Abstract
Interaction between receptor activator of nuclear factor κB ligand (RANKL) and RANK/osteoprotegerin (OPG) plays a dominant role in osteoclast activation and possibly in plasma cell survival in multiple myeloma (MM). We measured soluble RANKL (sRANKL), OPG, and bone remodeling markers in 121 patients with newly diagnosed MM to evaluate their role in bone disease and survival. Serum levels of sRANKL were elevated in patients with MM and correlated with bone disease. The sRANKL/OPG ratio was also increased and correlated with markers of bone resorption, osteolytic lesions, and markers of disease activity. The sRANKL/OPG ratio, C-reactive protein (CRP), and β2-microglobulin were the only independent prognostic factors predicting survival in multivariate analysis. We generated a prognostic index based on these factors that divided our patients into 3 risk groups. The low-risk group had a 96% probability of survival at 5 years, whereas the intermediate-risk and the high-risk groups had probabilities of survival of 52% and 0%, respectively. Not only do these results confirm for the first time in humans the importance of sRANKL/OPG in the development of bone disease, they also highlight the role of this pathway in the biology of plasma cell growth as reflected by its influence on survival.
Introduction
Multiple myeloma (MM) is a B-cell malignancy characterized by the accumulation of monoclonal plasma cells in the bone marrow. The bone marrow microenvironment plays an important role in myeloma growth and survival, as demonstrated by the effect of novel therapies that target the interactions of myeloma cells with stromal cells.1,2 Osteoclasts are activated through these interactions, which result in increased resorptive activity without a comparable increase in bone formation, thus leading to the development of osteolytic lesions characteristic of myeloma bone disease.3,4 Increased bone resorption is illustrated by the elevated serum levels of tartrate-resistant acid phosphatase isoform 5b (TRACP-5b), a novel resorption marker only produced by activated osteoclasts, and the elevated levels of the bone collagen degradation products, including the N-terminal cross-linking telopeptide of type 1 collagen (NTX) that can predict early progression of bone disease in MM.5-8 Bone alkaline phosphatase (bALP) and osteocalcin (OC), markers of bone formation, are strongly suppressed in MM patients.9 The overproduction of cytokines—including interleukin-6 (IL-6), IL-1α, tumor necrosis factor-α (TNF-α), and TNF-β—by stromal and myeloma cells had been recognized as the major factors for activating osteoclasts.10 Characterization of the receptor activator of nuclear factor κB ligand (RANKL) and its decoy receptor osteoprotegerin (OPG) system has facilitated understanding the pathophysiology of bone disease in MM.11 In vitro studies have shown that the increased ratio of RANKL/OPG in MM is the dominant, final mediator of osteoclastogenesis and is associated with increased osteoclast activation.12 RANKL is produced mainly by osteoblasts and marrow stromal cells as a membrane-bound protein; subsequently, it is cleaved into a soluble form (sRANKL) by the metalloprotease-disintegrin TNF-α–converting enzyme.13,14 In animal models, the inhibition of osteoclast activity by blocking RANKL is associated with the inhibition of myeloma cell growth, suggesting that this molecule is implicated not only in the development of bone disease but also in tumor growth and survival.12,15 OPG is decreased in MM and correlates with the extent of bone destruction, but it inhibits bone resorption when it is administered in myeloma patients.16,17 However, there is no information about the levels of sRANKL in patients with MM or any correlation with bone disease and survival.
The primary aim of this study was to evaluate the role of sRANKL, OPG, and serum markers of bone turnover (TRACP-5b, NTX, bALP, and OC) in myeloma bone disease and overall survival (OS) in 121 patients with newly diagnosed MM. A secondary aim was to evaluate the interrelationship between these markers and any correlation with markers of disease activity, including C-reactive protein (CRP), β2-microglobulin, paraprotein, and IL-6, to better understand the biology of myeloma bone disease and myeloma growth.
Patients, materials, and methods
Patients
One hundred twenty-one patients (61 men, 60 women) with newly diagnosed MM were studied. All diagnoses were made between 1992 and 2000, and patients' sera and urine were frozen at -70°C. Evidence of bone involvement was documented retrospectively; old radiology reports were used. Patients were considered to have bone involvement if radiographic findings were consistent with MM bone disease, including osteoporosis, osteolytic lesions, and fractures. Although bone pain was documented, the presence of bone pain alone was not considered indicative of bone disease in the absence of abnormal radiographic findings. Bone morbidity was graded in 3 groups according to the radiographic evaluation of the skeleton. Group A included patients with no lytic lesions or osteoporosis alone; group B included patients with 1 to 3 osteolytic lesions; group C included patients with more than 3 osteolytic lesions, pathologic fracture resulting from MM, or both. The following biochemical parameters were evaluated: sRANKL, OPG, bone resorption markers (TRACP-5b, NTX), bone-formation markers (bALP, OC), IL-6, paraprotein, CRP, and β2-microglobulin.
To establish a range for normal values for the above biochemical parameters of bone turnover, 45 healthy control donors of similar age and sex distributions to the patients were also tested. The median age of these controls was 61.5 years (range, 34-79 years). The medical record of each control was reviewed to ensure that they had no evidence of bone disease and that they were not taking any medication that could alter the normal bone turnover. This study was conducted with the approval of the Ethical Committee of Hammersmith Hospital (Faculty of Medicine, Imperial College London) and under the guidelines of the Declaration of Helsinki.
Measurement of markers of bone turnover
Levels of sRANKL and OPG in the serum were determined using enzyme-linked immunosorbent assay (ELISA; Biomedica Medizin-produkte, Gesellschaft GmbH, Vienna, Austria). The range of controls tested for sRANKL and OPG were 0 to 50 pM and 0 to 30 pM, respectively. Sensitivity levels for these markers were 0.4 pM for sRANKL and 0.14 pM for OPG. Intra-assay and interassay coefficients of variation (CV) were less than 10% for both tests. Serum TRACP-5b was measured using a solid-phase immunofixed enzyme activity assay (BoneTRAP assay; SBA, Oulu, Finland). Sensitivity of the assay was 0.06 U/L; intra-assay and interassay CVs were less than 6% and 8%, respectively. Normal values ranged from 0.5 to 3.8 U/L for men and premenopausal women, and they were 4.8 U/L for postmenopausal women. Urinary NTX excretion was also quantified using ELISA assay (Osteomark NTX urine; Ostex International, Seattle, WA) with intra-assay and interassay CVs of 7.6% and 4%, respectively. Normal values ranged from 20 to 55 nM bone collagen equivalent (BCE)/mM creatinine (up to 75 nM BCE/mM creatinine for postmenopausal women). Serum bALP was determined using an immunoassay with a monoclonal antibody directed against bALP purified from human SAOS-2 osteosarcoma cells as a standard, followed by a conventional colorimetric detection using paranitrophenyl phosphate (Alkphase-B; Metra Biosystems, Mountain View, CA). Sensitivity of the assay was 0.7 U/L; intra-assay and interassay CVs were less than 6% and 8%, respectively; normal values ranged from 45 to 130 U/L. An ELISA assay was also used to determine the values of OC (N/MID Osteocalcin; Osteometer BioTech A/S, Herley, Denmark) (normal ranges, 11-46 ng/mL for men and 25-48 ng/mL for postmenopausal women) and of IL-6 (Genzyme Diagnostics, San Carlos, CA; normal values, less than 3 pg/mL).
Statistical analysis
The primary end point for our study was to determine whether there was any association between survival and different levels of a given marker. Survival probabilities were calculated using the Kaplan-Meier method, and comparisons were made using the log-rank test to identify potential prognostic factors. Variables found to be statistically significant at the P < .2 level were entered into a proportional hazards regression analysis that used a backward-stepping procedure to identify the most statistically significant model. A prognostic index for each patient was then calculated by adding the scores of the 3 variables from the multivariate analysis. Associations between bone disease stage and biochemical markers were examined using the Kruskal-Wallis test, whereas the Spearman Rank correlation test was used to examine relationships between various parameters and clinical patient characteristics. Differences between patients and controls were evaluated using the Wilcoxon rank sum test. All P values were 2 sided, and confidence intervals referred to 95% boundaries.
Results
Patients
Table 1 summarizes the characteristics of the 121 patients. Of these, 111 (91.7%) patients had at least 1 lytic lesion on the skeletal survey, whereas 28 (23.1%) patients had evidence of pathologic fractures at diagnosis. Almost 10% of patients had increased calcium levels, whereas most patients had increased β2-microglobulin (greater than 3 mg/L) and serum IL-6 (greater than 10 pg/mL) levels. There were no data on cytogenetics in this cohort of patients.
Patient characteristics
. | No. . | % . |
|---|---|---|
| Patients | 121 | 100 |
| Age, median (range), y | 68 (31-89) | — |
| Sex | ||
| Male | 61 | 50.4 |
| Female | 60 | 49.5 |
| Stage | ||
| I | 4 | 3.3 |
| IIA | 31 | 25.6 |
| IIIA | 67 | 55.3 |
| IIIB | 19 | 15.7 |
| Immunologic subtype | ||
| IgG | 62 | 51.2 |
| IgA | 34 | 28.0 |
| IgD | 1 | 0.8 |
| BJ | 19 | 15.7 |
| Nonsecretory | 5 | 4.1 |
| Bone disease | ||
| A, no lytic lesions or osteoporosis | 10 | 8.2 |
| B, 1-3 lytic lesions | 28 | 23.1 |
| C, more than 3 lytic lesions or fractures | 83 | 68.5 |
| Hemoglobin level, less than 10 g/dL | 36 | 29.7 |
| Calcium level, greater than 3 mM | 12 | 9.9 |
| Albumin level, less than 30 g/L | 14 | 11.5 |
| β2-microglobulin level, greater than 3 mg/L | 64 | 52.8 |
| CRP level, greater than 10 mg/L | 32 | 26.4 |
| IL-6 level, greater than 30 pg/mL | 52 | 42.9 |
. | No. . | % . |
|---|---|---|
| Patients | 121 | 100 |
| Age, median (range), y | 68 (31-89) | — |
| Sex | ||
| Male | 61 | 50.4 |
| Female | 60 | 49.5 |
| Stage | ||
| I | 4 | 3.3 |
| IIA | 31 | 25.6 |
| IIIA | 67 | 55.3 |
| IIIB | 19 | 15.7 |
| Immunologic subtype | ||
| IgG | 62 | 51.2 |
| IgA | 34 | 28.0 |
| IgD | 1 | 0.8 |
| BJ | 19 | 15.7 |
| Nonsecretory | 5 | 4.1 |
| Bone disease | ||
| A, no lytic lesions or osteoporosis | 10 | 8.2 |
| B, 1-3 lytic lesions | 28 | 23.1 |
| C, more than 3 lytic lesions or fractures | 83 | 68.5 |
| Hemoglobin level, less than 10 g/dL | 36 | 29.7 |
| Calcium level, greater than 3 mM | 12 | 9.9 |
| Albumin level, less than 30 g/L | 14 | 11.5 |
| β2-microglobulin level, greater than 3 mg/L | 64 | 52.8 |
| CRP level, greater than 10 mg/L | 32 | 26.4 |
| IL-6 level, greater than 30 pg/mL | 52 | 42.9 |
Ig indicates immunoglobulin; BJ, Bence-Jones
Bone markers and evidence of bone involvement. Patients with MM had elevated median sRANKL, TRACP-5b, and NTX values compared with controls, but serum levels of OPG, OC, and bALP were lower than they were in controls (Table 2). The ratio of sRANKL/OPG was also significantly higher in MM patients than in the control group (P < .0001). Even patients with no lytic lesions (group A) had higher sRANKL/OPG ratios than controls (P = .031).
Serum markers of bone turnover of patients and controls
Serum marker . | MM patients median (range) . | Controls median (range) . | P . |
|---|---|---|---|
| sRANKL, pM | 7.06 (0.88-41.94) | 2.66 (0.81-4.73) | <.0001 |
| OPG, pM | 4.71 (0.31-16.66) | 7.98 (7.77-8.54) | .007 |
| sRANKL/OPG ratio | 1.58 (0.10-44.04) | 0.33 (0.10-0.59) | <.0001 |
| Osteoclastic activity markers | |||
| NTX (nM BCE/mM creatinine) | 132 (25-571) | 37.9 (11-76.2) | <.0001 |
| TRACP-5b (U/L) | 6.57 (1.32-47.12) | 3.11 (1-5) | <.0001 |
| Osteoblastic activity markers | |||
| bALP (U/L) | 43.1 (12-98.4) | 93.9 (45.3-124) | .01 |
| OC (ng/mL) | 16.0 (1-52) | 37 (25-52.8) | <.0001 |
Serum marker . | MM patients median (range) . | Controls median (range) . | P . |
|---|---|---|---|
| sRANKL, pM | 7.06 (0.88-41.94) | 2.66 (0.81-4.73) | <.0001 |
| OPG, pM | 4.71 (0.31-16.66) | 7.98 (7.77-8.54) | .007 |
| sRANKL/OPG ratio | 1.58 (0.10-44.04) | 0.33 (0.10-0.59) | <.0001 |
| Osteoclastic activity markers | |||
| NTX (nM BCE/mM creatinine) | 132 (25-571) | 37.9 (11-76.2) | <.0001 |
| TRACP-5b (U/L) | 6.57 (1.32-47.12) | 3.11 (1-5) | <.0001 |
| Osteoblastic activity markers | |||
| bALP (U/L) | 43.1 (12-98.4) | 93.9 (45.3-124) | .01 |
| OC (ng/mL) | 16.0 (1-52) | 37 (25-52.8) | <.0001 |
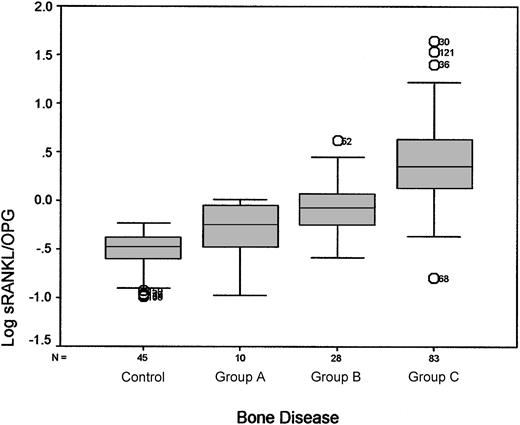
There was a strong correlation between the sRANKL/OPG ratio, the serum levels of TRACP-5b, NTX, and OC, and the extent of bone disease as graded (P < .0001, P < .0001, P < .001, and P = .023, respectively). Serum levels of sRANKL and OPG alone showed a correlation with MM bone disease. Figure 1 shows the relationship between the ratio of sRANKL/OPG and the extent of bone destruction. There was also a strong correlation between IL-6 levels and MM bone involvement (P < .01), but there was no correlation between bALP levels and the extent of bone disease (P = .91).
Association between log sRANKL/OPG ratio and extent of bone disease as assessed by radiographic evaluation. Median values (ranges) of the sRANKL/OPG ratio for controls and patients were as follows: controls, 0.33 (0.10-0.59); group A (no lytic lesions or osteoporosis), 0.57 (0.10-1.01); group B (1-3 lytic lesions), 0.84 (0.26-4.16); group C (more than 3 lytic lesions or fractures), 2.25 (0.43-44.04). Circled numbers referred to patient outliers.
Association between log sRANKL/OPG ratio and extent of bone disease as assessed by radiographic evaluation. Median values (ranges) of the sRANKL/OPG ratio for controls and patients were as follows: controls, 0.33 (0.10-0.59); group A (no lytic lesions or osteoporosis), 0.57 (0.10-1.01); group B (1-3 lytic lesions), 0.84 (0.26-4.16); group C (more than 3 lytic lesions or fractures), 2.25 (0.43-44.04). Circled numbers referred to patient outliers.
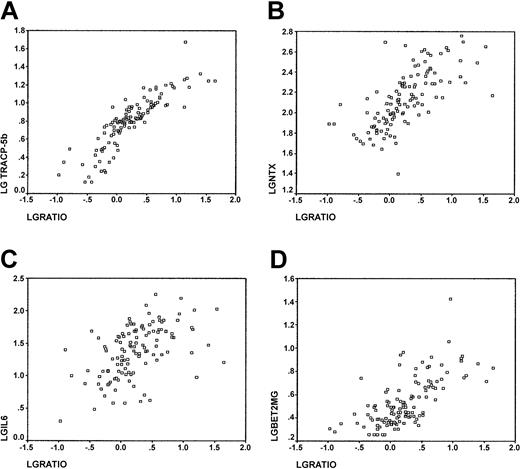
The sRANKL/OPG ratio also showed strong correlation with serum levels of TRACP-5b and NTX (P < .0001, and P < .0001, respectively; Figure 2A-B) and with OC levels (P = .023). There was no correlation between sRANKL/OPG and serum bALP levels (P = .73).
Correlations with sRANKL/OPG ratio. Correlations between sRANKL/OPG ratio and (A) TRACP-5b serum levels (r = 0.87; P < .0001); (B) NTX urinary levels (r = 0.69; P < .0001); (C) IL-6 serum levels (r = 0.52; P < .0001); and (D) β2-microglobulin levels (r = 0.68; P < .0001).
Correlations with sRANKL/OPG ratio. Correlations between sRANKL/OPG ratio and (A) TRACP-5b serum levels (r = 0.87; P < .0001); (B) NTX urinary levels (r = 0.69; P < .0001); (C) IL-6 serum levels (r = 0.52; P < .0001); and (D) β2-microglobulin levels (r = 0.68; P < .0001).
Correlation of bone markers with known prognostic factors in MM. We studied the correlation between serum markers of bone turnover and β2-microglobulin, CRP, IL-6, and stage of MM. There was a strong correlation between sRANKL/OPG ratio and both serum levels of β2-microglobulin and IL-6 (P < .0001, Figure 2C; P < .0001, Figure 2D, respectively) but not with CRP (P = .11). Serum levels of IL-6 also showed a significant association with markers of bone resorption (P < .0001 and P < .0001 for TRACP-5b and NTX, respectively) but not with markers of bone formation (P = .068 and P = .386 for OC and bALP, respectively). There was a strong correlation between β2-microglobulin and markers of bone resorption (P < .0001 and P < .0001 for TRACP-5b and NTX, respectively), IL-6 (P < .003), and OC (P = .003) but not with bALP (P = .15). The ratio of sRANKL/OPG also correlated with the stage of MM (P < .0001). Only 1 of 4 patients with stage I disease had a ratio greater than 0.59, the upper limit observed in the control group. Median values of the sRANKL/OPG ratio for stages II and III MM patients were 0.88 (range, 0.29-4.50) and 4.36 (range, 0.16-44.04), respectively, much higher than the controls (median value, 0.33; range, 0.10-0.59). Disease stage correlated with TRACP-5b and NTX (P < .0001 and P = .014, respectively), showing a borderline correlation with OC (P = .046) but no correlation with bALP (P = .73) or IL-6 (P = .08).
Prognostic significance of sRANKL/OPG ratio and other serum markers of bone turnover in MM
The median overall survival time for the 121 patients was 56.9 months. Eighty-two patients had received only conventional chemotherapy (1-5 lines of treatment), whereas 38 patients had been given high-dose therapy with autologous stem cell support; 1 patient underwent allogeneic stem cell transplantation. Sixteen patients were never administered bisphosphonates as part of antimyeloma treatment; however, 49 patients had been given pamidronate or clodronate from the beginning of their antimyeloma therapy, and 56 patients had been given pamidronate, zoledronic acid, or clodronate at some stage of their treatment.
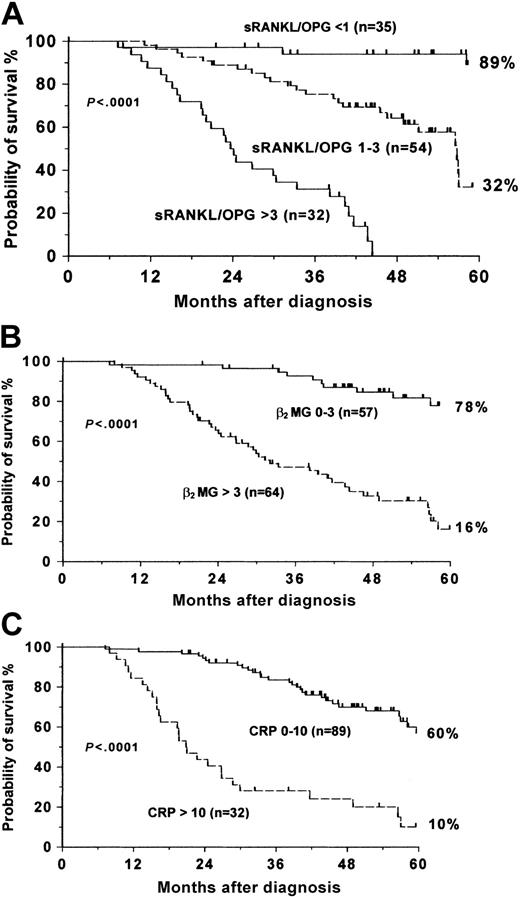
Eleven of the 17 variables that we analyzed showed a significant association with survival in the univariate analysis presented in Table 3. These parameters were stage of disease, treatment modality, myeloma bone disease, sRANKL, OPG, the sRANKL/OPG ratio, TRACP-5b, NTX, β2-microglobulin, CRP, and IL-6. However, in multivariate analysis, only sRANKL/OPG ratio, β2-microglobulin level, and CRP were found to be independent prognostic variables for survival (Table 4). Figure 3 shows the probability of survival of patients based on each of these 3 factors. The sRANKL/OPG ratio was the most important prognostic factor in this analysis, giving a 5-year probability for survival of 89% if its value was less than 1, 32% if the ratio was between 1 and 3, and 0% if it was more than 3 (Figure 3A).
Univariate analysis of potential prognostic factors
Parameter . | No. patients . | 5-y survival probability rate, % . | P . |
|---|---|---|---|
| Sex | .49 | ||
| Male | 61 | 39.2 | |
| Female | 60 | 52.2 | |
| Age, y | .11 | ||
| Younger than 60 | 20 | 65.4 | |
| 60-70 | 48 | 46.9 | |
| Older than 70 | 53 | 37.0 | |
| Stage | < .0001 | ||
| I | 4 | 100 | |
| II | 31 | 72.0 | |
| IIIA | 67 | 40.6 | |
| IIIB | 19 | 9.2 | |
| MM subtype | .21 | ||
| IgG | 62 | 42.5 | |
| IgA | 34 | 38.4 | |
| BJ | 19 | 57.9 | |
| NS | 5 | 100 | |
| Treatment | .02 | ||
| Chemotherapy alone | 82 | 39.8 | |
| HDT + ASCT | 38 | 60.5 | |
| Bone disease | .0002 | ||
| A | 10 | 88.9 | |
| B | 28 | 67.7 | |
| C | 83 | 31.7 | |
| sRANKL, pM | < .0001 | ||
| 0-8 | 75 | 65.7 | |
| Greater than 8 | 46 | 10.9 | |
| OPG, pM | .001 | ||
| Less than 3 | 30 | 6.9 | |
| 3-6 | 58 | 26.9 | |
| Greater than 6 | 33 | 78.8 | |
| sRANKL/OPG ratio | < .0001 | ||
| Less than 1 | 35 | 89.1 | |
| 1-3 | 54 | 32.1 | |
| Greater than 3 | 32 | 0 | |
| TRACP-5b, U/L | < .0001 | ||
| Less than 5 | 31 | 91.7 | |
| 5-10 | 66 | 30.5 | |
| Greater than 10 | 24 | 8.2 | |
| NTX, nm BCE/nM creatinine | < .0001 | ||
| Less than 80 | 27 | 87.9 | |
| 80-150 | 46 | 46.0 | |
| Greater than 150 | 48 | 20.8 | |
| OC, ng/mL | .072 | ||
| Less than 10 | 41 | 34.0 | |
| 10-20 | 29 | 55.2 | |
| Greater than 20 | 51 | 51.4 | |
| bALP, U/L | .678 | ||
| Less than 20 | 30 | 34.7 | |
| 20-50 | 54 | 53.4 | |
| Greater than 50 | 37 | 39.3 | |
| β2-microglobulin, mg/L | < .0001 | ||
| 0-3 | 57 | 77.9 | |
| Greater than 3 | 64 | 16.2 | |
| CRP, mg/L | < .0001 | ||
| 0-10 | 89 | 60.0 | |
| Greater than 10 | 32 | 10.0 | |
| IL-6, pg/mL | < .0001 | ||
| Less than 12 | 32 | 79.8 | |
| 12-30 | 37 | 46.0 | |
| 31-50 | 25 | 29.9 | |
| Greater than 50 | 27 | 0 | |
| Paraprotein (IgG, IgA, IgD MM) | 0.18 | ||
| 0-30 g/L | 55 | 51.0 | |
| Greater than 30 g/L | 42 | 32.4 |
Parameter . | No. patients . | 5-y survival probability rate, % . | P . |
|---|---|---|---|
| Sex | .49 | ||
| Male | 61 | 39.2 | |
| Female | 60 | 52.2 | |
| Age, y | .11 | ||
| Younger than 60 | 20 | 65.4 | |
| 60-70 | 48 | 46.9 | |
| Older than 70 | 53 | 37.0 | |
| Stage | < .0001 | ||
| I | 4 | 100 | |
| II | 31 | 72.0 | |
| IIIA | 67 | 40.6 | |
| IIIB | 19 | 9.2 | |
| MM subtype | .21 | ||
| IgG | 62 | 42.5 | |
| IgA | 34 | 38.4 | |
| BJ | 19 | 57.9 | |
| NS | 5 | 100 | |
| Treatment | .02 | ||
| Chemotherapy alone | 82 | 39.8 | |
| HDT + ASCT | 38 | 60.5 | |
| Bone disease | .0002 | ||
| A | 10 | 88.9 | |
| B | 28 | 67.7 | |
| C | 83 | 31.7 | |
| sRANKL, pM | < .0001 | ||
| 0-8 | 75 | 65.7 | |
| Greater than 8 | 46 | 10.9 | |
| OPG, pM | .001 | ||
| Less than 3 | 30 | 6.9 | |
| 3-6 | 58 | 26.9 | |
| Greater than 6 | 33 | 78.8 | |
| sRANKL/OPG ratio | < .0001 | ||
| Less than 1 | 35 | 89.1 | |
| 1-3 | 54 | 32.1 | |
| Greater than 3 | 32 | 0 | |
| TRACP-5b, U/L | < .0001 | ||
| Less than 5 | 31 | 91.7 | |
| 5-10 | 66 | 30.5 | |
| Greater than 10 | 24 | 8.2 | |
| NTX, nm BCE/nM creatinine | < .0001 | ||
| Less than 80 | 27 | 87.9 | |
| 80-150 | 46 | 46.0 | |
| Greater than 150 | 48 | 20.8 | |
| OC, ng/mL | .072 | ||
| Less than 10 | 41 | 34.0 | |
| 10-20 | 29 | 55.2 | |
| Greater than 20 | 51 | 51.4 | |
| bALP, U/L | .678 | ||
| Less than 20 | 30 | 34.7 | |
| 20-50 | 54 | 53.4 | |
| Greater than 50 | 37 | 39.3 | |
| β2-microglobulin, mg/L | < .0001 | ||
| 0-3 | 57 | 77.9 | |
| Greater than 3 | 64 | 16.2 | |
| CRP, mg/L | < .0001 | ||
| 0-10 | 89 | 60.0 | |
| Greater than 10 | 32 | 10.0 | |
| IL-6, pg/mL | < .0001 | ||
| Less than 12 | 32 | 79.8 | |
| 12-30 | 37 | 46.0 | |
| 31-50 | 25 | 29.9 | |
| Greater than 50 | 27 | 0 | |
| Paraprotein (IgG, IgA, IgD MM) | 0.18 | ||
| 0-30 g/L | 55 | 51.0 | |
| Greater than 30 g/L | 42 | 32.4 |
HDT indicates high-dose therapy; ASCT, autologous stem cell transplantation
Variables with independent prognostic importance for survival according to multivariate Cox analysis and scoring based on this analysis
Parameter . | N . | Relative risk (95% CI) . | P . | Prognostic score, points . |
|---|---|---|---|---|
| sRANKL/OPG ratio | ||||
| Less than 1 | 35 | 1.00 | 1 | |
| 1-3 | 54 | 7.8 (2.5-23.7) | < .001 | 2 |
| Greater than 3 | 32 | 24.7 (7.2-84.4) | < .001 | 4 |
| CRP | ||||
| 10 or less | 89 | 1.00 | 2 | |
| Greater than 10 | 32 | 2.9 (1.6-5.2) | .001 | 3 |
| β2-microglobulin | ||||
| 3 or less | 57 | 1.00 | 1 | |
| Greater than 3 | 64 | 2.2 (1.03-4.9) | .043 | 3 |
Parameter . | N . | Relative risk (95% CI) . | P . | Prognostic score, points . |
|---|---|---|---|---|
| sRANKL/OPG ratio | ||||
| Less than 1 | 35 | 1.00 | 1 | |
| 1-3 | 54 | 7.8 (2.5-23.7) | < .001 | 2 |
| Greater than 3 | 32 | 24.7 (7.2-84.4) | < .001 | 4 |
| CRP | ||||
| 10 or less | 89 | 1.00 | 2 | |
| Greater than 10 | 32 | 2.9 (1.6-5.2) | .001 | 3 |
| β2-microglobulin | ||||
| 3 or less | 57 | 1.00 | 1 | |
| Greater than 3 | 64 | 2.2 (1.03-4.9) | .043 | 3 |
Abbreviations are defined in the note to Table 3
Probability of survival of MM patients for each of the 3 independent prognostic variables. (A) sRANKL/OPG ratio. (B) β2-microglobulin. (C) CRP.
Probability of survival of MM patients for each of the 3 independent prognostic variables. (A) sRANKL/OPG ratio. (B) β2-microglobulin. (C) CRP.
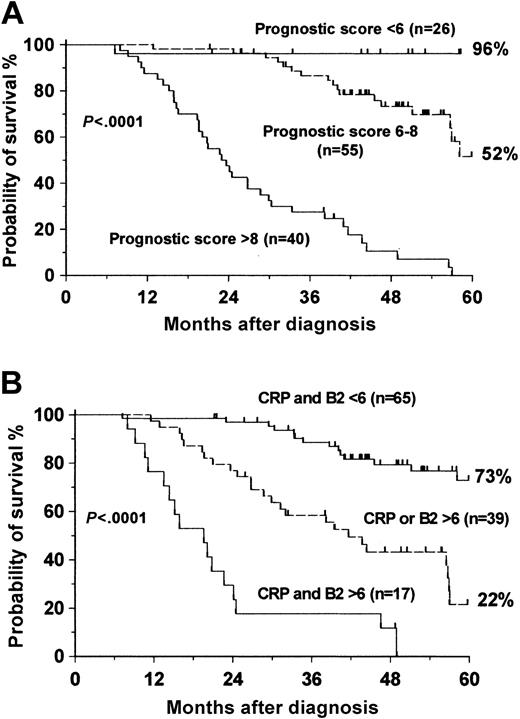
Based on the survival curves of each of the independent prognostic parameters, we created a risk score that is illustrated in Table 4 (Hammersmith Prognostic Index). An sRANKL/OPG ratio less than 1 and β2-microglobulin levels less than or equal to 3 mg/L were given 1 point; an sRANKL/OPG ratio between 1 and 3 and CRP levels less than or equal to 10 mg/L were given 2 points; CRP levels greater than 10 mg/L and β2-microglobulin levels greater than 3 mg/L were given 3 points; and an sRANKL/OPG ratio greater than 3 was given 4 points. According to this index, a patient could have a score between 4 and 10. This system subdivided our patients into 3 groups. The low-risk group was composed of 26 patients who had a prognostic score lower than 6; the intermediate-risk group was composed of 55 patients having a prognostic score between 6 and 8; and the high-risk group was composed of 40 patients with a prognostic score of greater than 8. The 5-year probability rates for survival were 96% in the low-risk group, 52% in the intermediate-risk group, and 0% in the high-risk group (Figure 4A).
Probability of survival of MM patients. (A) Based on the proposed Hammersmith Prognostic Index. (B) Based on the Bataille Prognostic Index.
Probability of survival of MM patients. (A) Based on the proposed Hammersmith Prognostic Index. (B) Based on the Bataille Prognostic Index.
Discussion
MM cell survival and proliferation depends on the interaction of MM cells with the bone marrow microenviroment.1,2 The attachment of myeloma cells with stromal cells, through a paracrine loop of cytokine production, leads to osteoclast activation and bone disease, one of the major clinical features of MM.3,4 The RANK/RANKL/OPG system has recently been recognized as the final, dominant mediator for osteoclastogenesis, whereas RANKL seems to have an effect on MM cell growth.12,15 The RANKL/OPG ratio is increased in MM because of the up-regulation of RANKL expression and the down-regulation and degradation of OPG by plasma cells, leading to osteoclast activation and increased bone destruction.11,18,19 Although the expression of RANKL in bone marrow stromal cells has been demonstrated by immunochemical examination of bone marrow biopsy samples from MM patients, the role of sRANKL in bone disease and survival in MM patients has not been investigated.20 OPG is decreased in patients with MM and seems to have no impact on survival, whereas degradation products of bone collagen, namely NTX and C-terminal telopeptide of collagen type 1 (ICTP), have been shown in previous reports to correlate with OS.16,21,22 The aim of this study was to evaluate the role of sRANKL, OPG, and serum markers of bone turnover in myeloma bone disease and to correlate them with clinical and laboratory characteristics of the patients, including overall survival.
This is the first report of the importance of serum levels of sRANKL in MM. Median serum sRANKL was 2.6-fold higher in MM patients than in controls. There was strong correlation between the levels of sRANKL, serum levels of TRACP-5b and NTX, and the extent of bone disease as assessed by radiographic images. These results provide the first evidence that in MM patients sRANKL correlates with osteoclast activation and bone resorption, as measured by TRACP-5 and NTX levels. TRACP-5b is a novel marker that is expressed only by resorbing osteoclasts.5 It is elevated in patients with osteoporosis; however, our group has shown that TRACP-5b levels were increased in MM and were suppressed by bisphosphonate administration.6,7,23 In our series of patients, TRACP-5b levels were significantly elevated compared with levels in controls, and they correlated with NTX and the number of osteolytic lesions. This is a further confirmation that bone resorption in myeloma is caused mainly by osteoclast activation and that TRACP-5b is a sensitive, novel bone marker for evaluating resorbing activity in MM.
Two previous reports show that serum OPG is decreased in MM.16,24 It has been proposed that myeloma cells inhibit OPG gene expression by osteoblasts and promote OPG binding to heparan sulfate chains of syndecan-1 expressed in MM cells, which internalize and degrade OPG.18,19 In our study, OPG levels were 36% lower in MM patients than in controls and were inversely related to the number of osteolytic lesions, TRACP-5b, and NTX levels. In the Nordic and American studies, OPG serum levels were 18% and 29% lower than those of controls, respectively.16,24 The difference between these studies and our study is possibly a result of the low number of stage I patients in our series. It is also interesting that, in contrast to previous reports, OPG levels correlated with β2-microglobulin, CRP, and IL-6 levels.
In several animal models of MM, the RANKL/OPG ratio is increased, reflecting the perturbed balance of these molecules in the bone marrow microenviroment.11,18,19 The increase in this ratio enhances osteoclastogenesis and osteoclast activation, thus leading to increased bone resorption, elevated collagen degradation products, and development of bone lesions (Figure 5). The sRANKL/OPG ratio correlated strongly with the extent of bone disease and with serum levels of TRACP-5b and NTX (Figures 1, 2A-B). Even MM patients with no osteolytic lesions or osteoporosis had higher values of sRANKL/OPG ratio than controls. These data confirm for the first time in humans the results of in vitro and animal model studies of the role of RANKL/OPG on the development of bone disease in MM. Moreover, there was a strong correlation between the sRANKL/OPG ratio with β2-microglobulin and Durie-Salmon stage of MM. Therefore, the ratio may serve not only as a marker of bone disease, it may reflect tumor burden as well—an observation that is further strengthened by the association of the ratio with the overall survival of MM patients, irrespective of the treatment modality. Patients whose ratio value was less than 1 had a 5-year probability of survival rate of 89%; no patient with a ratio level greater than 3 survived for more than 4 years (Figure 3A).
Role of the MM microenvironment in osteoclast activation. Adherence of MM cells to bone marrow stromal cells (BMSCs) enhances the production of RANKL and IL-6, whereas it suppresses the production of OPG (the decoy receptor of RANKL). RANK/RANKL interaction results in osteoclast differentiation, proliferation, and activation, leading to increased bone resorption (increased levels of TRACP-5b and NTX).
Role of the MM microenvironment in osteoclast activation. Adherence of MM cells to bone marrow stromal cells (BMSCs) enhances the production of RANKL and IL-6, whereas it suppresses the production of OPG (the decoy receptor of RANKL). RANK/RANKL interaction results in osteoclast differentiation, proliferation, and activation, leading to increased bone resorption (increased levels of TRACP-5b and NTX).
Our study also confirms previous observations that bone formation is severely inhibited in MM. Both OC and bALP were decreased compared with controls, but only OC correlated with the extent of bone disease. Neither OC nor bALP showed any correlation with overall survival.
MM is a heterogeneous disease, and a number of prognostic systems have been proposed. They are necessary for identifying patients with low- or high-risk disease, which would influence the treatment decision. To better discriminate between different risk groups of patients with MM, we tested 17 parameters; 11 of these were found to predict for survival using univariate analysis. As previously described, IL-6 and degradation products of bone collagen, such as NTX, were found to have prognostic significance.21,22 TRACP-5b and OPG also had significant prognostic value in the univariate system. TRACP-5b has been tested for the first time and has provided a reliable index for prognosis, giving a 5-year probability of survival of approximately 91% in patients with normal values. Serum OPG levels, in contrast to a previous observation, predicted survival with a 5-year survival probability of 78% in patients with values greater than 6 pM.16 However, this study showed that serum NTX, TRACP-5b, and OPG levels did not give independent prognostic information because of their significant correlation with the sRANKL/OPG ratio and β2-microglobulin. Serum levels of IL-6 also failed to be informative in the multivariate system because of the correlation with CRP. Multivariate analysis revealed that only the sRANKL/OPG ratio, CRP, and β2-microglobulin were independent prognostic factors in this cohort of patients (Table 4). We generated a scoring system based on this analysis, which subdivided our patients into 3 groups (Hammersmith Prognostic Index). The low-risk group achieved a 5-year probability of survival rate of 96%, and the intermediate-risk group achieved a 52% rate; all patients in the high-risk group died by 5 years. We compared our prognostic system with that proposed by Bataille et al,25 and we found that our model had better discriminating power in identifying patients at low or intermediate risk. Bataille's system allowed stratification of MM patients into 3 groups according to CRP and β2-microglobulin levels. The 5-year probability for survival rates for our cohort of patients, based on this system, were 73% for the low-risk group, 22% for the intermediate-risk group, and 0% for the high-risk group (Figure 4B), whereas in our system the respective values were 96%, 52%, and 0% (Figure 4A).
The favorable prognosis for patients with low sRANKL/OPG ratios gives credence to the rationale for developing novel antimyeloma drugs by targeting the RANKL/OPG interaction, either by inhibiting RANKL expression or by inducing OPG production. The blockage of RANKL by RANK-Fc antibody, a recombinant RANKL antagonist, in myeloma mice models (SCID ARH-77 xenograft, SCID-hu-MM mice) inhibited not only bone resorption but also myeloma cell growth.12,15,26 Moreover, recombinant OPG administration in murine models of MM resulted in decreased tumor growth and increased survival.27 The same effect was reported after OPG gene transfer in a SCID ARH-77 xenograft model.28 These observations are in keeping with our results regarding the prognostic value of the sRANKL/OPG ratio. It is of interest that neither RANK-Fc antibody nor recombinant OPG had a direct effect on myeloma cell survival, a fact that points out the importance of the interaction of MM cells with stromal cells for the accumulation and survival of plasma cells in the bone marrow. The exact mechanism by which the RANKL/OPG system interferes with myeloma cell survival is not yet clear. It has been postulated that RANKL may enhance the production of cytokines, such as IL-6, which are necessary for myeloma cell growth, by osteoclasts.12 In humans, recombinant OPG has been administered in menopausal women with osteoporosis, has caused a rapid suppression of bone resorption, as assessed by NTX levels, and has delayed the reduction of bone formation.29 A recent phase 1 study described the effect of recombinant OPG (AMGN-0007) in 28 patients with MM and 26 with breast cancer. OPG led to a dramatic and sustained decrease of NTX without severe side effects.17
Our results suggest that the RANKL/OPG system is essential not only for the development of myeloma bone disease but also for the biology of plasma cell growth, as reflected by its influence on survival. Although further studies with greater numbers of patients are needed to confirm these observations, we have identified the RANKL/OPG pathway as a potential target for novel therapeutic modalities in future clinical trials.
Prepublished online as Blood First Edition Paper, April 10, 2003; DOI 10.1182/blood-2003-02-0380.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal