Abstract
To determine if megakaryocytes are targeted by immune thrombocytopenic purpura (ITP) autoantibodies, as are platelets, we have studied the effects of ITP plasma on in vitro megakaryocytopoiesis. Umbilical cord blood mononuclear cells were incubated in the presence of thrombopoietin and 10% plasma from either ITP patients (n = 53) or healthy donors. The yield of megakaryocytic cells, as determined by flow cytometry, was significantly reduced in the presence of ITP plasma containing antiplatelet glycoprotein Ib (GPIb) autoantibodies (P < .001) as compared with both the control and patient plasma with no detectable anti-GPIIb/IIIa or anti-GPIb autoantibodies. Platelet absorption of anti-GPIb autoantibodies in ITP plasmas resulted in double the megakaryocyte production of the same plasmas without absorption, whereas platelet absorption of control plasma had no effect on megakaryocyte yield. Furthermore, 2 human monoclonal autoantibodies isolated from ITP patients, 2E7, specific for human platelet glycoprotein IIb heavy chain, and 5E5, specific for a neoantigen on glycoprotein IIIa expressed on activated platelets, had significant inhibitory effects on in vitro megakaryocytopoiesis (P < .001). Taken together, these data indicate that autoantibodies against either platelet GPIb or platelet GPIIb/IIIa in ITP plasma not only are involved in platelet destruction, but may also contribute to the inhibition of platelet production.
Introduction
Platelets play a central role in the maintenance of normal hemostasis and vascular repair. Decreased platelet numbers (thrombocytopenia) can result in bruising, petechiae, or even life-threatening bleeding. Immune-mediated thrombocytopenic purpura (ITP) is one of the most common forms of autoimmune disease affecting both adults and children.1-3 The thrombocytopenia of this disorder is associated with autoantibodies that are directed against various platelet membrane receptors, including platelet glycoproteins such as glycoprotein IIb/IIIa (GPIIb/IIIa) or GPIb/IX complexes.4-7 Binding of autoantibodies to these target antigens eventually results in platelet destruction by the reticuloendothelial system.1,2
Since the target antigens of these autoantibodies are present on both platelets and their precursors, megakaryocytes, it is possible that megakaryocytopoiesis and thrombopoiesis are also impaired during ITP,8-10 which could further aggravate the thrombocytopenia caused initially by increased peripheral destruction of platelets. This hypothesis has been supported by immmunoreactivity of ITP sera/plasma or isolated immunoglobulin with either purified human megakaryocytes or megakaryocytes in the marrow of several ITP animal models.11-13 Furthermore, morphologic alterations of ITP marrow megakaryocytes also support the hypothesis that megakaryocytopoiesis and thrombopoiesis may be disrupted in ITP.14-16 Various ultrastructural abnormalities of ITP megakaryocytes, including vacuoles, markedly distended demarcation membrane systems, mitochondrial swelling, and the emperipolesis of other marrow cells, have been previously reported.10,14-17 A substantial number of megakaryocytes, particularly those that had reached the stage of thrombocytopoiesis,10,17 had this kind of extensive damage, causing a compensatory increase in more immature progenitors.17
Although these observations suggest that autoantibodies in ITP plasma may affect megakaryocytopoiesis, very little has been reported demonstrating a direct effect of ITP plasma or purified ITP monoclonal autoantibodies on in vitro megakaryocytopoiesis. In this study, ITP plasma samples were collected and classified into different subgroups according to the presence or absence of GPIIb/IIIa or GPIb autoantibodies. Their effect on in vitro megakaryocytopoiesis was determined with the use of a liquid megakaryocytic culture system, which takes advantage of the recent availability of recombinant human thrombopoietin (rhTPO),18-20 as well as umbilical cord blood–derived stem and progenitor cells.21 The effect of human monoclonal antiplatelet glycoprotein autoantibodies isolated from ITP patients on in vitro megakaryocytopoiesis is also described.
Patients, materials, and methods
ITP patient and control plasma samples
Fifty-three ITP plasma samples from 53 randomly selected ITP patients were collected for analysis. None of the ITP patients had been pregnant or had received blood or platelet transfusion before the plasma samples were collected. All ITP patients were treated at Children's Hospital of Orange County (CHOC), Orange, CA. Platelet-poor plasma was prepared by centrifuging citrated whole blood (820g) at room temperature for 15 minutes. Fresh plasma was assayed for GPIIb/IIIa or GPIb antiplatelet autoantibody of either immunoglobulin G (IgG) or IgM isotype, by means of the enzyme-linked immunosorbent assay (ELISA), and the remainder stored frozen in aliquots at -80°C for later study.
Antibodies
Fluorescein isothiocyanate (FITC)–labeled murine antihuman platelet GPllb/IIIa complex (CD41, clone P2) monoclonal antibody (mAb) and FITC-labeled F(ab′)2 goat antimurine IgG were purchased from Beckman Coulter (Fullerton, CA). FITC-labeled murine IgG1 (antimurine keyhole limpet hemocyanin) isotype control was from Becton Dickinson Immunocytometry Systems (San Jose, CA). Murine anti-CD41 mAb (clone 5B12) was from DAKO (Carpinteria, CA). Murine IgG1,κ (MOPC-21) or IgG2a,κ (UPC-10) isotype controls; peroxidase-F(ab′)2 goat antihuman IgM (μ-chain specific); and purified human IgM were from Sigma Chemical (St Louis, MO). Peroxidase-F(ab′)2 goat antihuman IgG (γ-chain specific) was from Zymed Laboratories (South San Francisco, CA). Murine antihuman platelet GPIbα (CD42b, clone HIP1) mAb was from BD PharMingen (San Diego, CA). Murine antihuman platelet GPIb-IX complex (CD42, clone C7E10)22,23 mAb was produced as described previously.23 Murine antihuman platelet GPIIIa (CD61, clone AP5)24 mAb was generously provided by Dr T. J. Kunicki (Scripps Research Institute, La Jolla, CA).
Platelet lysate
Platelet-rich plasma (1 to 10 × 1012 platelets in 500 mL, containing 13 mM citrate) was obtained from the blood bank at St Joseph's Hospital of Orange, CA. Platelet pellets containing prostaglandin E1 (PGE1) (20 ng/mL) were isolated as previously described25 and then disrupted in lysis buffer (20 mM Tris-HCl [tris(hydroxymethyl)aminomethane–HCl], 100 mM NaCl, 1% Triton X-100, 10 mM N-ethylmaleimide, 1 μM leupeptin, 10 mM benzamidine, 2 mM phenylmethylsulfonyl fluoride). Insoluble proteins in the lysate were removed by ultracentrifugation (27 522g) at 4°C for 90 minutes. The protein concentration of the supernatant was determined by means of the Bio-Rad Protein Assay (Bio-Rad Laboratories, Hercules, CA).
ELISA for antiplatelet IgG or IgM antibody
The ELISA was carried out as previously described by Fujisawa et al,26 with the following modifications. Briefly, microtiter plate wells were coated overnight at 4°C with 50 μL murine anti-CD42 or anti-CD61 (5 μg/mL) per well. Negative control wells were coated with murine IgG1,κ (MOPC-21) or IgG2a,κ (UPC-10). Wells for IgG assay were blocked with Superblock (Pierce Chemical, Rockford, IL) in Ca++- and Mg++-free phosphate-buffered saline (PBS) and IgM assay wells with 0.05% Tween-20 in PBS (PBST) for 45 minutes. Platelet lysates were then added to each well at 1 mg/mL in PBS and incubated at room temperature for 2 hours. Plates were washed twice with PBST. After absorption with either the murine IgG1 or murine IgG2a to prevent nonspecific reactivity with murine antibodies, control and ITP patient plasma samples were serially diluted at 1:20 to 1:1280 in either PBS (IgG) or PBST (IgM), transferred to the wells, and incubated for 2 hours. The wells were then washed 3 times with PBST and incubated 1 hour with either peroxidase-F(ab′)2 goat antihuman IgG diluted 1:4000 in PBST or peroxidase-F(ab′)2 goat antihuman IgM diluted 1:2000 in PBST. Following 3 PBST washes, the plates were incubated in a substrate/chromogen solution containing 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) in citrate buffer and 0.015% H202. Absorbance was determined at 410 nm by means of an MR700 Microplate reader (Dynex Technologies, Chantilly, VA).
Purification of human monoclonal autoantibodies specific for platelet glycoproteins IIb and IIIa
Antibodies 2E7 (IgM), specific for human platelet glycoprotein IIb heavy chain, and 5E5 (IgM), specific for a neoantigen on glycoprotein IIIa expressed on activated platelets, were isolated from hybridoma culture supernatants as previously described.27,28 After centrifugation to pellet the cells, the resulting supernatants were passed over a Thiophilic Superflow Resin (Clontech, Palo Alto, CA) column. The fractions were monitored by absorbance at 280 nm, and the fractions containing sulfhydryl proteins were dialyzed against PBS, polyethylene glycol precipitated, and passed over a Bakerbond ABx high-performance liquid chromatography (HPLC) column (JT Baker, Phillipsburg, NJ) specific for antibody purification. The HPLC peaks were collected, and the fractions containing antibody were determined by Western blot analysis with the use of alkaline phosphatase–conjugated goat antihuman immunoglobulins (Biosource, Camarillo, CA). Purified 2E7, 5E5, and human IgM as isotype control as well as murine antihuman platelet GPIbα (CD42b, clone HIP1) mAb and murine IgG1,κ (MOPC-21) were dialyzed and quantified as previously described.29
Liquid culture system for in vitro megakaryocytopoiesis analysis
The culture was carried out as previously described,30,31 with modification as follows. Briefly, venous cord blood from the umbilical vessels of healthy, full-term infants was collected immediately after delivery and mixed with a quarter volume of 6% hydroxyethyl starch. Erythrocytes were allowed to settle at room temperature for 30 to 45 minutes. Mononuclear cells (MNCs) in the supernatant were then isolated by a Ficoll-Hypaque density gradient, as previously described,32 washed, and resuspended in culture. The culture system consisted of MNCs at 1 × 106/mL in Iscove modified Dulbecco Medium (Irvine Scientific, Irvine, CA), supplemented with 1% Nutridoma Hu (Roche Boehringer Mannheim, Indianapolis, IN), 10 U/mL heparin, various concentrations of rhTPO (288-TP-005; R&D Systems, Minneapolis, MN), and 10% plasma from either healthy AB blood group donors or ITP patients.31 The total number of suspension cells in culture was determined by a CELL-DYN 1600 multiparameter hematology analyzer (Abbott Laboratories, Abbott Park, IL). The quantity of megakaryocytic cells (CD41+ cells) in culture was initially determined by Wright/Geimsa and immunohistochemical staining at least every other day for up to 12 days. The day-8 cells were chosen for use in all subsequent experiments because the yield of CD41+ cells, as determined by staining, was well within the linear range (data not shown), consistent with previous reports.21,33,34
The effect of purified human monoclonal autoantibodies specific for platelet glycoproteins IIb and IIIa on in vitro megakaryocytopoiesis was examined with the use of the liquid culture system described. Umbilical cord blood MNCs were incubated with various concentrations of 2E7, 5E5, or purified human IgM in the presence of rhTPO, Nutridoma Hu, and 10% plasma from healthy AB blood group donors. The day-8 cells were analyzed by determining the quantity of CD41+ cells in all subsequent experiments.
Cytocentrifugation, Wright staining, and immunohistochemistry
Cytocentrifugation slides were prepared by loading 50 000 to 100 000 cultured cells per slide at 800 rpm for 2 minutes by a cytocentrifuge (Shandon Southern Instruments, Pittsburgh, PA). Slides were subjected to either Wright/Giemsa staining by a Sakura RSG-61 Hematology Stainer (Sakura Finetek, Torrance, CA), or immunohistochemical staining by means of either anti-CD41 mAb (5B12, 1:20 dilution) or anti-CD42 mAb (C7E10) with the LSAB 2 system (DAKO), following manufacturer's recommendations. Slides stained with murine IgG1 (MOPC-21) or without the primary antibody were used as negative controls. The percentage of CD41+ cells in cytocentrifugation slides was quantified with the use of an Olympus microscope and Miller ocular piece.
Yield of megakaryocytic cells in culture
The yield of megakaryocytic cells in culture was quantified by a 2-color flow cytometric technique.33,35-37 The day-8 cultured cells were harvested, washed with cold Ca++- and Mg++-free PBS containing 3% bovine serum albumin (BSA) (fraction V, Sigma Chemical) and 5 mM EDTA (ethylenediaminetetraacetic acid) to block nonspecific binding. After washing, 1 × 106 cells from each aliquot were labeled with 1 μg IgG1 FITC-labeled antihuman CD41 (P2) mAb diluted in 100 μL PBS with BSA and EDTA. Nonspecific antibody binding was monitored with the use of an FITC-labeled murine IgG1 isotype control. After incubation in the dark for 30 minutes at 4°C with gentle mixing, cells were washed with PBS, resuspended in 0.1% sodium citrate containing 50 μg/mL propidium iodide (PI) plus 100 μg/mL DNAse-free RNAse (Sigma Chemical), and incubated again on ice in the dark for 20 minutes. Following staining, cells were immediately analyzed by FACSCalibur flow cytometer (Becton Dickinson Immunocytometry Systems). At least 50 000 cells were acquired for each sample and analyzed by CellQuest software. CD41+PI+ cells were considered to be megakaryocytic cells. The ploidy distribution of each sample was determined by setting markers at the nadirs between peaks.37 Selected cell samples were also stained with another murine anti-CD41 mAb (5B12) to verify the results obtained with the use of mAb P2. Megakaryocyte buffer37 containing sodium citrate, BSA, and PGE1 was also used in selected samples to verify that the CD41 staining results were not buffer dependent.
To test whether ITP plasma was blocking the binding of the murine antihuman CD41 monoclonal Ab to the megakaryocytic cells, cells from day-8 cultures containing control plasma were harvested, washed, reincubated with basic culture medium containing 10% ITP plasma for 1 hour, and then processed and analyzed by the 2-color flow cytometric technique as described.
ELISA for TGF-β1
Transforming growth factor-β1 (TGF-β1) levels of ITP plasma were measured by sandwich ELISA kits (R&D Systems) according to the manufacturer's instructions.
Absorption of ITP plasma with fresh platelets
Fresh control platelet-rich plasma (1 to 10 × 1012 platelets in 500 mL, containing 13 mM citrate) was obtained from the CHOC apheresis unit. Platelet pellets containing PGE1 (20 ng/mL) were isolated as previously described.25 Patient plasma (0.5 mL) was then mixed with the washed platelets (0.9 to 1.5 × 1010/mL plasma) and incubated at 4°C for 1 hour.38 After centrifugation at 3000g for 5 minutes, the supernatant plasma was again absorbed with fresh platelets at 4°C for 1 to 1.5 hours. The absorbed plasma was then analyzed by ELISA for the presence of autoantibodies. Selected absorbed plasma was also analyzed for its effect on megakaryocytopoiesis in the liquid culture system.
Analysis of apoptosis
Selected cultures were incubated with either vehicle (dimethyl sulfoxide [DMSO]) or 80 μM general caspase inhibitor Boc-Asp (Ome)–FMK (FMK) (Enzyme System Products, Livermore, CA, and Oncogene Research Products, San Diego, CA) according to the manufacturers' recommendations and as previously described.39 The content of CD41+PI+ cells was then analyzed as described in previous section.
Human subjects
Materials from human subjects were obtained following informed consent as approved by the CHOC Institutional Review Board.
Statistics
Results were expressed as the means ± standard deviations (SDs) of 3 or more samples. Where appropriate, the probability of significant differences between 2 groups was determined by the unpaired Student t test, whereas the probability of significant differences among multiple groups was determined by analysis of variance, followed by the Tukey-Kramer multiple-comparison test to define the unique subsets within the study. Comparison among groups for frequency of an event was done by the Fisher exact test. The correlation between two sets of variables was determined by linear correlation test (Pearson test). P < .05 was considered significant. Statistical analyses were performed by the InStat (Graphpad, San Diego, CA) or Sigmastat (Jandel Scientific, San Rafael, CA) statistical software programs.
Results
Antiplatelet autoantibodies in ITP plasma
Fifty-three fresh, platelet-poor ITP plasma samples were collected for the current study from 44 acute ITP patients at diagnosis and 9 chronic ITP patients during the thrombocytopenic episodes (average platelet count, 15.3 ± 12.8 × 103/μL) (Table 1). All plasma samples were analyzed for the presence of antibodies against the major platelet autoantigens GPIIb/IIIa and GPIb by sandwich ELISA, and were then classified into 4 groups: negative for both anti-GPIlb/IIIa and anti-GPIb autoantibodies (group O); positive for both autoantibodies (group IIb/IIIa/Ib); positive for anti-GPIlb/IIIa alone (group IIb/IIIa); and positive for anti-GPIb alone (group Ib) (Table 1).
Demographics of ITP patients during thrombocytopenic phase
. | . | . | . | . | . | . | Autoantibody . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | . | . | . | Anti-GPIIb/IIIa . | . | Anti-GPIb . | . | |||
| Patient no. . | Sex . | Age . | Diagnosis . | Plt count, × 103/μL . | Treatment* . | Yield of CD41+PI+, % of control† . | IgG . | IgM . | IgG . | IgM . | |||
| Group O | |||||||||||||
| 1 | M | 4 y | Acute | 16 | No meds | 115.00 | - | - | - | - | |||
| 2 | M | 8 y | Acute | 15 | After IVIG | 94.00 | - | - | - | - | |||
| 3 | M | 11 mo | Acute | 5 | After IVIG test | 90.00 | - | - | - | - | |||
| 4 | M | 7 y | Acute | 19 | No meds | 69.00 | - | - | - | - | |||
| 5 | F | 1 y | Acute | 5 | No meds | 52.98 | - | - | - | - | |||
| 6 | F | 14 y | Acute | 5 | No meds | 116.07 | - | - | - | - | |||
| 7 | M | 8 y | Acute | 10 | No meds | 82.00 | - | - | - | - | |||
| 8 | F | 9 y | Acute | 28 | No meds | 60.00 | - | - | - | - | |||
| 9 | F | 9 y | Acute | 10 | No meds | 111.00 | - | - | - | - | |||
| 10 | F | 3 y | Acute | 54 | No meds | 130.00 | - | - | - | - | |||
| 11 | M | 3 y | Acute | 14 | No meds | 94.00 | - | - | - | - | |||
| 12 | F | 15 y | Acute | 11 | No meds | 64.00 | - | - | - | - | |||
| 13 | F | 1 y | Acute | 6 | No meds | 125.00 | - | - | - | - | |||
| 14 | F | 8 mo | Acute | 15 | No meds | 83.51 | - | - | - | - | |||
| 15 | F | 15 y | Acute | 20 | No meds | 86.00 | - | - | - | - | |||
| Group IIb/IIIa/Ib | |||||||||||||
| 16 | M | 3 m | Acute | 17 | 2 d after IVIG | 47.67 | + | - | + | + | |||
| 17 | F | 4 y | Acute | 8 | No meds | 55.00 | + | - | + | + | |||
| 18 | F | 7 y | Acute | 18 | No meds | 83.00 | - | + | + | + | |||
| 19 | M | 6 y | Acute | 7 | No meds | 30.00 | - | + | + | + | |||
| 20 | F | 3 y | Acute | 14 | No meds | 69.53 | + | + | - | + | |||
| 21 | M | 4 y | Acute | 3 | No meds | 19.87 | + | + | + | + | |||
| 22 | M | 3 y | Acute | 16 | No meds | 59.60 | + | - | + | - | |||
| 23 | F | 3 y | Acute | 4 | No meds | 79.00 | + | - | - | + | |||
| 24 | M | 11 y | Acute | 37 | No meds | 36.00 | + | - | + | - | |||
| 25 | M | 1 y | Acute | 37 | After IVIG | 33.84 | + | - | + | + | |||
| 26 | F | 1 y | Acute | 2 | No meds | 43.00 | - | - | - | + | |||
| 27 | F | 11 m | Acute | 7 | No meds | 37.09 | + | - | + | + | |||
| 28 | M | 2 y | Acute | 7 | No meds | 82.00 | + | - | + | + | |||
| 29 | M | 4 y | Acute | 15 | After IVIG | 15.00 | + | - | + | + | |||
| 30 | F | 5 y | Acute | 17 | 2 d after IVIG | 77.00 | - | + | - | + | |||
| 31 | M | 12 y | Chronic | 34 | No meds | 70.20 | + | - | + | - | |||
| 32 | M | 6 y | Chronic | 18 | Prednisone | 65.56 | + | - | + | + | |||
| 33 | M | 9 y | Chronic | 8 | No meds | 55.36 | - | + | - | + | |||
| 34 | F | 13 y | Acute | 21 | No meds | 24.00 | + | - | - | + | |||
| Group IIb/IIIa | |||||||||||||
| 35 | M | 6 y | Acute | 10 | No meds | 122.00 | + | - | - | - | |||
| 36 | M | 1 y | Acute | 6 | No meds | 114.00 | - | + | - | - | |||
| 37 | F | 4 y | Chronic | 57 | No meds | 84.00 | + | - | - | - | |||
| 38 | M | 3 y | Chronic | 36 | Prednisone | 47.88 | - | + | - | - | |||
| 39 | F | 14 y | Acute | 4 | No meds | 28.00 | + | - | - | - | |||
| Group Ib | |||||||||||||
| 40 | F | 7 y | Acute | 17 | No meds | 78.00 | - | - | + | - | |||
| 41 | M | 2 y | Acute | 18 | No meds | 48.75 | - | - | - | + | |||
| 42 | F | 1 y | Acute | 8 | No meds | 46.24 | - | - | - | + | |||
| 43 | M | 5 y | Acute | 9 | No meds | 49.00 | - | - | - | + | |||
| 44 | M | 3 y | Acute | 9 | No meds | 75.00 | - | - | + | + | |||
| 45 | M | 3 y | Acute | 7 | No meds | 69.00 | - | - | - | + | |||
| 46 | M | 4 y | Acute | 6 | No meds | 109.00 | - | - | - | + | |||
| 47 | M | 4 y | Acute | 3 | No meds | 69.00 | - | - | - | + | |||
| 48 | M | 2 y | Acute | 12 | No meds | 52.00 | - | - | - | + | |||
| 49 | M | 3 y | Acute | 13 | No meds | 45.00 | - | - | - | + | |||
| 50 | F | 16 y | Chronic | 49 | No meds | 30.13 | - | - | - | + | |||
| 51 | F | 16 y | Chronic | <10 | No meds | 6.00 | - | - | - | + | |||
| 52 | M | 4 y | Chronic | 7 | No meds | 53.00 | - | - | - | + | |||
| 53 | F | 8 y | Chronic | 9 | Prednisone | 16.00 | - | - | + | + | |||
. | . | . | . | . | . | . | Autoantibody . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | . | . | . | Anti-GPIIb/IIIa . | . | Anti-GPIb . | . | |||
| Patient no. . | Sex . | Age . | Diagnosis . | Plt count, × 103/μL . | Treatment* . | Yield of CD41+PI+, % of control† . | IgG . | IgM . | IgG . | IgM . | |||
| Group O | |||||||||||||
| 1 | M | 4 y | Acute | 16 | No meds | 115.00 | - | - | - | - | |||
| 2 | M | 8 y | Acute | 15 | After IVIG | 94.00 | - | - | - | - | |||
| 3 | M | 11 mo | Acute | 5 | After IVIG test | 90.00 | - | - | - | - | |||
| 4 | M | 7 y | Acute | 19 | No meds | 69.00 | - | - | - | - | |||
| 5 | F | 1 y | Acute | 5 | No meds | 52.98 | - | - | - | - | |||
| 6 | F | 14 y | Acute | 5 | No meds | 116.07 | - | - | - | - | |||
| 7 | M | 8 y | Acute | 10 | No meds | 82.00 | - | - | - | - | |||
| 8 | F | 9 y | Acute | 28 | No meds | 60.00 | - | - | - | - | |||
| 9 | F | 9 y | Acute | 10 | No meds | 111.00 | - | - | - | - | |||
| 10 | F | 3 y | Acute | 54 | No meds | 130.00 | - | - | - | - | |||
| 11 | M | 3 y | Acute | 14 | No meds | 94.00 | - | - | - | - | |||
| 12 | F | 15 y | Acute | 11 | No meds | 64.00 | - | - | - | - | |||
| 13 | F | 1 y | Acute | 6 | No meds | 125.00 | - | - | - | - | |||
| 14 | F | 8 mo | Acute | 15 | No meds | 83.51 | - | - | - | - | |||
| 15 | F | 15 y | Acute | 20 | No meds | 86.00 | - | - | - | - | |||
| Group IIb/IIIa/Ib | |||||||||||||
| 16 | M | 3 m | Acute | 17 | 2 d after IVIG | 47.67 | + | - | + | + | |||
| 17 | F | 4 y | Acute | 8 | No meds | 55.00 | + | - | + | + | |||
| 18 | F | 7 y | Acute | 18 | No meds | 83.00 | - | + | + | + | |||
| 19 | M | 6 y | Acute | 7 | No meds | 30.00 | - | + | + | + | |||
| 20 | F | 3 y | Acute | 14 | No meds | 69.53 | + | + | - | + | |||
| 21 | M | 4 y | Acute | 3 | No meds | 19.87 | + | + | + | + | |||
| 22 | M | 3 y | Acute | 16 | No meds | 59.60 | + | - | + | - | |||
| 23 | F | 3 y | Acute | 4 | No meds | 79.00 | + | - | - | + | |||
| 24 | M | 11 y | Acute | 37 | No meds | 36.00 | + | - | + | - | |||
| 25 | M | 1 y | Acute | 37 | After IVIG | 33.84 | + | - | + | + | |||
| 26 | F | 1 y | Acute | 2 | No meds | 43.00 | - | - | - | + | |||
| 27 | F | 11 m | Acute | 7 | No meds | 37.09 | + | - | + | + | |||
| 28 | M | 2 y | Acute | 7 | No meds | 82.00 | + | - | + | + | |||
| 29 | M | 4 y | Acute | 15 | After IVIG | 15.00 | + | - | + | + | |||
| 30 | F | 5 y | Acute | 17 | 2 d after IVIG | 77.00 | - | + | - | + | |||
| 31 | M | 12 y | Chronic | 34 | No meds | 70.20 | + | - | + | - | |||
| 32 | M | 6 y | Chronic | 18 | Prednisone | 65.56 | + | - | + | + | |||
| 33 | M | 9 y | Chronic | 8 | No meds | 55.36 | - | + | - | + | |||
| 34 | F | 13 y | Acute | 21 | No meds | 24.00 | + | - | - | + | |||
| Group IIb/IIIa | |||||||||||||
| 35 | M | 6 y | Acute | 10 | No meds | 122.00 | + | - | - | - | |||
| 36 | M | 1 y | Acute | 6 | No meds | 114.00 | - | + | - | - | |||
| 37 | F | 4 y | Chronic | 57 | No meds | 84.00 | + | - | - | - | |||
| 38 | M | 3 y | Chronic | 36 | Prednisone | 47.88 | - | + | - | - | |||
| 39 | F | 14 y | Acute | 4 | No meds | 28.00 | + | - | - | - | |||
| Group Ib | |||||||||||||
| 40 | F | 7 y | Acute | 17 | No meds | 78.00 | - | - | + | - | |||
| 41 | M | 2 y | Acute | 18 | No meds | 48.75 | - | - | - | + | |||
| 42 | F | 1 y | Acute | 8 | No meds | 46.24 | - | - | - | + | |||
| 43 | M | 5 y | Acute | 9 | No meds | 49.00 | - | - | - | + | |||
| 44 | M | 3 y | Acute | 9 | No meds | 75.00 | - | - | + | + | |||
| 45 | M | 3 y | Acute | 7 | No meds | 69.00 | - | - | - | + | |||
| 46 | M | 4 y | Acute | 6 | No meds | 109.00 | - | - | - | + | |||
| 47 | M | 4 y | Acute | 3 | No meds | 69.00 | - | - | - | + | |||
| 48 | M | 2 y | Acute | 12 | No meds | 52.00 | - | - | - | + | |||
| 49 | M | 3 y | Acute | 13 | No meds | 45.00 | - | - | - | + | |||
| 50 | F | 16 y | Chronic | 49 | No meds | 30.13 | - | - | - | + | |||
| 51 | F | 16 y | Chronic | <10 | No meds | 6.00 | - | - | - | + | |||
| 52 | M | 4 y | Chronic | 7 | No meds | 53.00 | - | - | - | + | |||
| 53 | F | 8 y | Chronic | 9 | Prednisone | 16.00 | - | - | + | + | |||
No meds indicates plasma samples were collected at diagnosis before the medication was given; After IVIG test, after IVIG; 2 d after IVIG, plasma samples were collected immediately after a test dose of IVIG, a full dose of IVIG, or 2 days after a full dose of IVIG; prednisone, that patient was taking prednisone regularly when plasma samples were collected
The average yield of CD41+PI+ of 4 group IIb/IIIa/Ib samples that received IVIG was 43.5 ± 26.1
Liquid culture system to assay in vitro megakaryocytopoiesis
In the absence of rhTPO, neither control nor ITP plasma could support the growth of megakaryocytes in liquid culture, and a majority of the cells had lysed by day 8 (data not shown). In contrast, when rhTPO was present in cultures containing control plasma, the cells remained 90% ± 7% viable after 8 days, as determined by trypan blue exclusion. Morphologically recognizable megakaryocytic cells at various stages of development were observed when cells from day-8 cultures containing rhTPO and control plasma were stained by Wright/Giemsa staining (Figure 1A). The megakaryocytic origin of these cells was further confirmed by their positive reactivity to anti-CD41 (Figure 1B) and CD42 mAbs (data not shown). Negative control slides displayed no immunostaining, suggesting a very low level of background binding (data not shown). The additional cells present in culture were macrophage-like, recognized as large cells with a single round, relatively small nucleus and an abundant, foamy cytoplasm, and some small lymphocytes as shown by Wright/Giemsa staining (Figure 1A). No immunostaining was found in those contaminating cells, nor were platelet-coated contaminating cells observed by immunohistochemistry (Figure 1B).
Micrographic pictures of megakaryocytic cells obtained from liquid culture of umbilical cord blood MNCs. Cells were cultured for 8 days in the presence of 10 ng/mL rhTPO and either control or ITP plasma. (A) Wright staining of a cytocentrifugation slide with cells from culture containing control plasma. Morphologically recognizable megakaryocytic cells at various stages of development were observed. Macrophage-like cells and small lymphocytes were the only major contaminating cells. Original magnification, × 400. (B) Immunohistochemistry staining (CD41, mAb 5B12) of cells from culture containing control plasma. Megakaryocytic cells are stained red. Original magnification, × 200. (C) Immunohistochemistry staining (CD41, mAb 5B12) of cells from culture containing group IIb/IIIa/Ib ITP plasma collected from an acute ITP patient at diagnosis. MNCs from the same umbilical cord blood was used for the cultures in panels B and C. None of the cells in this field stained positive for CD41. Original magnification, × 200.
Micrographic pictures of megakaryocytic cells obtained from liquid culture of umbilical cord blood MNCs. Cells were cultured for 8 days in the presence of 10 ng/mL rhTPO and either control or ITP plasma. (A) Wright staining of a cytocentrifugation slide with cells from culture containing control plasma. Morphologically recognizable megakaryocytic cells at various stages of development were observed. Macrophage-like cells and small lymphocytes were the only major contaminating cells. Original magnification, × 400. (B) Immunohistochemistry staining (CD41, mAb 5B12) of cells from culture containing control plasma. Megakaryocytic cells are stained red. Original magnification, × 200. (C) Immunohistochemistry staining (CD41, mAb 5B12) of cells from culture containing group IIb/IIIa/Ib ITP plasma collected from an acute ITP patient at diagnosis. MNCs from the same umbilical cord blood was used for the cultures in panels B and C. None of the cells in this field stained positive for CD41. Original magnification, × 200.
Yield of megakaryocytic cells in culture was monitored by 2-color flow cytometry. The yields of CD41+PI+ cells detected by either clone P2 or clone 5B12 anti-CD41 mAb were indistinguishable and quantitatively correlated to each other (r = 0.91, P < .0001, n = 11). Yields of CD41+PI+ cells detected by flow cytometry with the use of 2 different staining systems were very similar and significantly correlated to each other (r = 0.997, P < .0001, n = 6). The yield of flow cytometrically detectable CD41+PI+ cells was also significantly correlated with the yield of immunohistochemically detectable CD41+ cells (r = 0.86, P < .01, n = 8). On the basis of these results, megakaryocytic cells were defined as flow cytometrically detectable CD41+PI+ cells in all subsequent experiments.
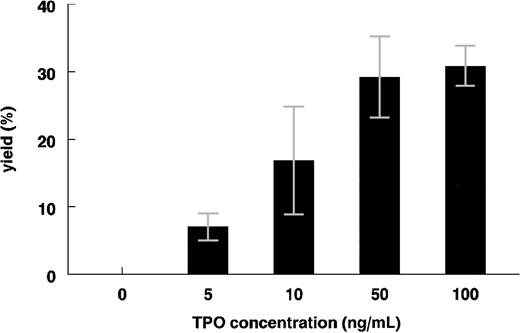
Megakaryocytic cell growth from cord blood MNCs was sensitive to increasing concentrations of rhTPO (Figure 2). The yield of CD41+PI+ cells reached a maximal 35% of total cells in day-8 cultures containing 50 ng/mL TPO with control plasma. The average yield of CD41+ cells in the day-8 cultures with control plasma (10 ng/mL TPO) was 16.3% ± 8% (n = 4), well within the linear range of the TPO concentration/CD41+PI+ yield curve (Figure 2). A concentration of 10 ng/mL TPO was selected for use in all subsequent experiments so that changes in the CD41+PI+ cell number could readily be detected.
Dose response of rhTPO to the growth of CD41+PI+ cells from liquid culture of umbilical cord blood MNCs. Cells were cultured for 8 days in the presence of various concentrations of rhTPO and 10% control plasma. The yields of CD41+PI+cells in cultures were determined by means of the 2-color flow cytometric technique (means ± SDs; n was at least 2 for each dose).
Dose response of rhTPO to the growth of CD41+PI+ cells from liquid culture of umbilical cord blood MNCs. Cells were cultured for 8 days in the presence of various concentrations of rhTPO and 10% control plasma. The yields of CD41+PI+cells in cultures were determined by means of the 2-color flow cytometric technique (means ± SDs; n was at least 2 for each dose).
Effects of ITP plasma on in vitro megakaryocytopoiesis
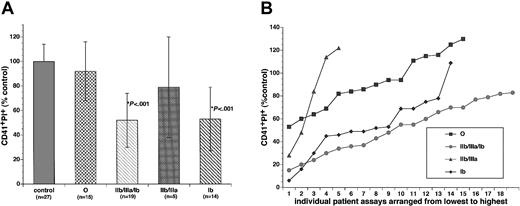
The yield of CD41+PI+ cells in cultures containing different groups of ITP plasma were compared with control or with each other in a blinded study in which the investigators were not aware of the autoantibody status of the ITP plasma while performing the in vitro megakaryocytopoiesis assay. To control for variation in stem cell quantity from different cord blood MNC preparations as well as other assay variations, plasma samples from 3 healthy adults were processed identically to the patient plasma samples in each experiment. Results from patient samples were then calculated and presented as a percentage of the control. The yield of CD41+PI+ cells was 92% ± 24% for group O (n = 15, P = not significant [NS]); 56% ± 27% for groups IIb/IIIa/Ib, IIb/IIIa, and Ib combined together (n = 38, P < .001); 52% ± 22% for group IIb/IIIa/Ib alone (n = 19, P < .001); 79% ± 41% for group IIb/IIIa alone (n = 5, P = NS); and 53% ± 26% for group Ib alone (n = 14, P < .001) as compared with 100% ± 14% (n = 27) for the control (Table 1; Figure 3). While 11 out of 15 samples (73%) in group O yielded CD41+PI+ cells of at least 80% or higher than the control level, only 3 out of 33 samples (9.4%) in groups IIb/IIIa/Ib and Ib did so (P < .01; Table 1). The ploidy distributions of CD41+PI+ cells in cultures containing either control or ITP plasma were not significantly different. A majority of the CD41+PI+ cells in day-8 cultures were 2N and 4N (data not shown), consistent with previous reports.21,33,34 The yield of CD41+cells determined by immunohistochemistry (Figure 1B-C) was quantitatively correlated with that of flow cytometry (r = 0.75, P < .0001, n = 62; Figure 4). There were no significant differences in the total number of cells per milliliter between cultures containing either control or any of the ITP plasmas (control, 100% ± 9.5%, n = 15; O, 92% ± 13%, n = 10; IIb/IIIa/Ib plus Ib, 90% ± 12%, n = 19, P = NS), indicating that ITP plasma probably did not affect overall survival of the MNCs.
Yields of CD41+PI+ cells in cultures containing control or ITP plasma collected when patients were in thrombocytopenic phase. Umbilical cord blood MNCs were cultured for 8 days in the presence of rhTPO and either control or ITP plasma. The ITP plasma samples were classified into 4 subgroups as described in the text. (A) The yields of CD41+PI+cells in the cultures were determined with the use of the 2-color flow cytometric technique. Results were presented as the percentage of the controls (means ± SDs). The yields of CD41+PI+cells in groups IIb/IIIa/Ib and Ib culture were significantly lower than that of the control (P < .001). (B) The yields of CD41+PI+cells in the cultures containing individual patient plasma from 4 subgroups. The data were arranged from lowest yield to the highest.
Yields of CD41+PI+ cells in cultures containing control or ITP plasma collected when patients were in thrombocytopenic phase. Umbilical cord blood MNCs were cultured for 8 days in the presence of rhTPO and either control or ITP plasma. The ITP plasma samples were classified into 4 subgroups as described in the text. (A) The yields of CD41+PI+cells in the cultures were determined with the use of the 2-color flow cytometric technique. Results were presented as the percentage of the controls (means ± SDs). The yields of CD41+PI+cells in groups IIb/IIIa/Ib and Ib culture were significantly lower than that of the control (P < .001). (B) The yields of CD41+PI+cells in the cultures containing individual patient plasma from 4 subgroups. The data were arranged from lowest yield to the highest.
Correlation between flow cytometrically detectable CD41+PI+ cells and immmunohistochemically detectable CD41+ cells. Umbilical cord blood MNCs was cultured for 8 days in culture containing 10 ng/mL rhTPO and either control or ITP plasma. Results were presented as the percentage of the controls. A significant correlation (r = 0.75; P < .0001; n = 62) was found by Pearson analysis.
Correlation between flow cytometrically detectable CD41+PI+ cells and immmunohistochemically detectable CD41+ cells. Umbilical cord blood MNCs was cultured for 8 days in culture containing 10 ng/mL rhTPO and either control or ITP plasma. Results were presented as the percentage of the controls. A significant correlation (r = 0.75; P < .0001; n = 62) was found by Pearson analysis.
To rule out the possibility that ITP plasma might block binding of the murine monoclonal Ab to the megakaryocytic cells and, therefore, interfere with the detection of those cells, control culture cells were harvested, washed, reincubated with the medium containing either 10% control or ITP plasma for an hour, and then processed and analyzed by 2-color flow cytometry. There was no significant difference between reincubation with control versus ITP plasma (control, 100% ± 12%, n = 4; ITP, 103% ± 15%, n = 15, P = NS), demonstrating that ITP plasma did not block the binding of the antihuman CD41 monoclonal Ab to the cells. To rule out the possibility that ITP plasma may have been uniquely deficient in some nutritional factors that could not be replaced by Nutridoma, selected cultures with ITP plasma were supplemented with additional BSA, sonicated lipids, and α-thioglycerol as described by Iscove et al,40 which have been shown to effectively substitute for serum/plasma in our own (data not shown) as well as other megakaryocytopoiesis culture systems.21,41,42 The reduced yield of CD41+ cells could not be corrected by the addition of those extra supplements (Nutridoma only, 63.8% ± 26.9% of the control, n = 11; extra supplements, 61.9% ± 31% of the control, n = 11, P = NS; there is also a significant correlation between the yield of Nutridoma only versus extra supplements: r = 0.844, P < .0011, n = 11), suggesting that inhibition of in vitro megakaryocytopoiesis by ITP plasma was not due to a deficiency in any of those factors. Additionally, to rule out the possibility that TGF-β1, a known negative regulator of megakaryocytopoiesis, might be elevated in ITP compared with control plasma, owing to its release from platelet granules43 as a result of either the disease or the plasma collection process, TGF-β1 levels were assayed in ITP versus control plasma by ELISA. These were not found to be elevated in ITP (control, 12.3 ± 3.6 ng, n = 2; ITP, 2.4 ± 1.3 ng, n = 4). TGF-β1 levels were, in fact, lower in the ITP plasma, consistent with a previous report.43
Effect of platelet-absorbed ITP plasma on in vitro megakaryocytopoiesis
To confirm that autoantibodies in ITP plasma were indeed reactive with accessible epitopes of the glycoproteins, 18 ITP plasma samples (group IIb/IIIa/Ib, n = 4; group IIb/IIIa, n = 3; group Ib, n = 11) were absorbed twice with washed platelets from healthy donors, resulting in depletion of anti-GPIb and/or anti-GPIIb/IIIa autoantibodies in all of the patient plasmas, as determined by sandwich ELISA (Table 2), suggesting that the detected autoantibodies were specific for platelet surface membrane antigens. To determine if inhibition of in vitro megakaryocytopoiesis by the ITP plasma was anti–GP autoantibody mediated, 2 of the absorbed group Ib ITP plasmas with reduced titers of anti-GPIb autoantibodies were analyzed further in culture. CD41+PI+ cell production was approximately double that of the same ITP plasmas without absorption, whereas CD41+PI+ yield was not altered by platelet absorption of control plasma (Table 3). These results suggest that anti-GPIb autoantibody may indeed be responsible, at least in part, for the reduced yield of CD41+PI+ cells in culture.
Reduction of plasma autoantibodies against GPIb/IX and GPIIb/IIIa after absorption with platelets
. | Plasma autoantibodies after treatment . | . | |
|---|---|---|---|
| Plasma autoantibodies before treatment . | Autoantibodies completely removed . | Autoantibodies partially removed . | |
| Against GPIb/IX | |||
| 2 IgG | 1 IgG | 1 IgG | |
| 14 IgM | 4 IgM | 10 IgM | |
| Against GPIIb/IIIa | |||
| 4 IgG | 2 IgG | 2 IgG | |
| 3 IgM | 1 IgM | 2 IgM | |
. | Plasma autoantibodies after treatment . | . | |
|---|---|---|---|
| Plasma autoantibodies before treatment . | Autoantibodies completely removed . | Autoantibodies partially removed . | |
| Against GPIb/IX | |||
| 2 IgG | 1 IgG | 1 IgG | |
| 14 IgM | 4 IgM | 10 IgM | |
| Against GPIIb/IIIa | |||
| 4 IgG | 2 IgG | 2 IgG | |
| 3 IgM | 1 IgM | 2 IgM | |
There were 18 ITP plasmas (group IIb/IIIa/Ib, n = 4; group IIb/IIIa, n = 3; group Ib, n = 11). Patient plasma (0.5 mL) was mixed with the washed platelets (0.9 to 1.5 × 1010/mL plasma) and incubated at 4°C for 1 hour. After centrifugation at 3000g for 5 minutes, the supernatant plasma was again absorbed with fresh platelets at 4°C for 1 to 1.5 hours. The absorbed plasma was then analyzed by ELISA for the presence of autoantibodies on the following day
Reduction of plasma autoantibodies against GPIb/IX is associated with increased yield of CD41+PI+ cells
. | Before absorption . | . | . | |
|---|---|---|---|---|
. | Process 1* . | Process 2† . | After absorption . | |
| Acute ITP no. 1; Plt, 2 × 103/μL | ||||
| IgM titer | >160 | >160 | 80 | |
| CD41+PI+, % of control | 25 | 25 | 50 | |
| Acute ITP no. 2; Plt, 18 × 103/μL | ||||
| IgM titer | >20 | >20 | Undetectable | |
| CD41+PI+, % of control | 82 | 66 | 120 | |
| Healthy control no. 1; Plt, control | ||||
| IgM titer | Undetectable | Undetectable | Undetectable | |
| CD41+PI+, % of control | 100 | 94 | 100 | |
| Healthy control no. 2; Plt, control | ||||
| IgM titer | Undetectable | Undetectable | Undetectable | |
| CD41+PI+, % of control | 100 | 108 | 100 | |
. | Before absorption . | . | . | |
|---|---|---|---|---|
. | Process 1* . | Process 2† . | After absorption . | |
| Acute ITP no. 1; Plt, 2 × 103/μL | ||||
| IgM titer | >160 | >160 | 80 | |
| CD41+PI+, % of control | 25 | 25 | 50 | |
| Acute ITP no. 2; Plt, 18 × 103/μL | ||||
| IgM titer | >20 | >20 | Undetectable | |
| CD41+PI+, % of control | 82 | 66 | 120 | |
| Healthy control no. 1; Plt, control | ||||
| IgM titer | Undetectable | Undetectable | Undetectable | |
| CD41+PI+, % of control | 100 | 94 | 100 | |
| Healthy control no. 2; Plt, control | ||||
| IgM titer | Undetectable | Undetectable | Undetectable | |
| CD41+PI+, % of control | 100 | 108 | 100 | |
Frozen plasma samples were defrosted in ice, and analyzed immediately in culture for their effect on in vitro megakaryocytopoiesis
Frozen plasma samples were defrosted in ice, processed same as absorbed plasma samples except that no platelets were added to the plasma, and then analyzed in culture
Inhibition of in vitro megakaryocytopoiesis by human monoclonal autoantibodies isolated from ITP patients
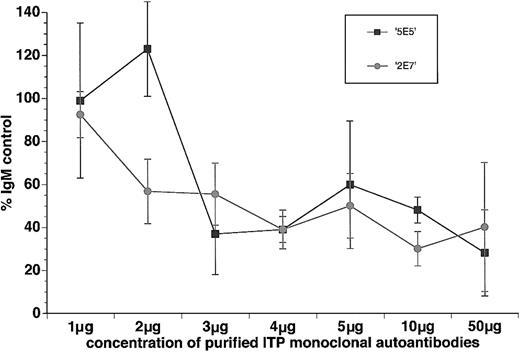
Since only a few of the ITP plasmas in this study belong to group IIb/IIIa, it was difficult to conclusively establish whether these plasmas significantly inhibited in vitro megakaryocytopoiesis and whether such inhibition is anti–GP autoantibody mediated or not. To provide more direct evidence that human autoantibodies from ITP patients in general, and autoantibodies specific for platelet GPIIb and GPIIIa in particular, inhibit in vitro megakaryocytopoiesis, 2 human antiplatelet GPIIb and GPIIIa monoclonal autoantibodies isolated from patients with ITP,27,28 2E7 and 5E5, were purified, and their effect on in vitro megakaryocytopoiesis was assayed. In the presence of 10% human AB plasma, CD41+PI+ cell growth from cord blood MNCs was sensitive to increasing concentrations of either 2E7 or 5E5 (Figure 5). The yield of CD41+PI+ cells in the cultures containing 1 μg/mL (1.1 nM) of either 2E7 or 5E5 was not significantly different from the same concentration of human control IgM (92.5% ± 10.7% for 2E7 and 98.9% ± 36.1% for 5E5 as compared with 100% for the control, n = 4, P = NS). However, the yield of CD41+PI+ cells in cultures containing 3 μg/mL (3.3 nM) or more (4, 5, 10, 50 μg/mL) of either 2E7 or 5E5 was reduced to about half of the IgM control (Figure 5). At 5 μg/mL (5.5 nM), the yield of CD41+PI+ cells was 50.1% ± 14.7% with 2E7 (n = 6, P < .01), and 59.8% ± 29% with 5E5 (n = 6, P < .01), compared with 100% in the IgM control. The inhibition of in vitro megakaryocytopoiesis by either 2E7 or 5E5 also appeared to be human plasma dependent. At either 1 (data not shown) or 5 μg/mL, neither 2E7 nor 5E5 was able to inhibit the in vitro megakaryocytopoiesis in the plasma-free culture system described by Iscove et al40 (5 μg/mL 2E7 and 5E5: 118% ± 27% and 106.5% ± 9.2% of the IgM control, n = 2). In addition, when both 2E7 and 5E5 were included in the same culture at 3 or 5 μg/mL of each, inhibition of megakaryocytopoiesis (48% ± 6% of the IgM control, n = 2) was comparable to that of either antibody added separately, suggesting that there was no additive effect between 2E7 and 5E5 on megakaryocytopoiesis inhibition. For comparison, dialyzed murine antihuman platelet GPIbα (clone HIP1) mAb was also inhibitory at a concentration of 1 μg/mL (6.7 nM) (56.3% ± 14% of the IgG control, n = 3), consistent with a previous report.29
Dose response of purified human antiplatelet GPIIb and GPIIIa monoclonal autoantibodies, 2E7 and 5E5, to the growth of CD41+PI+ cells in the liquid culture of umbilical cord blood MNCs. Cells were cultured for 8 days in the presence of rhTPO, 10% control plasma, and various concentrations of 2E7 and 5E5. The yields of CD41+PI+cells in cultures were determined by the 2-color flow cytometric technique (means ± SDs; n was at least 2 for each dose).
Dose response of purified human antiplatelet GPIIb and GPIIIa monoclonal autoantibodies, 2E7 and 5E5, to the growth of CD41+PI+ cells in the liquid culture of umbilical cord blood MNCs. Cells were cultured for 8 days in the presence of rhTPO, 10% control plasma, and various concentrations of 2E7 and 5E5. The yields of CD41+PI+cells in cultures were determined by the 2-color flow cytometric technique (means ± SDs; n was at least 2 for each dose).
Effect of general caspase inhibitor on in vitro megakaryocytopoiesis
To investigate whether the inhibition of in vitro megakaryocytopoiesis by ITP autoantibodies/plasma was mediated by increased apoptosis, we examined the effect of the general caspase inhibitor, FMK, on in vitro megakaryocytopoiesis in the presence of either control or ITP plasma (groups IIb/IIIa/Ib and Ib). The presence of FMK may increase megakaryocyte production in day-8 cultures with ITP plasma if megakaryocytopoietic inhibition is mediated by enhanced apoptosis. A small increase in the yield of CD41+PI+ cells was indeed observed in the presence of the inhibitor for both control and ITP groups at day 8 (control with vehicle, 100.3% ± 12.7%, n = 6; control with FMK, 113.2% ± 16.6%, n = 6, P = NS; ITP with vehicle, 42.6% ± 25.8%, n = 9; ITP with FMK, 57.2% ± 39.8%, n = 9, P = NS). However, the increase in the production of CD41+PI+ cells was not statistically significant for either the control or the ITP groups. Moreover, FMK treatment clearly could not completely reverse the inhibitory effect of the ITP plasma. Yield of CD41+PI+ cells in ITP samples treated with FMK was still significantly lower than that of control samples treated with FMK (P < .001).
Discussion
This report provides a systematic analysis of ITP plasma effects on rhTPO-induced in vitro megakaryocytopoiesis. Umbilical cord blood–derived MNCs were used as an efficient source of cells for multiple quantitative analyses. The hydroxyethyl starch sedimentation step prior to the Ficoll density centrifugation not only significantly reduced erythrocyte contamination in the cord blood MNC preparation, as expected, but also was consistently associated with a virtual absence of platelet contamination in the day-8 cultures, as determined by Wright and immunohistochemical staining. Thus, this step became critical for obtaining a pure MNC preparation for further analysis.
Prior to the discovery of TPO, the few studies examining the effects of ITP sera/plasma on in vitro megakaryocytopoiesis by means of colony assays reported conflicting results.44-47 Four ITP sera analyzed by one group had either a negative or a neutral effect on megakaryocyte colony formation.44 In contrast, another group found that 8 ITP sera increased colony formation.47 Neither the autoantibody status of the plasma nor the clinical stages of the patients were described in detail by these previous studies. When ITP plasma was classified into different subgroups, as described in the present study, and examined in culture, it became clear that the different subgroups of ITP patient plasma had heterogenous influences on rhTPO-induced megakaryocytopoiesis, which may partially explain the previous conflicting results. In the current study, only the presence of anti-GPIb autoantibodies in the ITP plasma collected during thrombocytopenic phase was associated with significantly reduced in vitro megakaryocytopoiesis. Autoantibody absorption further confirmed that the anti-GPIb autoantibodies may be responsible, at least in part, for the reduced yield of megakaryocytopoiesis in group Ib cultures. A recent study has shown that murine monoclonal antihuman platelet GPIbα antibody also inhibits the in vitro human megakaryocyte colony formation,29 which was confirmed with the use of the liquid culture system in the current study. However, it could have been argued that such inhibition was due to the subtle interspecies differences between the human and the murine platelet/megakaryocyte membrane, especially in light of the significant amino acid sequence differences between the extracytoplasmic domains of the human and murine platelet GPIbα that have recently been reported.48 The epitope(s) reacting with the murine mAb, therefore, may not be pathophysiologically relevant in human ITP. Our results, obtained with the use of human autoantibodies in ITP plasma, would appear to rule out that possibility. Furthermore, in contrast to the murine mAb that recognizes a single epitope of GPIbα,29 antiplatelet human GPIb autoantibodies in group IIb/IIIa/Ib and group Ib ITP plasma differ in their immunoglobulin classes, titers, and, most likely, idiotypic specificity as well.38,49 Nonetheless, they are all associated with reduced in vitro megakaryocytopoiesis, suggesting that more than one epitope of the platelet GPIb/IX complex may be recognized by the autoantibodies. Taken together, these results suggest a critical role for GPIb as a target for autoantibody-mediated down-regulation of megakaryocytopoiesis.
The results obtained using the 2 purified human monoclonal autoantibodies, 2E7 and 5E5, not only provide direct evidence that purified ITP antiplatelet autoantibodies are indeed able to inhibit megakaryocytopoiesis, but also help to clarify the effect of antiplatelet GPIIbIIIa autoantibodies on in vitro megakaryocytopoiesis, which was difficult to assess using group IIb/IIIa ITP plasma owing to the small number of samples available. While both 2E7 and 5E5 were inhibitory to megakaryocytopoiesis, higher concentrations of the autoantibodies appeared to be necessary for inhibition comparable to that of antiplatelet GPIb. Unlike the murine antihuman platelet GPIbα mAb, which was inhibitory at 1 μg/mL,29 neither 2E7, 5E5, nor the murine antiplatelet GPIIb/IIIa mAb described in the earlier study29 had any effect on human megakaryocytopoiesis at that concentration. This, however, does not necessarily imply that antiplatelet GPIIb/IIIa autoantibodies are less deleterious to megakaryocytopoiesis than antihuman platelet GPIbα autoantibodies. Unlike the GPIb-V-IX complex, which is not expressed on the megakaryocytic membrane until several days after the umbilical cord blood culture is begun in response to cytokine induction, GPIIb/IIIa is one of the early expressed genes of megakaryocytopoiesis, already present on significant numbers of stem cells at birth.50 Therefore, it may be difficult to accurately assess the relative effect of the ITP autoantibodies against the GPIIb/IIIa complex compared with the GPIb-V-IX complex in culture. Furthermore, when the molar concentration of the antibodies is taken into account, the larger 2E7 and 5E5 IgM autoantibodies may be even more potent inhibitors than the smaller, antiplatelet GPIb IgG autoantibody.
The exact mechanism that underlines ITP autoantibody–mediated down-regulation of megakaryocytopoiesis remains unclear. However, previous studies indicated that some ITP patients have increased amounts of platelet-associated complement, suggesting in vivo complement activation.51 Furthermore, certain ITP autoantibodies were found to activate complement-mediated platelet lysis in vitro,52 and purified IgG from ITP patients also showed complement-dependent cytotoxicity toward megakaryocyte progenitors.53 Our results demonstrating that healthy control plasma is required for the inhibitory effect of purified 2E7 or 5E5 seem to be consistent with these previous findings that biologically active factors other than autoantibodies in the plasma, such as complement, might facilitate the inhibitory effect of the autoantibodies. Variations in the amount of these factors between plasma samples may explain why some samples in our study were more inhibitory to megakaryocytopoiesis than others even when their autoantibody titers were similar (data not shown). It was also interesting to investigate whether the inhibition of megakaryocytopoiesis by ITP plasma/autoantibodies was mediated by the reduced proliferative capacity or increased apoptosis. Since previous studies using ITP plasma or murine monoclonal antihuman platelet GPIbα antibody had found a reduction in megakaryocytic progenitors,29,44 the present investigation was designed to determine whether or not enhanced apoptosis of megakaryocytic cells in the presence of ITP plasma might also play a role. Our results using the caspase inhibitor, FMK, suggest that increased apoptosis is probably not required to produce the inhibitory effect of ITP plasma observed, which is also consistent with 2 previous studies. The first study indicated that only senescent cultured megakaryocytic cells (after 12 days of culture) showed significant apoptosis, whereas the percentage of apoptosis was at background level (less than 10%) prior to day 9.54 In the second study, the presence of apoptosis was directly measured in freshly purified bone marrow GPIIb/IIIa+ megakaryocytic cells by means of flow cytometry and electron microscopy. The mean percentage of apoptotic megakaryocytic cells was found to be not significantly different between the control and ITP patients.55 It has been reported recently that nonapoptotic programmed cell death of megakaryocytopoiesis, such as paraapoptosis, may be enhanced by ITP in vivo.17 Whether or not inhibition of megakaryocytopoiesis by ITP plasma/autoantibodies in vitro is also mediated by para-apoptosis requires further electron microscopic investigation.
Although megakaryocyte levels are often increased or remain normal in ITP,2,3 recent studies of autologous platelet turnover have indicated that effective delivery of the nascent platelets into circulation is either reduced or normal, rather than increased as would be expected, in a substantial number of ITP patients.56-58 The exact mechanism of such decreased platelet production is unclear. Our data, together with those of the earlier studies demonstrating that a substantial number of megakaryocytes were damaged in ITP,10,17 may provide a partial explanation for the reduced platelet production. It is possible that the platelet autoantibodies may interfere with the maturation of the megakaryocytes or even destroy them, resulting in reduced platelet production, contributing to the severity of thrombocytopenia in some ITP patients. Such a deleterious effect of the autoantibodies may be transient in a majority of the ITP children, since their platelet counts recover quickly. However, autoantibody-mediated suppression of megakaryocytopoiesis may play a more critical role in patients with persistent thrombocytopenia, such as in chronic ITP.
Prepublished online as Blood First Edition Paper, April 3, 2003; DOI 10.1182/blood-2002-05-1475.
Supported by the Children's Hospital of Orange County, Foundation for Children and Padrinos, and National Institutes of Health grant HL61846 (D.J.N.).
Presented in part at the Annual Meeting of the American Society of Hematology, December 1999, New Orleans, LA; December 2000, San Francisco, CA; and December 2002, Philadelphia, PA.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors gratefully acknowledge Joseph Trotter of Scripps Research Institute; Dr Stephen Hou of University of California at Irvine; Sandra Kulczyk CLS, Gay Joyce CLS, and the staff of clinical laboratory at St Joseph Hospital; Department of Obstetrics at St Joseph Hospital; Blood and Donor Services of CHOC; Blood and Marrow Transplantation Laboratory of CHOC; Dr Erfen Zhu, Violeta D. Dadufalza, and Amalia I. Lam for technical assistance and advice; and Dr Robert McMillan and Dr Thomas. J. Kunicki of Scripps Research Institute, Dr Jonathan G. Drachman of Puget Sound Blood Center, and Dr Monique Berman of CHOC for advice and helpful discussion.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal