We read with interest the article by Nishikori et al,1 which reported that expression levels of the bcl-3 gene product distinguish anaplastic lymphoma kinase (ALK)–positive anaplastic large cell lymphoma (ALCL) from Hodgkin lymphoma (HL). The bcl-3 proto-oncogene originally was identified by its involvement in the t(14;19)(q32;q13) observed in a small subset of chronic lymphocytic leukemia (CLL) cases.2,3 BCL-3 protein functions as a p50 subunit-specific I-kappa B (I-κB)–like inhibitor of the NF-kappaB (NF-κB) transcription factor.4,5
Using 4 ALCL and 3 HL cell lines and the Atlas Human 1.2 cDNA microarray, Nishikori and coworkers1 demonstrated that BCL-3 mRNA levels were significantly higher in ALCL compared with HL cell lines, and confirmed their data using Northern blot analysis, real-time reverse transcriptase–polymerase chain reaction (RT-PCR), and Western blot analysis. Using a similar approach and a “pathway” array (1126 genes) developed by the Cancer Genomics Core Laboratory of our institution, we also compared the gene expression profile of an ALK-positive ALCL cell line, Karpas 299, with an HL cell line established in our laboratory. After appropriate normalization, we found a 6-fold higher BCL-3 mRNA level in Karpas 299 cells compared with HL cells, confirmed by Western blot analysis (data not shown).
Nishikori and coworkers1 also reported that bcl-3 mRNA levels were higher in 3 ALK-positive ALCL compared with 3 ALK-negative ALCL tumor samples. However, the authors did not assess BCL-3 protein levels. We have assessed BCL-3 expression in a series of 40 systemic ALCL tumors (14 ALK positive, 26 ALK negative). Although the ALK-negative ALCL cases were strongly and uniformly CD30 positive, others have suggested that these ALK-negative neoplasms are better classified as peripheral T-cell lymphomas.6 The clinical characteristics and therapy of these patients are similar to that reported elsewhere.7 BCL-3 expression was assessed immunohistochemically using a tissue microarray8 that included 4 cores from each tumor. We used a monoclonal antibody (clone 1E8, Novocastra, Newcastle upon Tyne, United Kingdom) and methods previously described.7
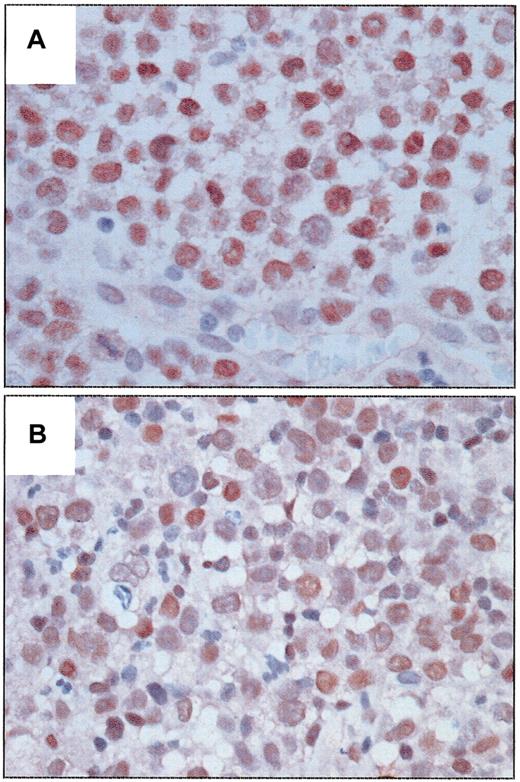
BCL-3 was detected in the nucleus of tumor cells in a subset of ALCL tumors and was significantly associated with ALK expression (Figure 1). Ten of 14 (71.4%) ALK-positive tumors strongly expressed BCL-3 compared with weaker expression in 3 of 26 (11.5%) ALK-negative tumors (P = .0002, Fisher exact test). This association further supports the data of Nishikori et al.1 In our study BCL-3 expression was more frequent in younger patients (P = .04), probably due to its association with ALK. An inverse correlation between BCL-3 and BCL-2 expression also was observed (P = .017). BCL-3 expression was not significantly associated with progression-free or overall survival in patients with ALK-positive or ALK-negative tumors after a median follow-up of 46 months for survivors.
Expression of BCL-3 protein in systemic ALCL tumors. (A) An ALK-positive ALCL tumor with strong nuclear immunoreactivity in almost all tumor cells. (B) An ALK-negative ALCL tumor with relatively weaker nuclear immunoreactivity in many tumor cells. (Immunoperoxidase, DAB, hematoxylin counterstain, original magnification × 400.)
Expression of BCL-3 protein in systemic ALCL tumors. (A) An ALK-positive ALCL tumor with strong nuclear immunoreactivity in almost all tumor cells. (B) An ALK-negative ALCL tumor with relatively weaker nuclear immunoreactivity in many tumor cells. (Immunoperoxidase, DAB, hematoxylin counterstain, original magnification × 400.)
Of interest, all 8 ALK-positive/BCL-3–positive ALCL tumors with available data expressed nuclear p50 and were positive for phosphorylated p65 protein. High nuclear expression levels of BCL-3 may sequester (p50)2 homodimers in the nucleus, thus preventing active p65/p50 heterodimers from binding to nuclear κB sites as suggested by Nishikori et al.1 This hypothesis provides a possible explanation for the differential effects of CD30 activation on apoptosis in ALCL and HL.9
In 2 of the 13 patients with BCL-3–positive tumors conventional cytogenetics was performed. The t(14;19)(q32;q13) was not present in either case. Nishikori and colleagues1 have reported that the mechanism of BCL-3 overexpression in ALK-positive ALCL is related to amplification of the gene or demethylation of CpG islands.
In conclusion, our results show that BCL-3 protein is expressed in most ALK-positive ALCL tumors but only in a small subset of ALK-negative tumors, thus providing further in vivo evidence for the differential BCL-3 expression among CD30-positive tumors.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal