the GATA-2 locus, replacing the endogenous GATA-2 coding sequences in the second exon (Figure 1A). We expected that in the KI mouse, GFP expression would faithfully recapitulate the endogenous expression profile of GATA-2. Green fluorescence was observed widely in various tissues of E11.5 KI mouse embryos (Figure 1H). In the AGM region, green fluorescence was observed along the aortic wall (Figure 1I, asterisk) and in the mesonephros (m). Immunostaining with anti-GFP antibody confirmed GFP expression in the lining of the aorta and in neighboring mesenchymal cells (Figure 1J, arrows). The green fluorescence was also detected in the lining of the dorsal aorta of E8.0 KI mouse embryos (Figure 1K).
Comparison of the expression profile of TG-GFP with that of KI-GFP revealed that TG-GFP expression does not recapitulate fully the expression of KI-GFP or of the endogenous GATA-2 gene. While bright fluorescence of KI-GFP was observed in the urogenital tissues of E11.5 embryos (Figure 1H-I) and in the aortic endothelial cells of E8.0 embryos, the fluorescence was not detected in the corresponding tissues of transgenic embryos (Figure 1B-C,F). These results suggest an absence in the TG construct of transcription regulatory elements that activate GATA-2 gene expression in urogenital and endothelial cells.37 The absence of GATA-2 expression in a wide range of cells is an important advantage in the identification of hematopoietic precursor cells expressing GATA-2.
We tested whether the expression of GFP coincides with the actual expression of GATA-2 by an immunochemical analysis using an anti–GATA-2 antibody (Figure 1L-M). Immunostaining with FITC-conjugated anti–GATA-2 antibody demonstrated GATA-2 expression along the dorsal aorta and in the urogenital ridge (Figure 1L, left), whereas no obvious fluorescence was observed in the AGM region stained with the control antibody (Figure 1L, right). Immunohistochemical analysis showed that the endogenous GATA-2 expression is in the cells lining the dorsal aorta and in the neighboring mesenchymal cells (Figure 1M). Thus, the profile of KI-GFP expression in the AGM region was consistent with that of endogenous GATA-2 expression.
GATA-2 expression in CD45+ and CD45- cells in the AGM region
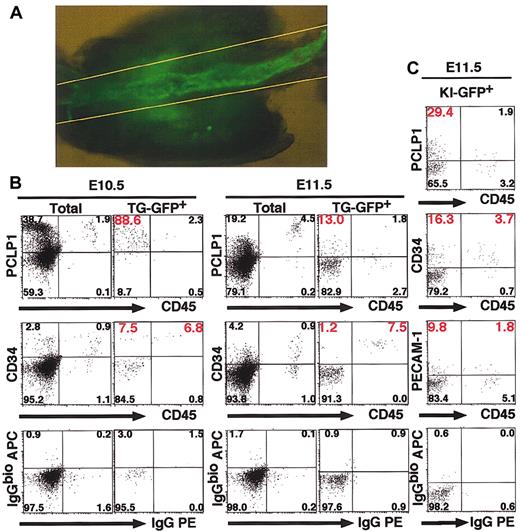
The results so far indicate that TG-GFP and endogenous GATA-2 are both expressed in the endothelial cells of the dorsal aorta and in neighboring mesenchymal cells. Since GATA-2 is expressed in bone marrow hematopoietic stem cells,46 we expected that GATA-2 would also be expressed in the hematopoietic stem cells and their precursors in the AGM region. To further characterize the TG-GFP+ cells, we dissected the AGM region between 2 yellow lines in Figure 2A of TG and KI embryos at E10.5 and E11.5. Cells were dispersed from this tissue to analyze surface markers. TG-GFP+ cells comprised 1.0% to approximately 2.5% of the total cells in the E10.5 AGM region (Table 1). CD45+/CD34+ cells, which include hematopoietic progenitors,11 were 6.8% (E10.5) and 7.5% (E11.5) of total TG-GFP+ cells in the AGM region (Figure 2B). On the other hand, CD45- cells accounted for more than 90% of the TG-GFP+ cell fraction, and significant numbers of the TG-GFP+/CD45- cells are also positive for PCLP1 or CD34 (Figure 2B; Table 1), both markers of hematogenic precursors. This is in contrast to the situation in the fetal liver, since TG-GFP+ cells are predominantly CD45+/CD34+ in the E11.5 fetal liver (data not shown).
TG-GFP+ and KI-GFP+ cells in the AGM region express CD34 and PCLP1 antigens. (A) TG-GFP expression in the AGM region of E11.5 embryo (line no. 685). The cells in the region between 2 yellow lines were analyzed. Original magnification, × 50. (B-C) Flow cytometric analysis of 3.1ISIGII GFP transgenic embryos (line no. 685, B) and KI-heterozygous embryos (C).
TG-GFP+ and KI-GFP+ cells in the AGM region express CD34 and PCLP1 antigens. (A) TG-GFP expression in the AGM region of E11.5 embryo (line no. 685). The cells in the region between 2 yellow lines were analyzed. Original magnification, × 50. (B-C) Flow cytometric analysis of 3.1ISIGII GFP transgenic embryos (line no. 685, B) and KI-heterozygous embryos (C).
Hematopoietic colonies derived from GFP-expressing cells
. | . | . | . | No. of colonies/10 000 cells . | . | |
|---|---|---|---|---|---|---|
| Samples (line no.) . | Region . | Fraction . | Percentage . | Day 4 . | Day 7 . | |
| E11.5 (685) | AGM | Total | 100 | < 5 | < 5 | |
| E11.5 (685) | AGM | TG-GFP+/CD45+ | 0.09 | 6100 | ++ | |
| E11.5 (685) | AGM | TG-GFP+/CD45- | 1.97 | 180 | 550 | |
| E11.5 (798) | AGM | Total | 100 | 0 | 15 | |
| E11.5 (798) | AGM | TG-GFP+/CD45+ | 0.09 | 4200 | 3700 | |
| E11.5 (798) | AGM | TG-GFP+/CD45- | 1.58 | 120 | 1400 | |
| E10.5 (685) | AGM | Total | 100 | 16 | 70 | |
| E10.5 (685) | AGM | TG-GFP+/CD45+ | 0.05 | 700 | 2400 | |
| E10.5 (685) | AGM | TG-GFP+/CD45- | 2.08 | 700 | 3550 | |
| E10.5 (798) | AGM | Total | 100 | 13 | 80 | |
| E10.5 (798) | AGM | TG-GFP+/CD45+ | 0.02 | 4000 | ++ | |
| E10.5 (798) | AGM | TG-GFP+/CD45- | 0.37 | 635 | ++ | |
| E9.5 (685) | P-Sp | Total | 100 | < 1 | < 1 | |
| E9.5 (685) | P-Sp | TG-GFP+/CD45+ | 0.38 | < 50 | < 50 | |
| E9.5 (685) | P-Sp | TG-GFP+/CD45- | 0.59 | 5 | 15 | |
| E9.5 (798) | P-Sp | Total | 100 | < 1 | < 1 | |
| E9.5 (798) | P-Sp | TG-GFP+/CD45+ | 0.06 | < 50 | 750 | |
| E9.5 (798) | P-Sp | TG-GFP+/CD45- | 0.46 | 65 | 235 | |
| E9.5 (685) | YS | Total | 100 | 23 | < 1 | |
| E9.5 (685) | YS | TG-GFP+/CD45+ | 0.28 | 100 | 750 | |
| E9.5 (685) | YS | TG-GFP+/CD45- | 1.77 | 10 | < 5 | |
| E9.5 (798) | YS | Total | 100 | 19 | 6 | |
| E9.5 (798) | YS | TG-GFP+/CD45+ | 0.14 | 10 | 300 | |
| E9.5 (798) | YS | TG-GFP+/CD45- | 2.30 | < 5 | < 5 | |
. | . | . | . | No. of colonies/10 000 cells . | . | |
|---|---|---|---|---|---|---|
| Samples (line no.) . | Region . | Fraction . | Percentage . | Day 4 . | Day 7 . | |
| E11.5 (685) | AGM | Total | 100 | < 5 | < 5 | |
| E11.5 (685) | AGM | TG-GFP+/CD45+ | 0.09 | 6100 | ++ | |
| E11.5 (685) | AGM | TG-GFP+/CD45- | 1.97 | 180 | 550 | |
| E11.5 (798) | AGM | Total | 100 | 0 | 15 | |
| E11.5 (798) | AGM | TG-GFP+/CD45+ | 0.09 | 4200 | 3700 | |
| E11.5 (798) | AGM | TG-GFP+/CD45- | 1.58 | 120 | 1400 | |
| E10.5 (685) | AGM | Total | 100 | 16 | 70 | |
| E10.5 (685) | AGM | TG-GFP+/CD45+ | 0.05 | 700 | 2400 | |
| E10.5 (685) | AGM | TG-GFP+/CD45- | 2.08 | 700 | 3550 | |
| E10.5 (798) | AGM | Total | 100 | 13 | 80 | |
| E10.5 (798) | AGM | TG-GFP+/CD45+ | 0.02 | 4000 | ++ | |
| E10.5 (798) | AGM | TG-GFP+/CD45- | 0.37 | 635 | ++ | |
| E9.5 (685) | P-Sp | Total | 100 | < 1 | < 1 | |
| E9.5 (685) | P-Sp | TG-GFP+/CD45+ | 0.38 | < 50 | < 50 | |
| E9.5 (685) | P-Sp | TG-GFP+/CD45- | 0.59 | 5 | 15 | |
| E9.5 (798) | P-Sp | Total | 100 | < 1 | < 1 | |
| E9.5 (798) | P-Sp | TG-GFP+/CD45+ | 0.06 | < 50 | 750 | |
| E9.5 (798) | P-Sp | TG-GFP+/CD45- | 0.46 | 65 | 235 | |
| E9.5 (685) | YS | Total | 100 | 23 | < 1 | |
| E9.5 (685) | YS | TG-GFP+/CD45+ | 0.28 | 100 | 750 | |
| E9.5 (685) | YS | TG-GFP+/CD45- | 1.77 | 10 | < 5 | |
| E9.5 (798) | YS | Total | 100 | 19 | 6 | |
| E9.5 (798) | YS | TG-GFP+/CD45+ | 0.14 | 10 | 300 | |
| E9.5 (798) | YS | TG-GFP+/CD45- | 2.30 | < 5 | < 5 | |
++ indicates numerous; P-Sp, para-aortic splanchno-pleura; and YS, yolk sac
We found that 9% of the cells isolated from the AGM region were KI-GFP+ (data not shown) and the KI-GFP+ fraction contained 4.4% to approximately 6.9% of CD45+ cells; in particular, 3.7% of KI-GFP+ cells were CD45+/CD34+ (Figure 2C). These results indicate that GATA-2 is expressed in the CD45+/CD34+ immature hematopoietic cells. The remaining KI-GFP+ cells were CD45- and this KI-GFP+/CD45- cell fraction contains 29.4% and 16.3% PCLP1+ and CD34+ cells, respectively (Figure 2C). These marker profiles of KI-GFP+ cells are consistent with those of TG-GFP+ cells. The existence of CD45-/CD34+ and CD45-/PCLP1+ cells in both the TG-GFP+ and KI-GFP+ cell fractions suggests that the TG-GFP+/CD45- cell fractions of the AGM region may contain hematogenic cells, whereas the CD45+ cell fraction represents hematopoietic progenitors.
Hematogenic cells in the TG-GFP+ cell fraction
To test the hematogenic activity of TG-GFP+/CD45- cells in the AGM region, we sorted TG-GFP+ cells from the AGM region of E11.5 transgenic embryos (Figure 3A) and cultured the cells with OP-9 stromal cells.13 As expected, TG-GFP+/CD45- cells in the AGM region contained a significant number of hematopoietic colony-forming cells (Table 1; Figure 3C). Although the TG-GFP+/CD45+ cell fraction contained many hematopoietic progenitors (Figure 3B), the low percentage of TG-GFP+/CD45+ cells in the E11.5 AGM region (Table 1) precludes the possibility that contaminating CD45+ cells might be the source of the hematopoietic colonies in the TG-GFP+/CD45- fraction.
TG-GFP+ cells from the AGM region have hematogenic activity. (A) TG-GFP+/CD45+ and TG-GFP+/CD45- cell fractions of the AGM region were sorted by FACS. (B-C) Phase-contrast microscopy analysis of hematopoietic colonies with a cobblestone-like appearance after 4 days of coculture with OP-9 cells. Cells in panel B are from the TG-GFP+/CD45+ fraction, while cells in panel C are from the TG-GFP+/CD45- fraction. The number of colony-forming cells is shown in Table 1. (D) Appearance of TG-GFP+/CD45- cell colonies. TG-GFP+/CD45- cells were cultured in medium containing OSM, SCF, b-FGF, and LIF. Note that colonies contain both adherent cells (dark cells) and floating cells (bright cells). (E) Flow cytometric analysis of the cells in panel D. Majority of the cells show abundant CD45 expression without GFP fluorescence. Original magnification, panels B-D, × 100.
TG-GFP+ cells from the AGM region have hematogenic activity. (A) TG-GFP+/CD45+ and TG-GFP+/CD45- cell fractions of the AGM region were sorted by FACS. (B-C) Phase-contrast microscopy analysis of hematopoietic colonies with a cobblestone-like appearance after 4 days of coculture with OP-9 cells. Cells in panel B are from the TG-GFP+/CD45+ fraction, while cells in panel C are from the TG-GFP+/CD45- fraction. The number of colony-forming cells is shown in Table 1. (D) Appearance of TG-GFP+/CD45- cell colonies. TG-GFP+/CD45- cells were cultured in medium containing OSM, SCF, b-FGF, and LIF. Note that colonies contain both adherent cells (dark cells) and floating cells (bright cells). (E) Flow cytometric analysis of the cells in panel D. Majority of the cells show abundant CD45 expression without GFP fluorescence. Original magnification, panels B-D, × 100.
It is reasonable to suppose that OP-9 stromal cells provide significantly different conditions than do the stromal cells in the AGM region. The latter augments hematogenesis, whereas the former mainly supports hematopoietic progenitor activity through the activity of secreted growth factors and/or interacting molecules.47 Therefore, we cultured the sorted TG-GFP+/CD45- cells by supplementing with LIF, SCF, b-FGF, and OSM, according to the previously described method,40 to test the hematogenic potential of the AGM region. In this culture, the sorted cells were first polygonal in shape, plastic adherent, and proliferated vigorously. The cells then gradually detached from the dish surface and started to express CD45 antigen and to lose GATA-2/TG-GFP expression (Figure 3D-E). These results indicate that GATA-2 is expressed in hematogenic cells in the AGM region, which are recovered in the TG-GFP+/CD45- fraction. After culture, only 0.5% of the cells were TG-GFP+, and the remaining cells lost TG-GFP expression during differentiation to CD45+ cells (Figure 3E).
Coordinate expression of transcription factors in AGM cells
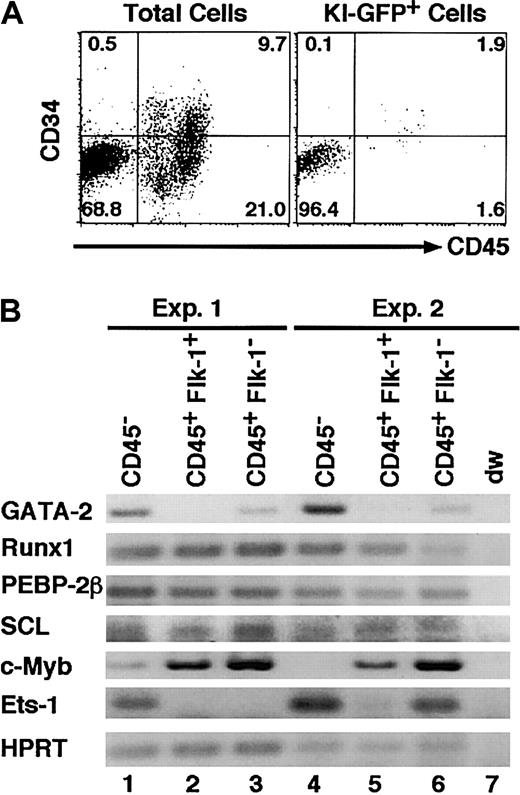
To elucidate whether the disappearance of TG-GFP truly reflects declining GATA-2 gene expression, we examined KI-GFP and GATA-2 mRNA expression in primary cultures of AGM region–derived cells. Expression of KI-GFP was detected in 8.6% of the cultured cells (data not shown), and 96.5% of the KI-GFP+ cells were CD45- (Figure 4A) and plastic adherent. RT-PCR analysis revealed that the GATA-2 mRNA level was high in the CD45- fraction but low in the CD45+ fraction (Figure 4B). This observation is consistent with expression of TG-GFP and KI-GFP (Figure 2). Reduced GATA-2 mRNA expression was accompanied by an increment in c-myb expression and the regression of ets-1 expression (Figure 4B), indicating that the expression of several transcription factors changes coordinately during the hematogenic cell differentiation. These results also suggest that GATA-2 plays an important role in early hematogenesis.
GATA-2 is expressed in CD45- cells in the primary cultured AGM region. (A) Flow cytometric analysis of 7-day–cultured AGM cells of KI-heterozygous embryos. (B) RT-PCR analysis of the cells in each sorted fraction from the primary culture of the wild-type AGM cells. Representative results of 2 of 4 mRNA analyses are shown.
GATA-2 is expressed in CD45- cells in the primary cultured AGM region. (A) Flow cytometric analysis of 7-day–cultured AGM cells of KI-heterozygous embryos. (B) RT-PCR analysis of the cells in each sorted fraction from the primary culture of the wild-type AGM cells. Representative results of 2 of 4 mRNA analyses are shown.
GATA-2 inhibits differentiation of hematogenic cells
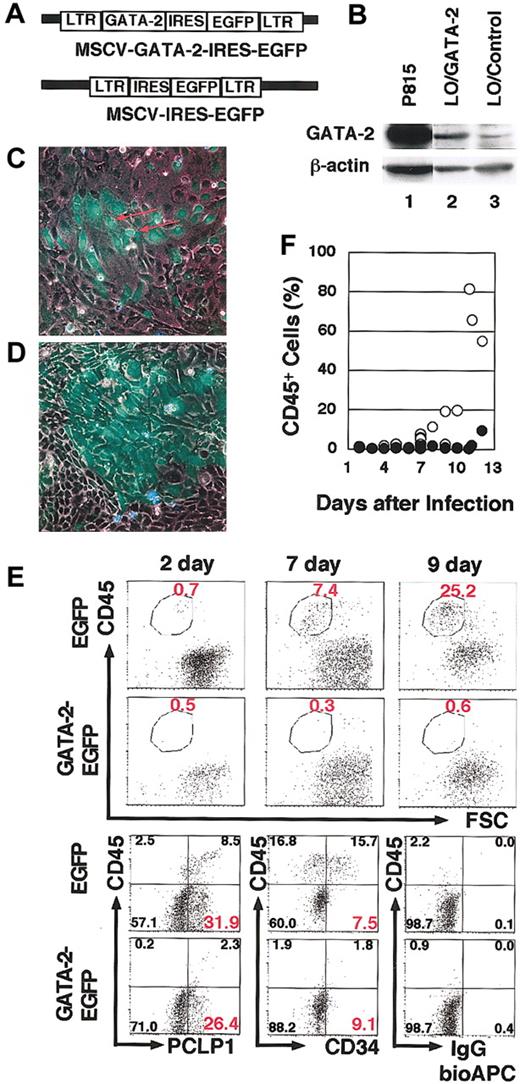
To assess the possibility that reduced GATA-2 expression is a prerequisite for the start of hematogenesis, we exogenously expressed GATA-2 by infecting cultured AGM cells with the MSCV–GATA-2–IRES-EGFP virus, which induces simultaneous expression of GATA-2 and EGFP (Figure 5A). Western blotting and RT-PCR analysis of the infected cells showed modest GATA-2 expression (Figure 5B). MSCV-IRES-EGFP virus, which induced EGFP expression but not GATA-2 expression, was used as a negative control in these experiments.
Retroviral expression of GATA-2 in hematogenic cells suppresses hematopoietic cell production. (A) Structure of the MSCV–GATA-2–IRES-EGFP and MSCV-IRES-EGFP retrovirus vectors. (B) Western blotting analysis showing modest GATA-2 expression in LO cells8 infected with the MSCV–GATA-2–IRES-EGFP virus (lane 2) and low level endogenous GATA-2 expression in the cells infected with MSCV-IRES-EGFP virus (lane 3). P815 mast cells were used as a positive control for this experiment (lanes 1). (C-D) Fluorescent microscopy analysis of V-GFP+ cells 9 days after culture. Cells infected by MSCV-IRES-EGFP (C) and MSCV–GATA-2–IRES-EGFP (D) are shown. The red arrow in panel C indicates the round-shaped V-GFP+ cells detached from the dishes. (E) Surface-marker profile of V-GFP+ cells infected with MSCV–GATA-2–IRES-EGFP virus or MSCV-IRES-EGFP virus. At day 7, 5.6% or 3.7% of cells were infected by MSCV–GATA-2–IRES-EGFP V-GFP+ virus or MSCV-IRES-EGFP virus, respectively. The bottom 3 panels demonstrate the CD34 and PCLP1 expression on the V-GFP+ cells at day 7. (F) The percentage of CD45+ cells. CD45+ cells in the fraction infected with either MSCV–GATA-2–IRES-EGFP virus (•) or MSCV-IRES-EGFP virus (○) in primary AGM culture. Original magnification, panels C-D, × 100.
Retroviral expression of GATA-2 in hematogenic cells suppresses hematopoietic cell production. (A) Structure of the MSCV–GATA-2–IRES-EGFP and MSCV-IRES-EGFP retrovirus vectors. (B) Western blotting analysis showing modest GATA-2 expression in LO cells8 infected with the MSCV–GATA-2–IRES-EGFP virus (lane 2) and low level endogenous GATA-2 expression in the cells infected with MSCV-IRES-EGFP virus (lane 3). P815 mast cells were used as a positive control for this experiment (lanes 1). (C-D) Fluorescent microscopy analysis of V-GFP+ cells 9 days after culture. Cells infected by MSCV-IRES-EGFP (C) and MSCV–GATA-2–IRES-EGFP (D) are shown. The red arrow in panel C indicates the round-shaped V-GFP+ cells detached from the dishes. (E) Surface-marker profile of V-GFP+ cells infected with MSCV–GATA-2–IRES-EGFP virus or MSCV-IRES-EGFP virus. At day 7, 5.6% or 3.7% of cells were infected by MSCV–GATA-2–IRES-EGFP V-GFP+ virus or MSCV-IRES-EGFP virus, respectively. The bottom 3 panels demonstrate the CD34 and PCLP1 expression on the V-GFP+ cells at day 7. (F) The percentage of CD45+ cells. CD45+ cells in the fraction infected with either MSCV–GATA-2–IRES-EGFP virus (•) or MSCV-IRES-EGFP virus (○) in primary AGM culture. Original magnification, panels C-D, × 100.
On day 1 or 2 of culture, adherent AGM cells were infected with each virus. The infected cells remained adherent and proliferated for several days. After 6 to 9 days in the dishes infected with MSCV-IRES-EGFP virus, round cells showing virus-derived GFP (V-GFP) fluorescence detached from the dishes (Figure 5C). In contrast, we did not find such V-GFP+/CD45+ cells in the dishes infected with the MSCV–GATA-2–IRES-EGFP virus (Figure 5D). Instead, there were patches of proliferating V-GFP+/CD45- cells, which were morphologically identical to the surrounding adherent cells. The CD45 expression profile of V-GFP+–infected cells was then examined (Figure 5E, top). Flow cytometric analysis revealed the existence of V-GFP+/CD45+ cells in the dishes infected with MSCV-IRES-EGFP virus, while the numbers of V-GFP+/CD45+ cells were substantially reduced in the dishes infected with the MSCV–GATA-2–IRES-EGFP virus. In contrast, the expression of PCLP1 and CD34 markers in V-GFP+/CD45- cells infected with MSCV–GATA-2–IRES-EGFP virus was comparable to that in the V-GFP+/CD45- cells infected with MSCV-IRES-EGFP virus (Figure 5E, bottom).
Noninfected cells in both culture dishes produced comparable numbers of CD45+ cells, and no significant difference was found between the dishes infected with the MSCV-IRES-EGFP virus and the MSCV–GATA-2–IRES-EGFP virus either in the percentages of V-GFP+ cells or in the cellular morphologies of the cultures (data not shown). When cells in the late stage of culture, which contained CD45+ cells, were infected with GATA-2–expressing virus, the production of hematopoietic cells was not impaired (data not shown). These results suggest that GATA-2 acts to preserve the immaturity of infected cells and the inhibitory effect of GATA-2 is functional in the early hematogenic precursors, not in hematopoietic progenitors. Hence, TG-GFP fluorescence recapitulated GATA-2 expression in hematogenic precursors, and GATA-2 overexpression in these hematogenic precursor cells inhibited their differentiation into hematopoietic cells.
Accelerated production of CD45+ cells in the yolk sacs of mice with a disrupted GATA-2 gene
One plausible explanation for this GATA-2 function during early hematogenesis is that it acts to inhibit differentiation, thereby maintaining a proper number of immature cells in developing embryos. To ascertain whether the inhibitory role of GATA-2 is important for hematogenesis or not, we decided to decrease the expression level of GATA-2. To this end, we exploited both KI-homozygous and KI-heterozygous mutant mouse embryos because the insertion of GFP disrupted the GATA-2 gene in these mice. An expected scenario here is that, if the inhibitory effect of GATA-2 on hematogenesis plays an essential role for the maintenance of a proper number of immature hematogenic cells, impairment of GATA-2 expression would enhance the differentiation of hematogenic cells in early embryos, thereby causing depletion of hematogenic precursors.
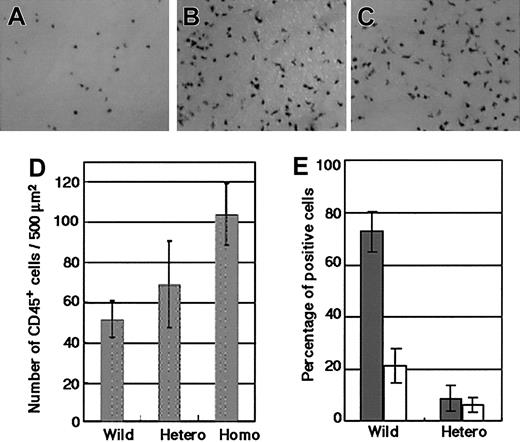
Although the details of yolk sac hematopoiesis are still unclear,45 definitive hematopoiesis is known to commence in the yolk sac 1 day earlier than it does in the AGM region.9,48 Because embryos homozygous for the GATA-2 KI allele die between E9.5 and E11.5, which is the midpoint of AGM hematopoiesis, we analyzed yolk sac–definitive hematopoiesis. Consistent with the KI-GFP expression in the AGM region (Figure 2; data not shown), the expression of KI-GFP in the heterozygous yolk sac correlated with the expression of c-kit and CD34 (data not shown). Immunohistochemical analysis with anti-CD45 antibody of E9.5 yolk sacs of wild-type, KI-heterozygous, and KI-homozygous conceptuses having 27 to 31 somites, showed significantly increased numbers of CD45+ cells in homozygous mutants (Figure 6A-C).
GATA-2 gene dosage has an influence on preservation of hematogenic precursor cells. (A-C) Immunohistochemical analysis of the E9.5 yolk sacs of a wild-type (A), KI-heterozygous (B), and KI-homozygous (C) conceptuses with CD45 antibody. These embryos have 27 to 31 somites. A significantly increased number of CD45+ cells were observed in the homozygous mutants. Original magnification, panels A-C, × 100. (D) The number of CD45+ cells/500 μm2of wild-type, KI-homozygous (4 embryos each), and KI-heterozygous (3 embryos) yolk sacs are presented as the mean ± standard deviation. (E) Hematopoietic cell production of AGM cells from 3 E11.5 KI-heterozygous mutant embryos and their wild-type littermates. The percentages of CD45+ (▦) and CD34+ (□) cells harvested on day 10 of culture are plotted.
GATA-2 gene dosage has an influence on preservation of hematogenic precursor cells. (A-C) Immunohistochemical analysis of the E9.5 yolk sacs of a wild-type (A), KI-heterozygous (B), and KI-homozygous (C) conceptuses with CD45 antibody. These embryos have 27 to 31 somites. A significantly increased number of CD45+ cells were observed in the homozygous mutants. Original magnification, panels A-C, × 100. (D) The number of CD45+ cells/500 μm2of wild-type, KI-homozygous (4 embryos each), and KI-heterozygous (3 embryos) yolk sacs are presented as the mean ± standard deviation. (E) Hematopoietic cell production of AGM cells from 3 E11.5 KI-heterozygous mutant embryos and their wild-type littermates. The percentages of CD45+ (▦) and CD34+ (□) cells harvested on day 10 of culture are plotted.
When the CD45+ cells were counted, the numbers in KI-homozygous yolk sacs were significantly increased compared with the yolk sac of the wild-type conceptuses at a similar stage of development (Figure 6D). The numbers of CD45+ cells in KI-heterozygous conceptuses were also slightly increased. In contrast, the increment in CD45+ cells was not observed in the KI-homozygous yolk sac of the conceptus with a greater number of somites. Flow cytometric analyses revealed that the percentages of CD45+ cells were comparable in wild-type (6.92% and 7.83%), heterozygous (4.30% and 5.59%), and homozygous (2.53% and 5.59%) yolk sacs. The number of CD45low cells was significantly lower in the GATA-2–null yolk sacs (0.24% and 0.60%) compared with that in the wild-type (2.86% and 2.66%) and heterozygous (1.18% and 0.60%) yolk sacs. These CD45low cells were c-kit+/KI-GFP+ and supposed to be at the transition stage from CD45- to CD45+ cells. One plausible interpretation of this data is that the lack of GATA-2 expression enhanced the first-wave emergence of CD45+ cells in the yolk sac, resulting in depletion of their precursor cells. CD45+ and CD34+ cell production was also impaired in the cultures of E11.5 AGM cells of KI-heterozygous mutants (Figure 6E). These results suggest that CD45- precursors may be depleted in the AGM region of these heterozygous embryos, consistent with GATA-2 acting to preserve the pool of immature cells.
The inhibitory function of GATA-2 resides in a nonfinger domain
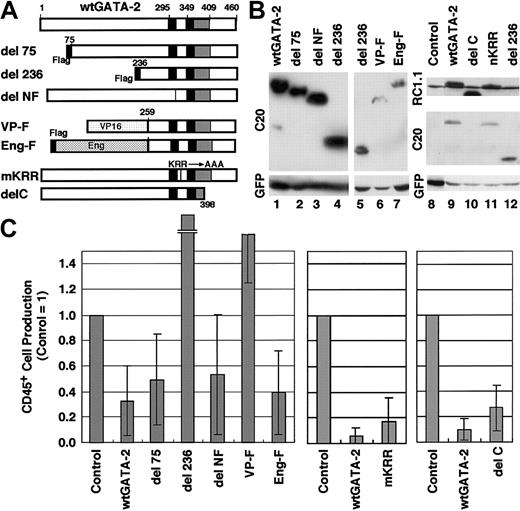
We next attempted to determine the domain of GATA-2 that is responsible for the suppression of hematopoietic cell production. To this end, we created various GATA-2 mutant cDNA constructs (Figure 7A) and these cDNAs were integrated into a retrovirus vector. These retroviruses were then infected into cells in AGM primary cultures. The expression levels of these mutant GATA-2 proteins were shown in Figure 7B. For the immunoblot analyses, we used both anti–GATA-2 polyclonal antibody C-20 and monoclonal antibody RC1.1.1. to compensate for the possible loss of epitopes in the deletion/mutation analysis. Most of these viruses induced the expression of GATA-2 mutant proteins at levels comparable to that of wild-type GATA-2.
The function of GATA-2 is domain-specific in primary cultures of AGM cells. (A) Schematic representation of the GATA-2 domain mutants integrated into the genome of MSCV-IRES-EGFP virus. (B) Western blot analysis of infected LO cells with anti–GATA-2 antibodies, C-20 and RC.1.1.1, and anti-GFP antibody. Note comparable expression of each mutant protein. (C) Numbers of CD45+ cells in cultures of AGM cells infected with each virus. Results are shown relative to control cultures. Each point and bar represents the mean ± standard deviation of 3 independent experiments. The results of del236 and VP-F are 5.21 ± 3.33 and 1.99 ± 0.72, respectively; and del236 and VP-F resulted in significantly higher numbers of CD45+ cells than wild-type GATA-2 (P < .05, Student t test). The significance of the differences was confirmed by repeated experiments (wild-type del 236, n = 12, P < .0001; wild-type VP, n = 7, P < .0001; Student t test).
The function of GATA-2 is domain-specific in primary cultures of AGM cells. (A) Schematic representation of the GATA-2 domain mutants integrated into the genome of MSCV-IRES-EGFP virus. (B) Western blot analysis of infected LO cells with anti–GATA-2 antibodies, C-20 and RC.1.1.1, and anti-GFP antibody. Note comparable expression of each mutant protein. (C) Numbers of CD45+ cells in cultures of AGM cells infected with each virus. Results are shown relative to control cultures. Each point and bar represents the mean ± standard deviation of 3 independent experiments. The results of del236 and VP-F are 5.21 ± 3.33 and 1.99 ± 0.72, respectively; and del236 and VP-F resulted in significantly higher numbers of CD45+ cells than wild-type GATA-2 (P < .05, Student t test). The significance of the differences was confirmed by repeated experiments (wild-type del 236, n = 12, P < .0001; wild-type VP, n = 7, P < .0001; Student t test).
Overexpression of wild-type GATA-2 significantly repressed the production of CD45+ cells in the primary culture system of AGM cells. An important finding here is that the Flag236 mutant did not show inhibitory activity but markedly enhanced the production of CD45+ cells compared with the control virus infection (Figure 7C). In contrast, neither N-terminus (Flag75) nor N-finger (delNF) deletion mutants affected the inhibitory activity of GATA-2. Therefore, the inhibitory function of GATA-2 in CD45+ cell production from primary AGM cultures resides in a domain extending from amino acid residues 75 to 235. This negative regulatory domain has no apparent homology to GATA-1, -4, -5, or -6 but has 46% homology to GATA-3, and the corresponding region of GATA-3 has been reported as a transcription activation domain.49
To further elucidate the activity of this regulatory domain, we constructed 2 additional mutant cDNAs. VP-F mutant is an activation mutant of GATA-2, in which the activation domain of VP-1642 replaces the N-terminal 236 amino acid residues of GATA-2, whereas Eng-F mutant is a repressor mutant, in which the repressor domain of engrailed43 is fused to the 2 zinc finger domains of GATA-2 (Figure 7A). The former (activating) mutant did not suppress the production of CD45+ cells, whereas the latter (repressor) mutant showed inhibitory activity of GATA-2 comparable to that of wild-type GATA-2 (Figure 7C). Thus, the inhibition of CD45+ cell production appears not to be mediated by a transcriptional activation of the target genes, but by a transcription repression activity within a nonfinger domain of GATA-2.
We also tested 2 additional GATA-2 mutant proteins in this system. The mKRR mutant lacks the putative acetylation sites50 and the delC mutant lacks the C-terminal 66 amino acid residues (Figure 7A). Both mutants were found to retain inhibitory activity similar to that of the wild-type GATA-2 (Figure 7C), indicating that these regions are not crucial for the inhibitory activity of GATA-2. These results suggest that the negative regulatory domain (extending from amino acid residues 74 to 235) is primarily responsible for the inhibitory regulation of CD45+ hematopoietic cell production.
Discussion
The transgenic mouse lines containing the GFP reporter gene under the influence of the GATA-2 hematopoietic regulatory domain enabled us to identify GATA-2 gene expression in hematogenic cells in the mouse AGM region and unveiled a new function for the GATA-2 nonfinger domain. This study thus demonstrates that transgenic mice containing specific gene-regulatory regions are powerful tools for marking specific lineages of cells. Indeed, we detected TG-GFP expression predominantly in the hematogenic cells, while KI-GFP and endogenous GATA-2 expression was observed in a broad range of cells in the AGM region (Figure 1). If we had marked cells using an approach that more comprehensively recapitulates endogenous GATA-2 expression, such as the GFP knock-in mouse approach, a large number of GATA-2–expressing cells with diverse characteristics would have been recovered in the KI-GFP+ cell fraction and this recovery would have obscured the results of the sorting and culture experiments. In addition, since we found hematopoietic haploinsufficiency in the GFP knock-in embryos (Figure 6), TG-GFP+ cells also have an advantage in this respect over the KI-GFP+ cells when monitoring the appearance and fate of the GATA-2–expressing cells.
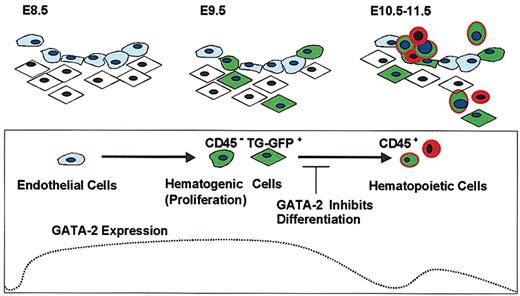
We propose that the expression level of GATA-2 is high in the cells with hematogenic potential, decreases upon the start of differentiation, and is up-regulated again after the cells have acquired characteristics of hematopoietic stem/progenitor cells. This expression profile of GATA-2 is summarized in Figure 8. Supporting this model, we have identified a TG-GFP+/CD45- hematogenic precursor fraction in the dorsal aorta and surrounding tissues. However, we found that TG-GFP expression is decreased after differentiation of TG-GFP+/CD45- hematogenic cells into CD45+ hematopoietic cells in primary culture. We also found that TG-GFP (and KI-GFP) is expressed in CD45+/CD34+ cells in the liver of E11.5 embryos, and expression of GATA-2 in bone marrow stem cells has been reported.46
GATA-2 functions as a gatekeeper in hematogenic precursor cells. GATA-2 is expressed in endothelial cells in the early stage of angiogenesis, and TG-GFP specifically recapitulated GATA-2 expression in endothelial and/or mesenchymal cells with the potential to undergo hematopoietic differentiation. The high-level expression of GATA-2 in hematogenic precursor cells blocks subsequent differentiation of the cells into hematopoietic lineages. Cells with a light-blue cytoplasm represent endothelial cells. Cells with rhomboid shape and spheroid shape represent mesenchymal and hematopoietic cells, respectively. The blue nuclei and the green cytoplasm represent the cells expressing GATA-2 and TG-GFP, respectively.
GATA-2 functions as a gatekeeper in hematogenic precursor cells. GATA-2 is expressed in endothelial cells in the early stage of angiogenesis, and TG-GFP specifically recapitulated GATA-2 expression in endothelial and/or mesenchymal cells with the potential to undergo hematopoietic differentiation. The high-level expression of GATA-2 in hematogenic precursor cells blocks subsequent differentiation of the cells into hematopoietic lineages. Cells with a light-blue cytoplasm represent endothelial cells. Cells with rhomboid shape and spheroid shape represent mesenchymal and hematopoietic cells, respectively. The blue nuclei and the green cytoplasm represent the cells expressing GATA-2 and TG-GFP, respectively.
We suggest that GATA-2 is expressed in hematogenic cells and inhibits their differentiation, thereby preventing the unregulated differentiation of the precursor cells and preserving the pool of immature cells. In the present study, retroviral overexpression of GATA-2 suppressed the differentiation of CD45- hematogenic cells into hematopoietic cells. The number of CD45+ cells was increased in the E9.5 yolk sacs of KI-homozygous conceptuses (Figure 6D). This may be due to the accumulation of CD45+ hematopoietic cells in the early-stage yolk sacs, since loss of GATA-2 expression is supposed to enhance the differentiation of hematogenic cells. However, the early-stage yolk sac might not provide a suitable environment for the production of hematopoietic stem cells.45 By this means, therefore, the hematogenic cells and immature hematopoietic cells would be finally depleted in the later stages of development. Consistent with this contention, the increment of CD45+ cells was not observed in the KI-homozygous yolk sac at an advanced stage of development (data not shown). We also found that hematopoietic cell production was reduced in cultures of E11.5 AGM cells from KI-heterozygous mutant embryos (Figure 6E).
GATA-2 gene disruption experiments have shown the profound reduction in blast/mixed colonies in GATA-2-/- yolk sacs.19,20 These data suggested that the function of GATA-2 is to expand immature cell populations in the definitive hematopoietic system. Our present result is consistent with the previous reports19,20 and extends the analysis to the function of GATA-2 in early hematogenic cells. Retrovirally infected, GATA-2–expressing cells from 5-FU–treated bone marrow did not proliferate, did not show colony-forming activity, and did not repopulate the transplanted bone marrow.33 This suppression of hematopoietic cell production by GATA-2 may have some similarities to the function of GATA-2 in hematogenic cells in the AGM region, although immature hematogenic cells (or hemangioblasts) in the AGM region are different entities from hematopoietic stem cells in the bone marrow.51
We found a new nonfinger domain of GATA-2 that acts negatively in the differentiation of CD45- hematogenic precursors to hematopoietic cells. We obtained evidence suggesting that this differentiation block is due to a transcriptional repression activity of GATA-2, since the engrailed-repressor–GATA-2–finger fusion protein can block production of hematopoietic cells from CD45- cells but the VP16-activator–GATA-2 fusion cannot (Figure 7). This demonstrates that in hematogenesis there is an indispensable role for GATA-2 as a transcriptional repressor. The Flag236 mutant without this regulatory domain largely enhanced the production of hematopoietic cells (Figure 7), and this suggests the presence of other molecular interactions of the GATA-2 zinc finger domain that enhance hematopoietic cell production.
In contrast to the present study, an engrailed-repressor–GATA-2–finger fusion protein showed an activity opposite to wild-type GATA-2 in RNA injection experiments in Xenopus embryos in that the fusion protein induced dorsalization of the embryos.52 Whereas the engrailed–GATA-2 fusion protein differs from ours at the fusion site in the engrailed repressor domain, the Xenopus study and our result in combination suggest that GATA-2 acts either as an activator or a repressor, depending on context. It is still unknown, however, which function of GATA-2 this fusion protein abrogates in Xenopus embryos, and further studies are necessary to validate this contention.
GATA factors are expressed in an overlapping manner in various tissues,27-29,53,54 and there are many factors that reportedly interact with GATA factors, altering the transcriptional activity from repression to activation and vice versa.55-58 In the embryonic stem (ES) cell differentiation system cocultured with OP-9 stroma cells, GATA-2 overexpression induced persistent proliferation of hematopoietic cells.59 While GATA-2 overexpression in this ES cell system also enhanced hematopoietic cell differentiation,59 these results reflect GATA-2 functions in committed hematopoietic cells rather than in the hematogenic precursor cells we focused on in this study (Figure 8). Thus, we believe that the function and molecular mechanisms of GATA-2 should be investigated using cells in which the factor is expressed on physiologic levels.
To date, several transcription factors regulating the development of the hematopoietic system are known, including SCL,16,60 Runx1,4,5,14 c-Myb,18,61 and GATA-2.19,20 Intriguing observations include the following. (1) The expression profiles of SCL and Runx1 appear to be closely related to that of GATA-2, whereas c-Myb expression is a mirror image of GATA-2 expression being expressed in hematopoietic cells (Figure 4B).14,16,18 (2) GATA-2 is reported to regulate the expression of SCL,26 but GATA-2 expression appears to be independent of Runx1 regulation, as TG-GFP fluorescence is detected in the aortic region of E11.5 Runx1-/- embryos (N.M., unpublished observation, July 2, 2002). (3) GATA-2 inhibits the transactivation activity of c-Myb through a direct interaction,58 and c-Myb activity is required to produce CD45+ cells.18 These observations further support our contention that GATA-2 regulates the proliferation and differentiation of hematogenic precursor cells.
The multipotential of hematogenic cells/hematopoietic stem cells raises the possibility that they might be used to facilitate the regeneration of multiple organs.62 GATA-2 has a distinct function in the development and maintenance of hematopoietic stem cells. We expect that our current transgenic and knock-in mice will serve as useful tools to solve these mechanisms' involvement.
Prepublished online as Blood First Edition Paper, April 10, 2003; DOI 10.1182/blood-2002-12-3809.
Supported by grants-in-aid from The Ministry of Education, Science, Sports and Culture of Japan (N.M., M.Y.), JSPS-RFTF (M.Y.), ERATO (M.Y.), PROBRAIN (S.T.), and the Naito Foundation (M.Y.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Mr Hironori Yamagiwa for help in the generation of transgenic mouse lines, and Drs Akihiro Kume and Nobuyuki Takakura for help in the retroviral study and for providing the anti-Tie2 antibody, respectively. We also thank Drs Atsushi Iwama and Mitsujiro Osawa for construction of the GATA-2 mutant genes and Makoto Kobayashi for valuable discussion.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal