Abstract
Hematopoietic cells have secretory lysosomes that degranulate at the inflammatory site upon stimulation. We asked whether one could target exogenous proteins with a therapeutic potential to secretory lysosomes in hematopoietic cells. For this purpose, we expressed a soluble tumor necrosis factor (TNF) receptor form (sTNFR1) in hematopoietic cell lines. In order to accomplish targeting to secretory lysosomes, both endoplasmic reticulum (ER) retention and constitutive secretion have to be prevented. ER export was facilitated by addition of a transmembrane (tm) sequence, and constitutive secretion was overcome by incorporating a cytosolic sorting signal (Y) from CD63. This signal directed the resulting sTNFR1-tm-Y to secretory lysosomes. Confirmation of these results was provided by biosynthetic radiolabeling, subcellular fractionation, immunofluorescence microscopy, and immunoelectron microscopy. The tm-Y fragment was cleaved by proteolysis, resulting in generation of the membrane-free sTNFR1 in secretory lysosomes. Our results suggest a potential for using the storage organelles of hematopoietic cells as vehicles for targeting sites of inflammation with therapeutically active agents.
Introduction
Hematopoietic cells such as neutrophil granulocytes, natural killer (NK) cells, and cytotoxic T lymphocytes (CTLs) have a critical role in host defense. As a consequence, individuals who lack neutrophils can be stricken by serious bacterial infections since elimination of microorganisms relies on phagocytosis by these cells followed by the release of cytolytic proteins into the phagosome for killing.1 In contrast, NK cells and CTLs destroy virus-infected and malignant cells through regulated secretion of cytolytic proteins to the exterior.2,3 Although they have different biologic functions in host defense, the various hematopoietic cells are characteristically furnished with many bioactive agents. These are specific for each cell type and are stored in subsets of granules manufactured during hematopoietic differentiation. Bioactive agents play a critical role after they are released from the granules.4,5 Specific hematopoietic cells, such as neutrophils, have many subsets of storage granules among which the lysosome-like azurophil granules are the first to be manufactured. Such granules are equipped with antimicrobial proteins and cationic serine proteases as well as lysosomal hydrolases.4,5 At a later maturation stage, specific (secondary) granules are manufactured in these cells that contain a unique composition of antimicrobial proteins, matrix metalloproteases, and other constituents.4
Clearly, hematopoietic cells have lysosome-related organelles that package and store specialized cytolytic proteins together with lysosomal hydrolases.2,3 These organelles are designated secretory lysosomes, as they display a dual function and are distinguished from conventional lysosomes by the additional ability to undergo secretion upon stimulation.3 Compelling evidence indicating a common critical secretory mechanism for cells with secretory lysosomes comes from observations in the Chediak-Higashi syndrome, a syndrome characterized by mutation of the LYST gene.2,3 In this syndrome the immune function of cells with secretory lysosomes is impaired, while the nonsecretory function of conventional lysosomes is retained and is normal.
The granule targeting process requires retrieval of newly synthesized proteins from the constitutive secretory pathway. While the delivery of the membrane proteins into secretory lysosomes relies on cytoplasmic sorting peptide sequences,3 the mechanism for delivery of matrix proteins into these organelles is less well defined.5 Secretory lysosomes have the morphology of multivesicular bodies that commonly contain internal vesicles as well as dense cores. These bodies may serve different functions, for example, a lytic function in the case of internal vesicles and a storage function in the case of dense cores.3 A role of the endosomal pathway for organelle biogenesis is obvious and consistent with the involvement of the mannose-6-phosphate receptor (MPR) system normally used for targeting lysosomal enzymes.3 The secretory granzymes of NK cells and activated CTLs are sorted by means of the mannose-6-phosphate pathway.3 Neutrophil precursors, on the other hand, do not depend on this pathway for targeting into lysosome-related azurophil granules.5 Granule formation for the regulated secretory pathway in endocrine, neuroendocrine, and exocrine cells does not use the endosomal pathway. In these cells, proteins are routed to dense secretory vesicles within the trans Golgi network (TGN), followed by a granule maturation process involving removal and rerouting of mis-sorted, soluble proteins and membrane proteins to lysosomes.6-8 A similar pathway may be used, in addition to endosomal sorting, in hematopoietic cells.
The results reviewed in the previous paragraphs suggested to us that we could take advantage of the granule targeting in hematopoietic cells and devise a therapeutic intervention. If a “foreign” protein were targeted for storage, it could be delivered at an inflamed site during degranulation. Targeting should be possible, as we have observed that certain exogenous nonhematopoietic proteins can be retrieved and sorted in hematopoietic cells.9,10 The targeting mechanism(s) in hematopoietic cells may in fact not be unique for endogenous granule proteins. The coexistence of lysosomal enzymes and hematopoietic serine proteases with several antibiotic proteins in secretory lysosomes indicates that costorage is possible without autodigestion. To explore this possibility, we have expressed a soluble form of a TNF receptor (sTNFR1)11 for storage in hematopoietic granules. sTNFR1 was chosen for the experiments because anti-TNF therapy is beneficial to patients with certain inflammatory disorders. For instance, if a local release of sTNFR1 from hematopoietic cells could be achieved at an inflamed site where TNF needs to be inactivated, this approach may have a potential therapeutic effect in such disorders.
As a first step to accomplish targeting of sTNFR1, characterization of sorting and processing was performed after expression in the murine leukemia 32D12 and the rat basophilic leukemia (RBL)13 hematopoietic cell lines. These cells have been extensively used in studies of targeting and processing,5 and the RBL cells are known to exhibit regulated secretion upon stimulation. For successful targeting of sTNFR1 to secretory lysosomes, endoplasmic reticulum (ER) retention and constitutive secretion has to be prevented. ER export was facilitated by using a transmembrane form of sTNFR1, and constitutive secretion was overcome by the incorporation of a cytosolic sorting signal from CD63, a normal membrane protein of secretory lysosomes.14,15
Materials and methods
Materials
Constructs were created for eukaryotic expression using the vector pcDNA3 (Invitrogen, Groningen, The Netherlands) for transfection of RBL and 32D cells. [35S]methionine/[35S]cysteine (cell radiolabeling grade) were from ICN Pharmaceuticals (Costa Mesa, CA). Heat-inactivated fetal bovine serum (FBS) was from BioWhittaker (Verviers, Belgium). l-glutamine was from GIBCO BRL (Life Technologies, Grand Island, NY). Percoll and protein A-sepharose CL-4B were from Pharmacia (Uppsala, Sweden). 2-Mercaptoethanol was from Sigma-Aldrich (St Louis, MO). Geneticin, endoglycosidase H (Endo-H), N-glycosidase F (N-glycanase), and Complete (protease inhibitor cocktail tablets) were from Boehringer Mannheim (Mannheim, Federal Republic of Germany). Novex Pre-Cast Gels (10%-20% Tris [tris(hydroxymethyl)aminomethane]-glycine gel) were from Novex (San Diego, CA). Polyclonal antiserum against sTNFR1 was obtained by immunization of rabbits and used for immunoprecipitation.11 A monoclonal antibody, anti-sTNFR1 (SC8436) (Santa Cruz Biotechnology, Santa Cruz, CA) was used in detection of sTNFR1 by Western blotting. Rabbit anti-lgp120 antiserum was from Dr I. Mellman (Yale University School of Medicine, New Haven, CT).
The following buffer systems were used: lysis buffer for cells (1 M NaCl, 50 mM Tris-HCl, pH 8.0, 0.5% Triton X-100 [vol/vol]), radioimmune precipitation buffer (RIPA) (0.75 M NaCl, 0.15 M HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], pH 7.3, 0.5% SDS [sodium dodecyl sulfate] [vol/vol], 5% Triton X-100 [vol/vol], 5% sodium deoxycholate [wt/vol]), lysis buffer for immunoblotting (86 mM Tris, pH 6.8, 11% [vol/vol] glycerol, 2.3% [wt/vol] SDS, 1.2% [vol/vol] β-mercaptoethanol, 0.005% [vol/vol] bromophenol blue). Protease inhibitors were added to all buffers before use.
Construction of expression vectors
The cDNA fusion constructs sTNFR1-tm and sTNFR1-tm-Y were generated (Figure 1). The tm corresponds to the transmembrane domain of human TNFR1 with flanking sequences (VKGTEDSGTTVLLPLVIFFGLCLLSLLFIGLMYRYORWKSKLYSIV). Y represents the cytosolic tyrosine-sorting signal (SIRSGYEVM) for secretory lysosomes of CD63.2 All polymerase chain reactions (PCRs) were performed in 20-cycle reactions in a Perkin-Elmer 480 Thermal Cycler using Pfu polymerase (Stratagene, La Jolla, CA) according to the manufacturer's instructions. By design of the primers, the Kozak consensus leader sequence for maximal translational efficiency16 was introduced 5′ to the ATG initiation codon. All constructs were cloned into the pcDNA3 vector.
Schematic view of the cDNA constructs used. sTNFR1-tm corresponds to the sequence for sTNFR1 with a transmembrane domain (T). sTNFR1-tm-Y contains the cytosolic sorting signal from CD63 (Y).
Schematic view of the cDNA constructs used. sTNFR1-tm corresponds to the sequence for sTNFR1 with a transmembrane domain (T). sTNFR1-tm-Y contains the cytosolic sorting signal from CD63 (Y).
The sTNFR1-tm cDNA construct was created by adding flanking restriction enzyme sites for BamHI and EcoRI using pADTNF-R as a template. The upstream primer was 5′-TTCGGAGGATCCGCCACCATGGGCCTCTCCACCGTG (A1), and the downstream primer was 3′-TTCGGAGAATTCTCAAACAATGGAGTAGAGCTTGGAC (A4).
The sTNFR1-tm-Y cDNA construct was generated by using ADTNF-R as a template in a PCR reaction with the following primers: A1 plus 3′-TTCGGAGAATTCTCACATCACCTCGTAGCCACTTCTGATACTAACAATGGAGTAGAGCTTGGAC (A6 long) leading to the creation of sTNFR1-tm-GY with BamHI and EcoRI restriction enzyme sites.
Transfections
32D cl3 cells12 or RBL cells13 were transfected with the pcDNA3 constructs described using the Bio-Rad Gene Pulser (Bio-Rad, Hercules, CA), with electrical settings of 960 μF and 260-300 V as described previously.17,18 After electroporation, 1 mg/mL geneticin was added, and recombinant clones of pcDNA3 containing the geneticin resistance were selected. Antibiotic-resistant cell clones were expanded in suspension and screened for the expression of the transfected protein by biosynthetic radiolabeling. Clones with appropriate expression were chosen. Cells were incubated in 5% CO2 at 37°C in a fully humidified atmosphere. Exponentially growing cells were used in all experiments.
Biosynthetic radiolabeling
Biosynthetic radiolabeling of newly synthesized protein was carried out with [35S]methionine/[35S]cysteine as described previously.19
Immunoprecipitation
All steps were performed at 4°C. Cells (2 × 106/mL) were solubilized in lysis buffer for cells, followed by freezing and thawing. Percoll-containing subcellular fractions were diluted with one volume H2O and half a volume of 5-fold concentrated RIPA. All samples were cleared by centrifugation at 32 000g for 60 minutes. Antiserum, protein A–sepharose, and protein G-agarose were added, the mixture was rotated overnight, and the pellet was dissolved in electrophoresis sample buffer followed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) in Pre-Cast 10%-20% Tris-glycine gels. The gels were exposed to x-ray film at -80°C. Densitometry was performed in a Molecular Imager FX (BioRad). Endoglycosidase H (0.24 U) and N-glycosidase F (2 U) digestions were performed at 37°C for 24 hours on immunoprecipitates corresponding to 20 × 106 cells.
Subcellular fractionation
Subcellular fractionation was carried out on continuous gradients of Percoll, as described previously.18 The cell homogenate was centrifuged in a Percoll density gradient after which 9 fractions were collected. All the cytosol was contained in fraction 9. The distribution of secretory lysosomes and Golgi elements in the gradient was determined by assaying for β-hexosaminidase and galactosyl transferase, respectively.20,21 The peak activities of β-hexosaminidase and galactosyl transferase in Percoll fractions of RBL cells were localized in fractions 2 and 6, respectively.18
Western blotting
The enhanced chemiluminescence (ECL) Western blotting kit was used according to the manufacturer's instructions. Cells (5 × 106) were washed in phosphate-buffered saline (PBS), frozen, and sonicated in 100 μL lysis buffer for immunoblotting. Samples were boiled for 5 minutes and cleared by centrifugation at 14 000 × g at 4°C for 5 minutes. Lysate from 0.5 × 106 cells was loaded in each lane of a precast 10%-20% Tris-glycine gel. After electrophoresis, proteins were transferred to Hybond-P nitrocellulose membrane in blotting buffer. Detection was performed by exposing the membranes to Hyperfilm ECL for 10-30 seconds.
Immunoelectron microscopy
RBL cells were fixed for 24 hours in 4% paraformaldehyde in PHEM buffer (pH 6.9) containing 240 mM PIPES (piperazine diethane sulfonic acid), 100 mM HEPES, 8 mM MgCl2, and 40 mM EGTA (ethylene glycol tetra-acetic acid), and then processed for ultrathin cryosectioning as described before.22 Cryosections (45 nm) were cut at -125°C using diamond knives (Drukker, Cuijk, the Netherlands) in an ultracryomicrotome (Leica Aktiengesellschaft, Vienna, Austria) and transferred with a mixture of sucrose and methylcellulose onto formvar-coated copper grids. The grids were placed on 35-mm petri dishes containing 2% gelatin. Double immunolabeling was performed using the procedure described by Slot et al23 with 10- and 15-nm protein A–conjugated colloidal gold probes (EM Lab, Utrecht University, the Netherlands). After immunolabeling, the cryosections were embedded in a mixture of methylcellulose and uranyl acetate and examined with a Philips CM 10 electron microscope (Eindhoven, the Netherlands). For the controls, the primary antibody was replaced by a nonrelevant rabbit antiserum.
Immunofluorescence microscopy
Cells were put on ice, washed in 1.5 mL cold PBS, and fixed with 1 mL 2% (vol/vol) paraformaldehyde solution (Becton Dickinson, Franklin Lakes, NJ) for 15 minutes and subsequently incubated for an additional 45 minutes at room temperature. After fixation, the cells were permeabilized in 1 mL cytoskeletal buffer containing 100 mM KOH (potassium hydroxide), 2 mM MgCl2, 5 mM EGTA, 0.05% (vol/vol) Triton X-100, and 100 mM PIPES (pH 6.8) for 10 minutes on ice and thereafter incubated in blocking solution (PBS containing 0.1% BSA [bovine serum albumin] [wt/vol], 0.2% Tween 20 [vol/vol], and 5% [vol/vol] goat serum (Sigma) for 30 minutes at room temperature. Next, cells were incubated at room temperature with the primary antibody (dilution 1:400-1:500) for 3 hours in blocking solution. Following washing, cells were incubated with secondary antibody (goat anti–rabbit Alexa Fluor 488 F(ab)2 fragment, dilution 1:400, or goat anti–mouse Alexa Fluor 594 F(ab)2 fragment, dilution 1:400; Molecular Probes, Eugene, OR) for 1 hour in blocking solution. After washing twice, cells were adhered to poly-L-lysine–coated coverslips. The samples were then overlaid with ProLong Antifade reagent (Molecular Probes, Eugene, OR) before mounting. Images were recorded on a Nikon Eclipse TE300 inverted fluorescence microscope (Nikon, Tokyo, Japan) equipped with a Hamamatsu C4742-95 cooled CCD camera, using a Plan Apochromat 100 × objective and a high N.A. oil-condenser. Adobe Photoshop (Adobe Photoshop, San Jose, CA) was used to pseudocolor and overlay the recorded images.
Results
Expression of sTNFR1-tm
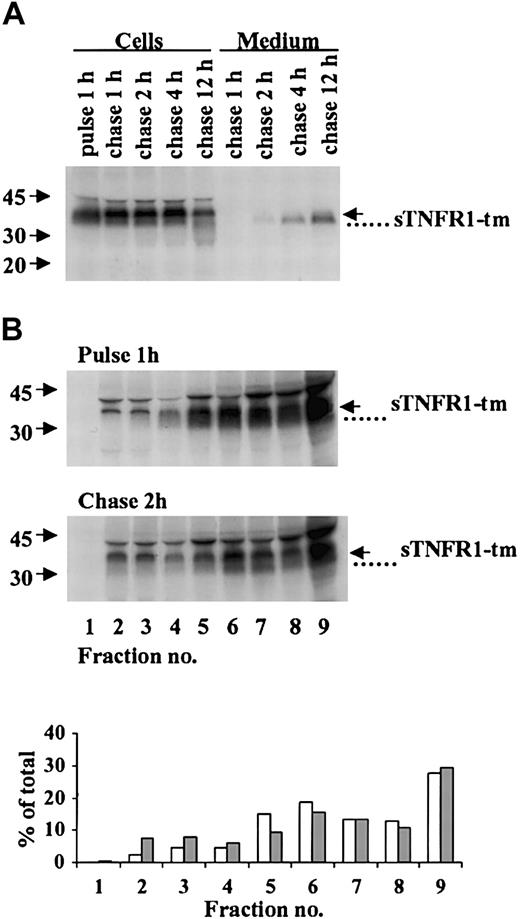
Pulse-chase-radiolabeling experiments were performed in RBL cells expressing sTNFR1-tm as shown in Figure 2. Immunoprecipitation of cell lysates showed a major band corresponding to full-length 37-kDa sTNFR1-tm that still predominated after 12 hours' chase (Figure 2A). A 34-kDa processed component also was observed. Secretion into the culture medium of the 34-kDa component was visible after 2-4 hours and increased during a 4- to 12-hour chase (Figure 2A). Densitometry performed at 12 hours (data not shown) indicated 59% of the immunoreactive species to be intracellular and 41% to be extracellular, the latter representing constitutive secretion.
Processing and subcellular distribution of sTNFR1-tm. (A) RBL cells expressing sTNFR1-tm were radiolabeled (pulse) for 1 hour and the radiolabel chased for up to 12 hours. At the indicated time points, 20 × 106 cells and incubation medium were withdrawn, immunoprecipitated, and analyzed as described in “Materials and methods.” The position of sTNFR1-tm is indicated with an arrow, and a processed form (that is, the secreted form) with a dotted line. Molecular weight markers are shown to the left in kDa. (B) Cells were biosynthetically radiolabeled for 1 hour (pulse), followed by a radiolabel chase for 2 hours. At the pulse and chase intervals, 100 × 106 cells were removed and homogenized, and the postnuclear supernatant was subcellularly fractionated by centrifugation in Percoll. Extraction, immunoprecipitation, and analysis were performed as described in “Materials and methods.” The densitometry data are shown for pulse (□) and chase (##) experiments as percent of total sTNFR1-tm and processed forms.
Processing and subcellular distribution of sTNFR1-tm. (A) RBL cells expressing sTNFR1-tm were radiolabeled (pulse) for 1 hour and the radiolabel chased for up to 12 hours. At the indicated time points, 20 × 106 cells and incubation medium were withdrawn, immunoprecipitated, and analyzed as described in “Materials and methods.” The position of sTNFR1-tm is indicated with an arrow, and a processed form (that is, the secreted form) with a dotted line. Molecular weight markers are shown to the left in kDa. (B) Cells were biosynthetically radiolabeled for 1 hour (pulse), followed by a radiolabel chase for 2 hours. At the pulse and chase intervals, 100 × 106 cells were removed and homogenized, and the postnuclear supernatant was subcellularly fractionated by centrifugation in Percoll. Extraction, immunoprecipitation, and analysis were performed as described in “Materials and methods.” The densitometry data are shown for pulse (□) and chase (##) experiments as percent of total sTNFR1-tm and processed forms.
Results from biosynthetic radiolabeling followed by subcellular fractionation (Figure 2B) indicated accumulation of the full-length 37-kDa sTNFR1-tm and a component of lower molecular weight (34 kDa) in fractions corresponding to the ER, Golgi, and plasma membrane. Only a minor accumulation in the densest secretory lysosome-containing fractions was observed after chase of the radiolabel, as is obvious also from the densitometry data shown in Figure 2B. In addition, results from Ip-Western of lysates from subcellular fractions support this interpretation (data not shown). In addition, no association of expressed sTNFR1 with dense organelles has been observed previously in subcellular fractionation experiments.10 We can therefore conclude that significant sorting of sTNFR1-tm into secretory lysosomes is unlikely to occur. sTNFR1-tm may instead be transferred to the cell membrane from which the 34-kDa sTNFR1 could be released after cleavage as suggested by the results in Figure 2A. In conclusion, these data show sTNFR1-tm to be synthesized followed by secretion of generated sTNFR1.
Expression of sTNFR1-tm-Y
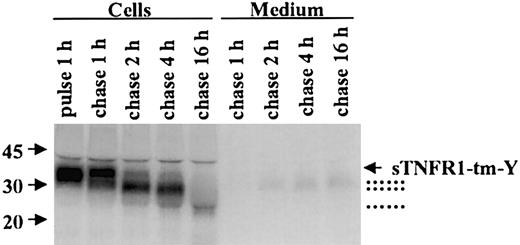
Pulse-chase radiolabeling of RBL cells expressing sTNFR1-tm-Y is shown in Figure 3. Immunoprecipitation visualized a 37-kDa sTNFR1-tm-Y after pulse radiolabeling. A series of processing steps were observed during the radiolabel chase period. Initially, a slight increase in molecular weight was observed, most likely corresponding to carbohydrate processing. In addition 34-kDa and 31-kDa forms were generated with time. A minor fraction of the 31/34-kDa processed forms was not retained but rapidly released into the culture medium. The 31/34-kDa processed forms are likely to consist of sTNFR1 generated by proteolysis of sTNFR1-tm-Y. After prolonged radiolabel chase a 24-kDa processed form also was visible. A similar processing pattern was observed in 32D cells without detectable secretion (data not shown).
Processing of sTNFR1-tm-Y. RBL cells were radiolabeled (pulse) for 1 hour followed by chase of the label for up to 16 hours. At the indicated time points, 20 × 106 cells and incubation medium were withdrawn, extracted, immunoprecipitated, and analyzed as described in “Materials and methods.” The position of sTNFR1-tm-Y is indicated by an arrow, and the various processed forms are indicated with dotted lines. Molecular weight markers are shown to the left in kDa.
Processing of sTNFR1-tm-Y. RBL cells were radiolabeled (pulse) for 1 hour followed by chase of the label for up to 16 hours. At the indicated time points, 20 × 106 cells and incubation medium were withdrawn, extracted, immunoprecipitated, and analyzed as described in “Materials and methods.” The position of sTNFR1-tm-Y is indicated by an arrow, and the various processed forms are indicated with dotted lines. Molecular weight markers are shown to the left in kDa.
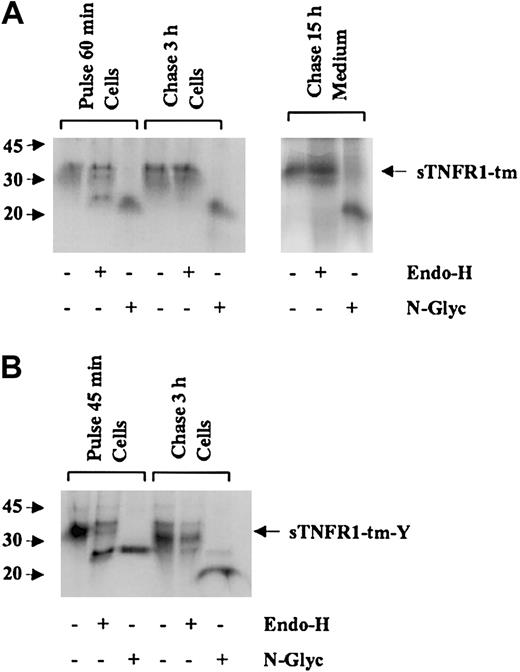
To confirm export from the ER of sTNFR1-tm and sTNFR1-tm-Y, carbohydrate processing also was investigated. Approximately half of the sTNFR1-tm observed after 60 minutes of radiolabeling was sensitive to digestion with Endo-H while the other half was resistant, indicating rapid ER export to Golgi of the latter part. Radiolabel chase for 3 hours resulted in total resistance to Endo-H (Figure 4A). This indicates a change from high mannose to complex glycoforms, consistent with ER export and transfer through the Golgi of sTNFR1-tm. The secreted species of sTNFR1-tm also was Endo-H resistant, indicating a similar transport through the secretory pathway. A major part of sTNFR1-tm-Y observed after 45 minutes' radiolabeling was sensitive to digestion with Endo-H as shown by a decrease in the molecular size from 37 to 29 kDa, indicating the presence of high mannose glycoforms that are normally produced in the ER (Figure 4B). A similar decrease in molecular size was achieved by digestion with N-glycanase that removes all N-linked carbohydrates, indicating that all N-linked glycans were of the high mannose type. After chase of the radiolabel for 3 hours, complete Endo-H resistance was observed in all processing forms. This suggested the presence of complex glycoforms that are normally manufactured in the Golgi by trimming of mannose and addition of new terminal sugars. In conclusion, these results show that both newly synthesized sTNFR1-tm and sTNFR1-tm-Y are rapidly exported to the Golgi for further processing.
Oligosaccharide side chains of processing forms for sTNFR1-tm and sTNFR1-tm-Y. (A) RBL cells transfected with sTNFR1-tm were biosynthetically radiolabeled for 60 minutes (pulse) and chased for 3 hours before analysis of cell extracts. Due to the slow secretion, the cell supernatant was obtained after a 15-hour chase to have enough secreted sTNFR1-tm for analysis. After pulse and chase, 20 × 106 cells were withdrawn, and after lysis, subjected to immunoprecipitation with anti-sTNFR1. Aliquoted immunoprecipitates were incubated either with Endoglycosidase-H (Endo-H) or N-glycosidase F (N-Glyc), or remained untreated as control, and analyzed by SDS-PAGE. The pulse cell lysate showed partial Endo-H sensitivity, while the chase cell lysate showed total Endo-H resistance. The cell supernatant showed Endo-H resistance. The position of sTNFR1-tm is indicated with an arrow. Molecular weight markers are shown to the left in kDa. (B) Similar experiments were performed with RBL cells transfected with sTNFR1-tm-Y. Cells were biosynthetically radiolabeled for 45 minutes (pulse) and chased for 3 hours before analysis of cell extracts. The pulse sample showed partial Endo-H sensitivity, while the chase sample showed total Endo-H resistance. Secretion was too low to yield enough material for analysis.
Oligosaccharide side chains of processing forms for sTNFR1-tm and sTNFR1-tm-Y. (A) RBL cells transfected with sTNFR1-tm were biosynthetically radiolabeled for 60 minutes (pulse) and chased for 3 hours before analysis of cell extracts. Due to the slow secretion, the cell supernatant was obtained after a 15-hour chase to have enough secreted sTNFR1-tm for analysis. After pulse and chase, 20 × 106 cells were withdrawn, and after lysis, subjected to immunoprecipitation with anti-sTNFR1. Aliquoted immunoprecipitates were incubated either with Endoglycosidase-H (Endo-H) or N-glycosidase F (N-Glyc), or remained untreated as control, and analyzed by SDS-PAGE. The pulse cell lysate showed partial Endo-H sensitivity, while the chase cell lysate showed total Endo-H resistance. The cell supernatant showed Endo-H resistance. The position of sTNFR1-tm is indicated with an arrow. Molecular weight markers are shown to the left in kDa. (B) Similar experiments were performed with RBL cells transfected with sTNFR1-tm-Y. Cells were biosynthetically radiolabeled for 45 minutes (pulse) and chased for 3 hours before analysis of cell extracts. The pulse sample showed partial Endo-H sensitivity, while the chase sample showed total Endo-H resistance. Secretion was too low to yield enough material for analysis.
Targeting of sTNFR1-tm-Y for secretory lysosomes
The subcellular fate of sTNFR1-tm-Y was investigated by biosynthetic pulse-chase radiolabeling followed by subcellular fractionation. Also, immunoprecipitation followed by Western blotting (IP-Western) of the subcellular fractions was performed, and the protein construct was visualized by immunofluorescence microscopy and immunogold electron microscopy.
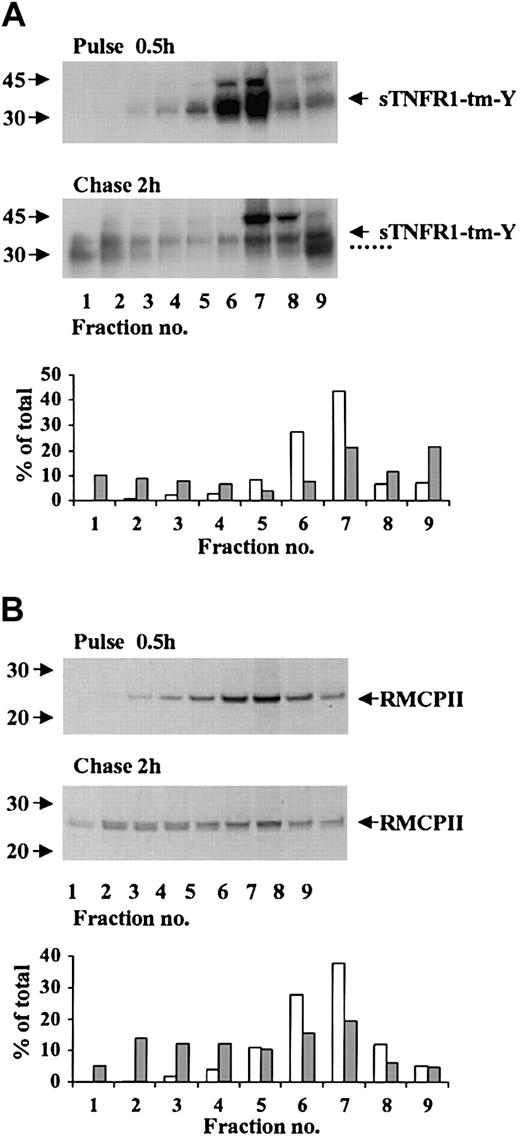
Possible targeting of sTNFR1-tm-Y for storage in the dense secretory lysosomes was investigated by pulse-chase radiolabeling experiments combined with subcellular fractionation. After 30 minutes of radiolabeling, most of the radiolabeled 37-kDa sTNFR1-tm-Y was in the light density fractions 6-7 corresponding to the ER and Golgi elements, and no radiolabeling was visible in the densest organelles (Figure 5A). After chase of the radiolabel for 2 hours there was a considerable decrease of the sTNFR1-tm-Y in the light fractions, concomitant with an accumulation of the 31-kDa product in the most dense organelles (Figure 5A). This was confirmed by densitometry analyses that showed an increase from 3% to 28% of radiolabeled sTNFR1-tm-Y and processing form in the densest fractions 1-3 (Figure 5A). At the same time, radiolabeled sTNFR1-tm-Y of fractions 6 and 7 decreased from 71% to 29%. The accumulation observed in the cytosol fraction of sTNFR1-tm-Y and processing form might represent leakage during the homogenization. Pulse-chase radiolabeling experiments also were performed with RMCP-II, an endogenous constituent of secretory lysosomes. After 30 minutes of radiolabeling, the RMCP-II was concentrated in light fractions (Figure 5B) similar to sTNFR1-tm-Y. Transfer to the densest fractions occurred upon radiolabel chase (Figure 5B). This was confirmed by densitometry analyses that showed an increase from 2% to 31% of radiolabeled RMCP-II in the densest fractions 1-3 (Figure 5B). At the same time, the radiolabeled RMCP-II of fractions 6 and 7 decreased from 66% to 35%. In conclusion, the results for RBL cells indicated export to the Golgi of newly synthesized sTNFR1-tm-Y, targeting for the densest organelles corresponding to secretory lysosomes, and concomitant proteolytic processing into lower molecular weight forms. Similar results were noticed for 32D cells (data not shown).
Subcellular distribution of radiolabeled sTNFR1-tm-Y, and endogenous RMCP-II. RBL cells were biosynthetically radiolabeled for 0.5 hours (pulse), followed by chase of the label for 2 hours (chase). At the pulse and chase intervals, 100 × 106 cells were removed and homogenized, and the postnuclear supernatant was subcellular fractionated by centrifugation in Percoll. The fractions were immunoprecipitated with anti-sTNFR1 (A) or anti–RMCP-II (B) and analyzed as described in “Materials and methods.” The densitometry data are shown for pulse (□) and chase (##) experiments as percent of total mature and processed forms of sTNFR1-tm-Y or RMCP-II. The position of sTNFR1-tm-Y and RMCP-II are indicated with arrows, and the various processed forms are indicated with dotted lines. Molecular weight markers are shown to the left in kDa.
Subcellular distribution of radiolabeled sTNFR1-tm-Y, and endogenous RMCP-II. RBL cells were biosynthetically radiolabeled for 0.5 hours (pulse), followed by chase of the label for 2 hours (chase). At the pulse and chase intervals, 100 × 106 cells were removed and homogenized, and the postnuclear supernatant was subcellular fractionated by centrifugation in Percoll. The fractions were immunoprecipitated with anti-sTNFR1 (A) or anti–RMCP-II (B) and analyzed as described in “Materials and methods.” The densitometry data are shown for pulse (□) and chase (##) experiments as percent of total mature and processed forms of sTNFR1-tm-Y or RMCP-II. The position of sTNFR1-tm-Y and RMCP-II are indicated with arrows, and the various processed forms are indicated with dotted lines. Molecular weight markers are shown to the left in kDa.
Results from IP-Western of subcellular fractions indicated a slight accumulation of the 31-kDa processed form of sTNFR1-tm-Y in the densest fractions of both RBL and 32D cells under steady-state conditions (data not shown). These observations are consistent with the results from the biosynthetic radiolabeling experiments.
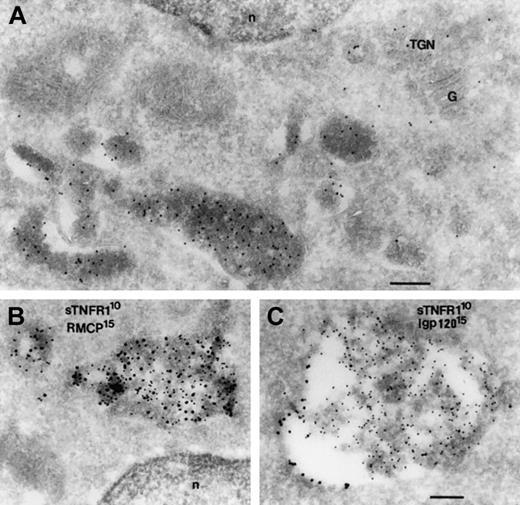
To confirm the targeting for storage in secretory lysosomes, we performed immunoelectron-microscopy with double immunogold labeling in order to verify colocalization of sTNFR1-tm-Y and constituents of these organelles in RBL cells. sTNFR1-tm-Y was visible in multivesicular bodies as well as in the trans-Golgi network (TGN) (Figure 6). Multivesicular bodies mature as prelysosomal compartments and correspond to the abnormal granules of RBL cells. sTNFR1-tm-Y colocalized with RMCP-II, the major constituent of the granules of RBL cells13 (Figure 6B). sTNFR1-tm-Y also colocalized with rat lysosomal glycoprotein 120 (lgp120) in the multivesicular bodies (Figure 6C). Lgp120 corresponds to hLAMP-1 and mLAMP-1 in humans and mice, respectively, indicating involvement of the endosomal/lysosomal pathway.24 sTNFR1-tm-Y was located mostly in the interior of the multivesicular bodies, while lgp 120 was along its outer membrane (Figure 6C). In conclusion, the data are consistent with targeting of sTNFR1-tm-Y to secretory lysosomes of RBL cells.
Colocalization of sTNFR1 with a matrix protein (RMCP) and a membrane-associated protein (lgp120) of secretory lysosomes. Ultrathin cryosections from RBL cells transfected with sTNFR1-tm-Y were labeled accordingly. (A) Labeled with rabbit anti-sTNFR1. (B) Double labeled with rabbit anti-sTNFR1 (10 nm) and mouse anti-RMCP (15 nm). (C) Double labeled with rabbit anti-sTNFR1 (10 nm) and rabbit anti-lgp 120 (15 nm). (A) Area of a cell showing granules highly labeled with sTNFR1. The Golgi (G) and the trans Golgi network (TGN) also are labeled. (B) Granules are shown double labeled for sTNFR1 and RMCP on the matrix. (C) A granule is seen with labeling for lgp 120 on the membrane and for sTNFR1 on the matrix. n indicates nucleus. Bar, 200 nm.
Colocalization of sTNFR1 with a matrix protein (RMCP) and a membrane-associated protein (lgp120) of secretory lysosomes. Ultrathin cryosections from RBL cells transfected with sTNFR1-tm-Y were labeled accordingly. (A) Labeled with rabbit anti-sTNFR1. (B) Double labeled with rabbit anti-sTNFR1 (10 nm) and mouse anti-RMCP (15 nm). (C) Double labeled with rabbit anti-sTNFR1 (10 nm) and rabbit anti-lgp 120 (15 nm). (A) Area of a cell showing granules highly labeled with sTNFR1. The Golgi (G) and the trans Golgi network (TGN) also are labeled. (B) Granules are shown double labeled for sTNFR1 and RMCP on the matrix. (C) A granule is seen with labeling for lgp 120 on the membrane and for sTNFR1 on the matrix. n indicates nucleus. Bar, 200 nm.
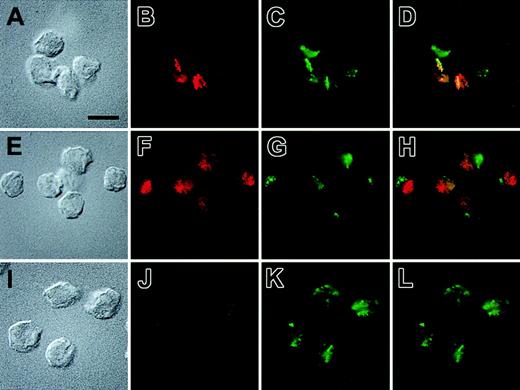
We then used immunofluorescence microscopy to confirm the colocalization of sTNFR1-tm-Y and the marker of secretory lysosomes, lgp120. Cells that expressed sTNFR1-tm-Y consistently showed a very similar staining pattern for the TNFR and for lgp120 (Figure 7A-D, where 2 of the cells express high levels of sTNFR1-tm-Y). This staining was frequently highly localized to one pole of the cells. On the other hand, cells expressing sTNFR1-tm did not exhibit colocalization with lgp120 (Figure 7E-H). Instead, the staining of the TNFR in these cells often appeared in smaller punctate structures, possibly representing the ER/endosomal apparatus. These structures were often accumulated in a different region of the cell than the staining for lpg120 (Figure 7E-H). In nontransfected cells, a low intensity and diffuse punctate staining was observed that could represent nonspecific staining or a low endogenous expression of TNFR (Figure 7I-L). The immunofluorescence analysis thus confirms the different targeting of sTNFR1-tm-Y and sTNFR1-tm in transfected RBL cells, and shows that sTNFR1-tm-Y accumulates in secretory lysosomes.
Subcellular localization of sTNFR1-tm and sTNFR1-tm-Y by immunofluorescence microscopy. Indirect immunolocalization of the TNF-R and a marker for secretory lysosomes, lgp120. The cells used were RBL cells transfected with sTNFR1-tm-Y (A-D), sTNFR1-tm (E-H), and wild-type RBL cells (I-L). The cells were fixed, permeabilized, and stained, as described in “Materials and methods,” before being attached to poly-lysine–coated coverslips and the images recorded. Briefly, the cells were incubated with a rabbit anti–lgp120 antibody and a mouse monoclonal antibody against the sTNFR1. Thereafter, the cells were stained with Alexa Fluor–labeled anti–rabbit and anti–mouse secondary antibodies. The localization of TNF-R and lgp120 is shown in red and green, respectively, whereas the rightmost images show an overlay of the red and green staining patterns. The corresponding Nomarski images also are shown. Results are representative of 4 separate experiments. Bar = 10 μM.
Subcellular localization of sTNFR1-tm and sTNFR1-tm-Y by immunofluorescence microscopy. Indirect immunolocalization of the TNF-R and a marker for secretory lysosomes, lgp120. The cells used were RBL cells transfected with sTNFR1-tm-Y (A-D), sTNFR1-tm (E-H), and wild-type RBL cells (I-L). The cells were fixed, permeabilized, and stained, as described in “Materials and methods,” before being attached to poly-lysine–coated coverslips and the images recorded. Briefly, the cells were incubated with a rabbit anti–lgp120 antibody and a mouse monoclonal antibody against the sTNFR1. Thereafter, the cells were stained with Alexa Fluor–labeled anti–rabbit and anti–mouse secondary antibodies. The localization of TNF-R and lgp120 is shown in red and green, respectively, whereas the rightmost images show an overlay of the red and green staining patterns. The corresponding Nomarski images also are shown. Results are representative of 4 separate experiments. Bar = 10 μM.
Discussion
We expressed a soluble TNF receptor form in hematopoietic cell lines with the aim of showing targeting of an exogenous protein to secretory lysosomes for storage and subsequent regulated secretion during degranulation. The major finding is that this was accomplished by using a transmembrane form of the protein to facilitate ER export and by incorporating a cytosolic sorting signal for secretory lysosomes to overcome constitutive secretion and achieve the targeting. This result suggests a potential for using the storage organelles of hematopoietic cells as vehicles for targeting sites of inflammation with therapeutically active agents.
In the first compartment of the secretory pathway, the ER, secretory, and membrane proteins acquire native conformations before export to the Golgi through the assistance of ER resident and cytosolic chaperones.25 As a consequence, conformation-based biosynthetic quality control identifies improperly folded or unassembled oligomeric proteins, enabling them to be diverted for proteolysis by the proteasomes instead of being exported to the Golgi. An initial problem with our goal was to prevent the retention and degradation of sTNFR1. What was ER exported was secreted through constitutive-like carriers and not retained.10 Other experience with truncated proteins has been similar, for example, deletion of the propeptide of myeloperoxidase (MPO) rendered the enzyme highly susceptible to degradation in the ER.19 In addition, we have observed that chimeric proteins, for example, MPO propeptide, fused to other proteins and were retained in the ER to a greater extent than the native proteins.10 However, anchoring sTNFR1 through a transmembrane domain facilitated ER export.
How is protein cargo selected in the Golgi and packaged into vesicles for dispatch to its final destination in hematopoietic cells? Timing of gene expression4 and selective posttranscriptional regulation26 determine the composition of the mixture of newly synthesized proteins that is offered at any given time. Consequently, the filling of forming granules depends, in the first place, on what is offered. The targeting for granule storage in the transformed cells used in this study may differ from that in normal hematopoietic cells whose granules are the final vehicles for delivering agents at an inflamed site. The secretory lysosomes of RBL cells, used in this work, link the endocytic and exocytic pathways to become the regulated secretory organelle.27,28 Further evidence indicates that, in these cells, the targeting may occur through either an MPR-dependent endosomal pathway or an MPR-independent nonendosomal pathway or both.27 The endosomal pathway, essential for MPR recycling, has however not been proven to be involved in filling of the azurophil granules of neutrophils. In addition, these granules lack the endosome-derived LAMP-1 and LAMP-2 membrane proteins.29,30 On the other hand, azurophil granules, as well as typical secretory lysosomes of hematopoietic cells, contain the membrane protein CD63 (LAMP-3). Consequently, the strategy employed here, which takes advantage of the sorting signal in CD63 for targeting of sTNFR1 to granules, should be generally applicable for targeting secretory lysosomes of hematopoietic precursors.
We have observed previously that retrieval and sorting are not exclusive for endogenous proteins in hematopoietic cells in as much as constitutive expression of exogenous proteins such as α1-microglobulin and lipopolysaccharide binding protein (LBP) resulted in equal sorting for storage.9,10 On the other hand, we have evidence to suggest that hematopoietic cells cannot route all proteins for storage. For instance, sTNFR1 that escaped ER retention and was exported to the Golgi was not sorted for storage but rather secreted.10 Moreover, when cleavage of transmembrane sTNFR1 was induced in the Golgi through a furin cleavage site, constitutive secretion of released sTNFR1 was again observed and not targeted (data not shown). For this reason it is suggested that not all that is offered is targeted to granules; what is not accepted for sorting may, in this case, be secreted. Granule targeting was, however, specifically accomplished by generating a transmembrane protein with a cytosolic signal for secretory lysosomes. This signal from CD63 was important, as transmembrane sTNFR1 without the signal was lost by degradation or constitutive secretion. Nonnative protein, if generated in post-ER compartments, may be eliminated by accelerated degradation. This seems to be the case for certain aggregated membrane proteins such as the integral membrane protein furin. The aggregated form of furin is directed to lysosomal degradation from the Golgi without reaching the plasma membrane.31 This pathway is consistent with a quality control for the disposal of aggregated proteins in compartments of the secretory pathway in addition to those of the ER.
A prerequisite for reliable storage of an exogenous protein prior to its release through degranulation is stability. Secretory lysosomes have a proteolytic environment, but proteins that are part of their characteristic dense cores may resist proteolysis. In addition, a polyanionic matrix of proteoglycan is likely to play a protective role for stored proteins.32 In fact, almost equal protection was observed in RBL cells for the endogenous protein RMCP-II, the major constituent of the granules of RBL cells, and sTNFR1-tm-Y (data not shown). Thus, sTNFR1-tm-Y seems to be rather stable in this environment, except for when the tm-Y fragment is cleaved by proteolysis, resulting in generation of the membrane-free-sTNFR1 in secretory lysosomes.
Although our results are based on studies of secretory lysosomes in hematopoietic cell lines, platelets, neutrophils, mast cells, macrophages, CTLs, and NK cells all use compartments with lysosomal properties for storage and for secretion.33 Thus, these cells, and the hematopoietic cell lines used in our experiments, have secretory lysosomes. Most of the secretory granules of RBL cells biochemically and functionally overlap with endosomes and lysosomes.27 Even though the environment is similar between the granules of the cellular models used and the secretory lysosomes of hematopoietic cells, the biogenesis of the respective granules may still be different. Therefore, the results reported need to be extended to neutrophil precursor cells and other nontransformed hematopoietic precursor cells. Many cytoplasmic sequences that are sufficient for targeting membrane proteins to conventional lysosomes also target P-selectin to the secretory lysosomes of RBL cells.28 While the secretory lysosome content of NK cells and CTLs is destined for extracellular discharge upon stimulation, the content of azurophil granules of activated neutrophils is released both into the phagosome and the extracellular space. Consequently, the azurophil granule may be particularly suitable for delivering proteins into phagosomes as a means of promoting antibacterial defense. NK cells and CTLs, on the other hand, may be particularly suitable for extracellular delivery as a means of promoting defense against tumor cells.
In summary, we asked whether one can target exogenous proteins with a therapeutic potential for granules in hematopoietic cells that might act as vehicles for sites of inflammation. The successful targeting of sTNFR1-tm-Y into secretory lysosomes of hematopoietic cells answers both questions. Another aspect of the results is that exposure to a proteolytic milieu, such as that of secretory lysosomes, is not necessarily destructive to an exogenous protein that is not normally adapted to this environment. If our results from hematopoietic cell lines can be adopted for cells such as neutrophils, the latter could be used as vehicle cells for site-specific delivery into inflamed tissue, enabling release both into the phagocytic vacuole and the exterior milieu. Furthermore, the principle may be adopted for other cells with secretory lysosomes, for example, NK cells and CTLs.
Prepublished online as Blood First Edition Paper, March 20, 2003; DOI 10.1182/blood-2002-10-3055.
Supported by the Swedish Cancer Foundation, the Swedish Childhood Cancer Foundation, the Alfred Österlund Foundation, and funds from Lund University Hospital.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We would like to thank Hans Janssen and Nico Ong for their expert technical assistance.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal