Abstract
Optimal activation of T cells requires delivery of both antigenic and costimulatory signals. It is unclear, however, if the function of the natural killer (NK) cells is also modulated by these 2 signals. Here we report that efficient control of solid allogeneic tumors by NK cells depends on codelivery of both B7-1 and major histocompatibility complex (MHC) class I on the tumor cells. The codelivery is required for optimal expansion and effector function of NK cells in response to both melanoma and plasmocytoma that expressed allogeneic MHC class I. Our results demonstrate that the 2 signals required for T-cell function also can regulate NK immunity and reveal an important similarity between the innate NK response and the adaptive T-cell response. (Blood. 2003;102:4456-4463)
Introduction
The immune system consists of both adaptive and innate components. The adaptive immune response involves selective expansion of T- and B-cell clones with precise recognition machinery capable of discriminating individual antigens.1 Coupling of the antigen-specific clonal expansion with acquisition of effector function and immunological memory permits not only the generation of powerful immunity against the primary invader, but also, perhaps more importantly, a faster and stronger response to the second invasion by the same pathogens.2 In contrast, NK cells, the activated lymphocytes that can be called upon to destroy appropriate target cells within 1-3 days of insult by pathogens or malignant cells,3,4 have been regarded as nonadaptive immune effectors.
With recent advances in analyzing NK cell recognition of targets,5 similarities between NK cells and T cells have emerged. For example, molecules that mediate both positive and negative regulation of the NK cell cytotoxicity are expressed on T cells. Accumulating evidence appears to support the notion that these molecules are also involved in controlling T-cell function.6-11 Two recent studies raised the intriguing possibility that NK immunity may have adaptive features. Glas et al12 observed preferential recruitment and activation of NK cells with less inhibitory receptor for the major histocompatibility complex (MHC) molecules on the inoculated tumor cells. Conversely, Dokun et al13 reported that NK cells expressing Ly49H, an activating receptor that recognizes an MHC-like molecule encoded by murine cytomegalovirus (MCMV)-infected cells,14,15 were preferentially expanded during the course of MCMV infection.
In addition to an MHC/peptide complex, optimal activation of T cells requires costimulation. While numerous costimulatory molecules have been identified,16,17 B7-1 and B7-2 are the prototypic costimulatory molecules expressed primarily on the antigen-presenting cells.18-23 Intriguingly, accumulating evidence also supports a role for costimulatory molecules in NK function. Approximately 10 years ago, Azuma and colleagues reported that a leukemia NK cell line expressed CD28 and that anti-CD28 promoted cytolysis by NK cells.24 Subsequent studies revealed that a subpopulation of human NK cells expressed CD28, although the CD28 on NK cells was apparently different from that on the T cells, as it was recognized by only a subset of anti-CD28 antibodies.25 Recently, using human NK cell clones, 2 groups revealed a role for B7-1 and B7-2 in promoting NK cell cytolysis.26,27 Data from animals used in experiments have offered additional evidence for involvement of costimulatory molecules in NK cell function. Thus, Nandi et al28 reported expression of CD28 on a subset of NK cells. Hunter et al showed that infection by Toxoplasma gondii induced expression of CD28 in a subset of NK cells.29 Since the parasite burden was substantially increased in the CD28(-/-) mice, it was suggested that B7-1:CD28 interaction may be responsible for NK cell function. Geldhof et al reported that B7-1-transfected T lymphoma was less metastatic in SCID mice, but not the SCID-beige mice.30 Since the beige mutation reduces NK activity, this work suggests that B7-1 can promote NK immunity in vivo. Interestingly, CD28(-/-) murine NK cells also preferentially kill target cells expressing either B7-1 or B7-2.31,32
Despite the evidence to date, a conceptual framework as to how costimulation contributes to NK function has not yet emerged. An important issue is whether NK cells are modulated by 2 signals, as T cells are. Since costimulatory molecules act in concert with the ligand for T-cell antigen receptor (TCR) during T-cell response, it is of interest to study whether costimulatory molecules act in concert with activating NK receptors. While many activating NK receptors have been identified, only 2 of them, Ly49D and Ly49H, are not expressed on all NK cells. Ly49H is involved in immunity against MCMV infection, and recent studies revealed that it recognizes an MHC-like molecule encoded by MCMV.14,15 Ly49D is the other activating receptor that is expressed on a subset of NK cells and has a known ligand, the H-2Dd.33 This makes it possible to investigate the effect of codelivering an activating NK receptor ligand (aNKRL) and costimulators on the fate of an identifiable NK subset.
In this study, we therefore used variants of 2 H-2d tumor lines, the plasmocytoma J558 and melanoma B16, to evaluate the function of aNKRL and the costimulatory molecule B7-1 on NK cells. Here we report that both B7-1 and MHC class I are required for expansion and effector function of NK cells in response to allogeneic tumors. These results suggest that much like T cells, the function and specificity of NK cells can be modulated by both NK ligand and costimulatory molecules, such as B7-1, and that these 2 “signals” act in concert to maintain NK cells with desired specificity and function.
Materials and methods
Tumor cell lines and experimental animals
Plasmocytoma J558, transfected with either vector alone (MHC+B7-) or costimulatory molecule B7-1 (MHC+B7+), has been described previously.34 A recurrent variant of J558-B7 that lacks cell surface MHC class I, MHC-B7, also has been described.35 RAG-1(-/-) C57BL6/j mice were purchased from the Jackson Laboratories (Bar Harbor, ME) and were used when 8-10 weeks old.
Antibodies and flow cytometry
Phycoerythrin (PE)-conjugated annexin V and fluorescent dye-conjugated antibodies specific for NK1.1, Ly49D (4E533 ), and bromodeoxyuridine (BrdU, PRB-1) were all purchased from BD Pharmingen (San Diego, CA). The Tmβ1 clone was a generous gift from Dr T. Tanaka of the Tokyo Metropolitan Institute of Medical Science, Japan. Single cells isolated from livers or spleens of mice were stained with fluorescent dye conjugated mAbs and were analyzed by flow cytometry (FACScalibur, Becton Dickinson, Mountain View, CA). The list mode data were analyzed using Flowjo software (Tree Star, La Jolla, CA).
Isolation of liver mononuclear leukocytes
Liver mononuclear cells (MNCs) were isolated as described.38 Briefly, livers were perfused through the portal vein with 10 mL phosphate-buffered saline (PBS) to remove blood cells. Then, individual livers were ground with a 3-mL syringe insert and suspended in 10 mL medium. The single-cell suspension was collected and centrifuged, and tissue debris was discarded. The cells were resuspended in 2 mL 40% Percoll (Pharmacia, Uppsala, Sweden), which was layered on 2.5 mL 70% Percoll in 15 mL conical tubes. MNCs were recovered at the interface between 40% and 70% Percoll after centrifuge at 1600 rpm at room temperature for 25 minutes. The remaining red blood cells in MNCs were lysed by ammonium chloride solution.
Analysis of NK cell cycle and death
To determine the effect of different tumor cell lines on NK cell proliferation, we injected 106/mouse of tumor cells intravenously into RAG-1(-/-) C57BL/6j mice. At the same time, BrdU (Sigma, St Louis, MO; 1 mg/0.1 mL/mouse) was injected intraperitoneally every 12 hours for 2 days. The spleen cells and liver MNCs were isolated and stained with fluorescent conjugated mAbs to BrdU, NK1.1, and Ly49D, using protocol provided by the manufacturer (BD PharMingen). For cell cycle and apoptosis analysis, the dye 7-AAD was added to the samples. For analysis of the early stage of cell apoptosis, the cells were stained with mAbs to surface markers and PE-conjugated annexin V, as described by manufacturer (BD PharMingen).
Tumorigenicity assays
RAG-1(-/-)C57BL6/j mice were inoculated subcutaneously with 5 × 106/mouse of either MHC+B7-, MHC+B7+, or MHC-B7+ in the flank as described.34 Both tumor incidences and tumor sizes were monitored by physical examination.
51Cr release assay
As effector cells, we used spleen cells from C57BL6/j mice that had been stimulated with 30-50 ng/mL of interleukin (IL)-15 for 4-8 days. Tumor cells were labeled with 51Cr and used as targets. The effector cells and the targets were coincubated for 6 hours, and the percent specific lyses were calculated based on the following formula: specific lysis % = 100 × (cpmsample - cpmmedim)/(cpmmax - cpmmedium).
Results
Two-signal-dependent rejection of tumor cells by natural killer cells
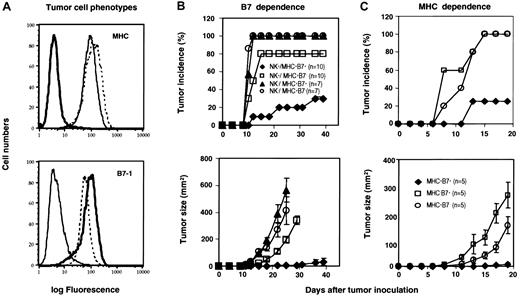
We used 3 cell lines derived from J558 for this study: J558 transfected with vector alone (MHC+B7-), J558 cell transfected with B7-1 (MHC+B7+), and a recurrent tumor cell line MHC-B7+ that was derived from MHC+B7+ tumor cells and had down-regulated multiple antigen-presentation genes including TAP-1/2, LMP-2/7, and lacked cell surface MHC (MHC-B7+).35 The cell surface expression of MHC and B7-1 was verified prior to this study, as shown in Figure 1A. We injected the tumor cells into the RAG-1(-/-) C57BL/6j mice, in which the Ly49D+ NK cells should be able to recognize the H-2Dd+ tumor cells. Surprisingly, 80% of the mice inoculated with MHC+B7- tumors developed palpable tumors within 14 days, while 10% of the mice that received the MHC+B7+ developed tumors in the same period (Figure 1B, upper panel). Although 30% of the mice that received the MHC+B7+ cells did develop tumors during the course of the study, the MHC+B7+ tumors grew substantially slower than the MHC+B7- tumors (Figure 1B, lower panel). To examine whether the rejection of MHC+B7+ in RAG-1(-/-) mice is NK cell dependent, the recipient mice were depleted of NK cells by NK cell-depleting mAb Tmβ139 (100 μg/mouse; intraperitoneally) prior to injection of MHC+B7+ or MHC+B7- tumor cells. This treatment eliminated all subsets of NK cells (data not shown). In control groups, the mice were treated with PBS. The depletion of NK cells increased tumorigenicity of both MHC+B7- and MHC+B7+ tumors (Figure 1B), which indicated that NK cells played an essential role in resistance to the J558 tumors. Importantly, the enhanced resistance to B7-1+ tumor cells was erased as a result of NK depletion (Figure 1B).
MHC and B7-1 promoted rejection of allogeneic tumors in RAG-1(-/-) mice. (A) Phenotypes of tumor cells used in this study. Tumor cells were stained with 28-14-8 (BD Pharmingen), a mAb specific for H-2Ld (upper panel) or 3A12, a mAb for B7-1 (lower panel).59 Solid thin lines: J558 cells were transfected with vector alone (hereby called MHC+B7-). Dashed lines: J558 cells transfected with B7-1 (MHC+B7+). Bold lines: an MHC loss variant of the MHC+B7+ tumor cell line (MHC-B7+). (B) B7-dependent rejection of tumor cells by NK cells. RAG-1(-/-) mice were treated with either PBS or the Tmβ1 mAb prior to tumor challenge. The incidences (upper panel) and tumor sizes (lower panel) were determined by physical examination. Data shown are a summary of 3 (no depletion) or 2 (NK depleted) independent experiments with a total of 7 (NK depleted) or 10 (PBS treated) mice per group. (C) B7-1 enhanced rejection of MHC class I+, but not MHC class I-, tumor cells by NK cells. RAG-1(-/-) mice were challenged with MHC+B7-, MHC+B7+, MHC-B7+ (n = 5). Data shown in panel C are representative of 2 independent experiments.
MHC and B7-1 promoted rejection of allogeneic tumors in RAG-1(-/-) mice. (A) Phenotypes of tumor cells used in this study. Tumor cells were stained with 28-14-8 (BD Pharmingen), a mAb specific for H-2Ld (upper panel) or 3A12, a mAb for B7-1 (lower panel).59 Solid thin lines: J558 cells were transfected with vector alone (hereby called MHC+B7-). Dashed lines: J558 cells transfected with B7-1 (MHC+B7+). Bold lines: an MHC loss variant of the MHC+B7+ tumor cell line (MHC-B7+). (B) B7-dependent rejection of tumor cells by NK cells. RAG-1(-/-) mice were treated with either PBS or the Tmβ1 mAb prior to tumor challenge. The incidences (upper panel) and tumor sizes (lower panel) were determined by physical examination. Data shown are a summary of 3 (no depletion) or 2 (NK depleted) independent experiments with a total of 7 (NK depleted) or 10 (PBS treated) mice per group. (C) B7-1 enhanced rejection of MHC class I+, but not MHC class I-, tumor cells by NK cells. RAG-1(-/-) mice were challenged with MHC+B7-, MHC+B7+, MHC-B7+ (n = 5). Data shown in panel C are representative of 2 independent experiments.
To determine if the B7-enhanced tumor resistance requires MHC class I expression on the tumor cell surface, we challenged the RAG-1(-/-)H-2b mice with either MHC+B7+ or MHC-B7+ variants of the J558 cells. The MHC+B7- tumor variant was used as control. Surprisingly, MHC-B7+ tumor cells grew substantially faster than the MHC+ cell line (Figure 1C). The failure to reject MHC class I- tumors suggests that J558 tumors do not have strong aNKRLs, other than the allogeneic MHC. Again, MHC+B7+ tumors grew substantially slower than the MHC+B7- tumors. These results demonstrate that optimal resistance of allogeneic J558 tumors depends on both MHC and costimulatory molecule B7-1.
Two-signal-dependent proliferation of NK cells induced by allogeneic tumor cells
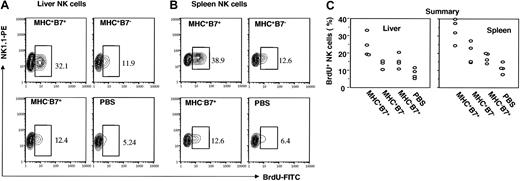
To test the role for B7-1 and MHC in proliferation of NK cells in vivo, we injected MHC+B7+, MHC+B7-, and MHC-B7+ tumor cells intravenously into RAG-1-deficient mice. On the day of tumor cell injection, the mice were pulsed with BrdU for 2 days, and incorporation of the BrdU was analyzed by flow cytometry using BrdU-specific mAb or isotype control. Profiles of NK cells from liver and spleen from one representative mouse in each group are presented in Figure 2A-B, while data from an experiment involving 4 mice per group are summarized in Figure 2C. Of the liver NK cells from mice that received PBS, 8% ± 2.45% incorporated BrdU over the 48-hour period. In mice that received either MHC+B7- or MHC-B7+ tumor cells, the population of dividing NK cells increased to 13.8% ± 2.03% (P = .01) and 15.1% ± 3.44% (P = .01), respectively. Importantly, a 3-fold increase in the percentages of BrdU+ NK cells, or 24.06% ± 5.67% of BrdU+ cells, was found among NK cells from the mice that received MHC+B7+ tumor cells. This was not only significantly higher than what was found in the mice that received PBS alone (P = .002), but also higher than what was found in mice that received MHC+B7- (P = .01) and MHC-B7+ (P = .03) tumor cells.
MHC and B7-1 promote incorporation of BrdU into NK cells. RAG-1(-/-)C57BL6/j mice were injected with 106/mouse of tumor cells intravenously and with 2 daily doses of BrdU intraperitoneally. Mice were killed at 48 hours after tumor cell challenge for analysis. Mononuclear leukocytes from liver (A) or spleen (B) were stained with NK1.1 mAb, and after permeabilization, incubated with anti-BrdU mAb. Data shown are profiles of gated NK1.1+ cells from one representative mouse in each group. The gates for BrdU+ cells were set based on isotype control, which gave less than 0.5% positive cells. The percentages of BrdU+ cells are shown in the panels. Data shown are representative of 2 independent experiments. (C) Composite data on percentages of BrdU+ cells of NK1.1+ cells from liver and spleen. Means, standard error, and statistical significance were described in the text.
MHC and B7-1 promote incorporation of BrdU into NK cells. RAG-1(-/-)C57BL6/j mice were injected with 106/mouse of tumor cells intravenously and with 2 daily doses of BrdU intraperitoneally. Mice were killed at 48 hours after tumor cell challenge for analysis. Mononuclear leukocytes from liver (A) or spleen (B) were stained with NK1.1 mAb, and after permeabilization, incubated with anti-BrdU mAb. Data shown are profiles of gated NK1.1+ cells from one representative mouse in each group. The gates for BrdU+ cells were set based on isotype control, which gave less than 0.5% positive cells. The percentages of BrdU+ cells are shown in the panels. Data shown are representative of 2 independent experiments. (C) Composite data on percentages of BrdU+ cells of NK1.1+ cells from liver and spleen. Means, standard error, and statistical significance were described in the text.
In the spleens from mice that received PBS alone, 11.33% ± 2.64% of NK cells were BrdU±. This was significantly increased in mice that had received MHC+B7- (20.1% ± 5.33%, P = .02) or MHC-B7+ tumor cells (17.5% ± 2.5%, P = .01). However, by far the most significant increase was found in mice that received the MHC+B7+ tumor cells (BrdU+ percent of NK cells = 33.4 ± 5.88; P vs PBS group = .001; P vs MHC+B7- group = .014; P vs MHC-B7+ group = .002). Thus, although tumor cells expressing either MHC or B7-1 were able to induce NK cell division in vivo, presentation of both aNKRL (MHC) and B7-1 was required for optimal proliferation of NK cells in vivo.
Preferential activation of Ly49D+ NK cells, the essential subset for tumor rejection
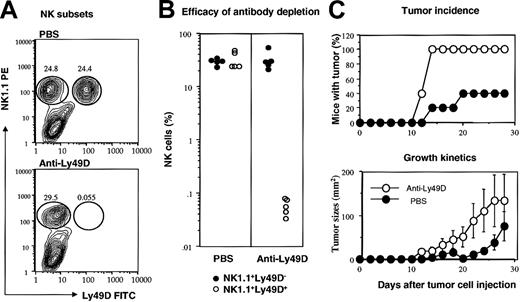
The requirement of MHC class I on the tumor cells for NK-mediated tumor resistance suggests that NK cells that recognize allogeneic MHC play a critical role. Since a substantial proportion of NK cells in the C57BL6/j mice express Ly49D, the only known activating receptor that recognizes the Dd molecule, it is possible that these cells may be critical for tumor rejection. To test this, we injected anti-Ly49D mAbs peritoneally prior to tumor challenge. As shown in Figure 3A-B, this mAb caused almost complete depletion of Ly49D+ NK cells, while leaving the Ly49D- subset intact. More importantly, the anti-Ly49D-treated mice were completely susceptible to the MHC+B7+ tumor cells, as judged by both tumor incidence and growth kinetics (Figure 3C-D).
Essential role of Ly49D+ NK cells in rejecting MHC+B7+ tumor cells. RAG-1(-/-)C57BL/6 mice were pretreated with either PBS or anti-Ly49D mAb. The MHC+B7+ tumor cells (5 × 106/mouse) were injected subcutaneously. (A) Representative contour graphs depicting NK subsets in PBS- or anti-Ly49D mAb-treated mice, as analyzed at 4 weeks after tumor cell challenge. The lack of Ly49D+ NK cells was not due to a blockade of fluorescein isothiocyanate (FITC)-conjugated anti-Ly49D antibody, as no Ig+ NK cells were found in the RAG-1(-/-) mice (J.X.G., unpublished observations, 2002). (B) Composite data that show almost complete depletion of Ly49D+ cells in vivo, 5 mice per group. (C) Tumor incidence (top) and growth kinetics (bottom) of MHC+B7+ tumors in PBS- or anti-Ly49D mAb-treated mice. Data shown are means and SEM of tumor diameters.
Essential role of Ly49D+ NK cells in rejecting MHC+B7+ tumor cells. RAG-1(-/-)C57BL/6 mice were pretreated with either PBS or anti-Ly49D mAb. The MHC+B7+ tumor cells (5 × 106/mouse) were injected subcutaneously. (A) Representative contour graphs depicting NK subsets in PBS- or anti-Ly49D mAb-treated mice, as analyzed at 4 weeks after tumor cell challenge. The lack of Ly49D+ NK cells was not due to a blockade of fluorescein isothiocyanate (FITC)-conjugated anti-Ly49D antibody, as no Ig+ NK cells were found in the RAG-1(-/-) mice (J.X.G., unpublished observations, 2002). (B) Composite data that show almost complete depletion of Ly49D+ cells in vivo, 5 mice per group. (C) Tumor incidence (top) and growth kinetics (bottom) of MHC+B7+ tumors in PBS- or anti-Ly49D mAb-treated mice. Data shown are means and SEM of tumor diameters.
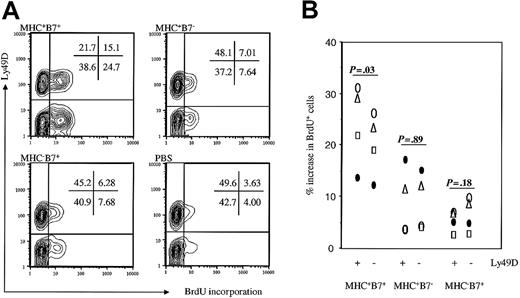
The essential role of Ly49D+ NK cells raised the possibility that the NK subset may be preferentially activated after challenge by MHC+B7+ tumor cells. To analyze this issue, we compared the percentages of Ly49D+ and Ly49D- NK cells that had incorporated BrdU. Again, representative fluorescence-activated cell-sorter scanner (FACS) profiles are shown in Figure 4A, and, after subtracting the average BrdU incorporation in PBS-challenged mice, the specific increase of BrdU+ cells is summarized in Figure 4B. Although there were more Ly49D- cells that incorporated BrdU in mice that received MHC+B7+ tumor cells, the percentage of BrdU+ cells among Ly49D+ subset was higher than those among the Ly49D- subsets, especially after the background incorporation in mice treated with PBS alone was subtracted. In mice that received either MHC+B7- or MHC-B7+ tumor cells, the increases in percentage of BrdU+ cells were comparable between Ly49D+ and Ly49D- NK subsets (P = .18 and .89 for mice receiving either MHC-B7+ or MHC-B7+ tumor cells, respectively). In contrast, a significantly higher increase was observed in BrdU+Ly49D+ cells compared with BrdU+Ly49D- cells in each of 4 mice that received MHC+B7+ tumor cells (P of paired t test = .03). Thus, in addition to promoting expansion of NK cells in general, codelivery of both MHC class I and the costimulatory molecule B7-1 also resulted in preferential expansion of the NK cells with the specific activating receptor Ly49D.
Preferential expansion of Ly49D+ NK cells in mice challenged with MHC+B7+ tumor cells. Mice were challenged with tumor cells and pulsed with BrdU as described in the legend to Figure 2. At 48 hours after tumor cell challenge, spleen cells were analyzed for NK cell subsets and BrdU incorporation. (A) Representative contour graphs from PBS control or tumor-challenged mice. Percentages of cells in the quadrants are presented in the panels. (B) Increase in percent of Ly49D+ and Ly49D- NK cells that were BrdU+ after tumor cell challenge. Data shown were percent of BrdU+ cells among spleen NK subsets from tumor cell-challenged mice minus the means of percent BrdU+ cells among spleen NK subsets in 4 PBS-treated mice.
Preferential expansion of Ly49D+ NK cells in mice challenged with MHC+B7+ tumor cells. Mice were challenged with tumor cells and pulsed with BrdU as described in the legend to Figure 2. At 48 hours after tumor cell challenge, spleen cells were analyzed for NK cell subsets and BrdU incorporation. (A) Representative contour graphs from PBS control or tumor-challenged mice. Percentages of cells in the quadrants are presented in the panels. (B) Increase in percent of Ly49D+ and Ly49D- NK cells that were BrdU+ after tumor cell challenge. Data shown were percent of BrdU+ cells among spleen NK subsets from tumor cell-challenged mice minus the means of percent BrdU+ cells among spleen NK subsets in 4 PBS-treated mice.
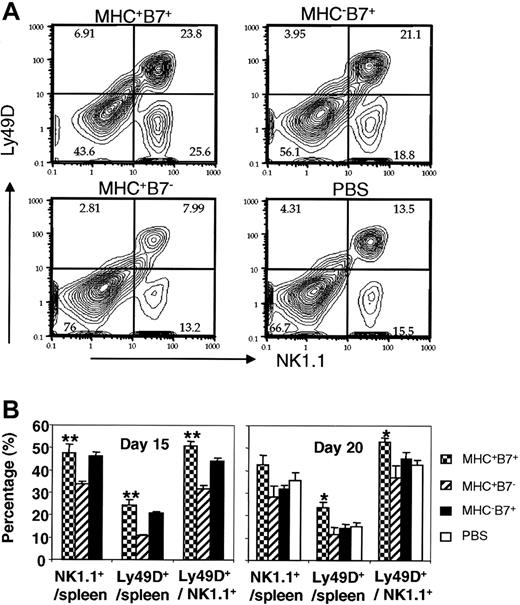
Surprisingly, among the mice that received different treatments, we did not find significant difference in the percentage of total NK cells and that of Ly49D+ NK cells on days 2 and 4 after tumor cell challenge (J.X.G., unpublished observations, 2002). To determine the long-term consequences of increased DNA synthesis on accumulation of NK cells and Ly49D+ NK cells, we compared, at 3 weeks after injection, the percentage NK1.1+ and NK1.1+Ly49D+ cells in mice challenged with PBS or tumor cells. Representative FACS profiles of spleen cells, on day 20 after tumor cell challenge, are presented in Figure 5A, while the summary data of days 15 and 20 after tumor cell challenge are presented in Figure 5B. On day 15, mice challenged with B7-1+ tumors (MHC+B7+ or MHC-B7+ tumor cells) had a significantly higher percentage of NK cells than did the mice challenged with the MHC+ B7- tumor cells (MHC+B7+ vs MHC+B7-, P = .01, MHC-B7+ vs MHC+B7-, P = .0005). Since MHC+B7- tumor cells and MHC-B7+ tumor cells induced similar levels of BrdU incorporation among NK cells, the selective reduction in percentage of NK cells in mice challenged with the MHC+B7- tumor cells cannot be explained by the rate of NK cell division. The increase of NK cells in mice challenged with the MHC-B7+ tumor cells was transient, as the percentage of NK cells was comparable to PBS and MHC+B7- tumor-challenged mice on day 20 (Figure 5B, right panel). Surprisingly, despite significant incorporation of BrdU into NK cells from mice challenged with either MHC+B7- or MHC-B7+ tumor cells, the proportion of NK cells was not increased in mice challenged with these 2 tumor cell lines compared with PBS-treated mice on day 20 (Figure 5B).
The effect of tumor expression of MHC class I and B7-1 on the proportion of Ly49D+ NK cells in the spleen on days 15 and 20 after tumor cell challenge. (A) Representative FACS profiles of NK1.1+ and Ly49D+ cells among total spleen cells isolated on day 20 after tumor cell inoculation. (B) Summary of NK cell subsets on days 15 and 20 after tumor cell inoculation. Data in left panels are means and SD of an experiment involving 4 (MHC+B7-) or 5 mice (MHC+B7+, MHC-B7+) per group. Data in the right panel are means and SE of 2 independent experiments. The number of mice are MHC+B7-(5), MHC+B7+ (8), MHC-B7+ (3), and PBS (4). *indicates P < .05; **, P < .01.
The effect of tumor expression of MHC class I and B7-1 on the proportion of Ly49D+ NK cells in the spleen on days 15 and 20 after tumor cell challenge. (A) Representative FACS profiles of NK1.1+ and Ly49D+ cells among total spleen cells isolated on day 20 after tumor cell inoculation. (B) Summary of NK cell subsets on days 15 and 20 after tumor cell inoculation. Data in left panels are means and SD of an experiment involving 4 (MHC+B7-) or 5 mice (MHC+B7+, MHC-B7+) per group. Data in the right panel are means and SE of 2 independent experiments. The number of mice are MHC+B7-(5), MHC+B7+ (8), MHC-B7+ (3), and PBS (4). *indicates P < .05; **, P < .01.
When the NK cells were analyzed for expression of Ly49D, it became clear that the MHC+B7+ tumors preferentially increased the Ly49D+ cells among the total NK cells compared with mice challenged with MHC+B7- (P = .0003 on day 15, and .0001 on day 20) or MHC-B7+ (P = .03 at both time points) tumor cells. Combining the increase of total NK cells and the selective enrichment of Ly49D+ subset among the NK cells, the frequency of Ly49D+ NK cells in mice challenged with MHC+B7+ tumor cells was 2-fold higher than that of the PBS-treated mice or of mice challenged with MHC+B7- (days 15 and 20) or MHC-B7+ (day 20 only) tumors. Interestingly, the group that had received MHC+B7- tumor cells had the lowest percentage of NK cells and Ly49D+ NK cells. Thus, codelivery of both B7-1 and MHC to NK cells increased the percentage of Ly49D+ NK cells in the spleen.
Activation-induced cell death of NK cells explains the slow accumulation or disappearance of NK cells in tumor cell-challenged mice
The lack of a significant increase in the NK cell population as well as the Ly49D+ NK subset within 4 days of tumor cell challenge appears inconsistent with tumor cell-induced incorporation of BrdU. One way to reconcile this is that activated NK cells may undergo activation-induced cell death. We used 2 different assays to determine the fate of NK cells on day 2 and day 4 after tumor cell challenge. Since results from the 2 time points were comparable, only those from day 2 after tumor cell challenge were presented in Figure 6.
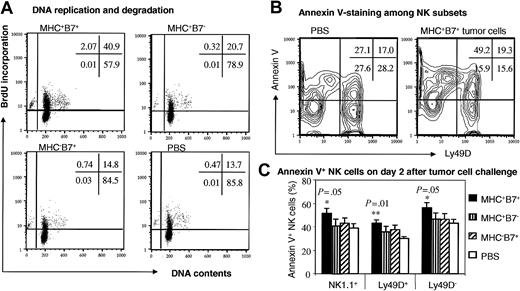
Programmed cell death of NK cells in response to tumor cell challenge. RAG-1(-/-)C57BL6/j mice were injected with 106/mouse of tumor cells intravenously and with 2 daily doses of BrdU intraperitoneally. Mice were killed at 48 hours after tumor cell challenge for analysis. (A) BrdU incorporation and DNA contents of NK cells. Spleen cells were stained with NK1.1 mAb and after fixation and permeabilization, stained with anti-BrdU mAb and 7-AAD for DNA content. Data shown were NK1.1+ cells with scatters of viable cells and are representative of 4 mice in each group. Note that all of the cells with subdiploid DNA contents were BrdU+. (B,C) Analysis of apoptosis by annexin V-staining of NK cells. Spleen cells were stained with mAbs specific for NK1.1 and Ly49D, or with annexin V. Data shown in panel B are contour graphs of gated NK1.1+ cells with scatters of viable lymphocytes. The percentages of annexin V+ cells among total NK population as well as Ly49D+ and Ly49D+ NK cells were presented in panel C. Only the mice that received MHC+B7+ tumor cells showed a significant increase in annexin V+ cells when compared with those mice that received PBS. No other comparison showed a statistically significant difference. Data shown are means ± SEM.
Programmed cell death of NK cells in response to tumor cell challenge. RAG-1(-/-)C57BL6/j mice were injected with 106/mouse of tumor cells intravenously and with 2 daily doses of BrdU intraperitoneally. Mice were killed at 48 hours after tumor cell challenge for analysis. (A) BrdU incorporation and DNA contents of NK cells. Spleen cells were stained with NK1.1 mAb and after fixation and permeabilization, stained with anti-BrdU mAb and 7-AAD for DNA content. Data shown were NK1.1+ cells with scatters of viable cells and are representative of 4 mice in each group. Note that all of the cells with subdiploid DNA contents were BrdU+. (B,C) Analysis of apoptosis by annexin V-staining of NK cells. Spleen cells were stained with mAbs specific for NK1.1 and Ly49D, or with annexin V. Data shown in panel B are contour graphs of gated NK1.1+ cells with scatters of viable lymphocytes. The percentages of annexin V+ cells among total NK population as well as Ly49D+ and Ly49D+ NK cells were presented in panel C. Only the mice that received MHC+B7+ tumor cells showed a significant increase in annexin V+ cells when compared with those mice that received PBS. No other comparison showed a statistically significant difference. Data shown are means ± SEM.
First, we analyzed the DNA content of the NK cells. Profiles of DNA content versus BrdU incorporation in the gated NK1.1+ cells, as shown in Figure 6A, revealed that the overwhelming majority of the BrdU+ NK cells were at G0 phase (2N), although a small fraction of them were present at the S to G2 phases. A clearly identifiable NK1.1+ subset had a subdiploid DNA content, which indicated that at least part of NK cells were undergoing apoptosis. Interestingly, essentially all of the apoptotic cells were BrdU+, which indicated that apoptotic NK cells must have been activated previously. Corresponding to the more vigorous activation, more apoptotic cells were found in NK cells from MHC+B7+ tumor cell-challenged mice.
Since the apoptotic cells are rapidly cleared by the macrophages and immature dendritic cells in vivo,40 the amount of subdiploid NK cells may be an underestimate of the proportion of NK cells undergoing apoptosis in vivo. We also used annexin V staining to detect cells with altered lipid configurations, an early sign of apoptosis. As shown in Figure 6B, in mice that received MHC+B7+ tumor cells, more than 50% of the NK cells exhibited early signs of apoptosis. This was significantly higher than what was found in the NK cells from control PBS-treated mice (P = .05). The increased apoptosis was observed in both Ly49D+ (P = .01) and Ly49D- (P = .05) NK cells. The high proportion of apoptotic cells in the MHC+B7+ tumor cell-challenged mice explains the lack of a major increase in the percentages of NK cells on day 2 and day 4, when more than 30% of the cells were actively synthesizing DNA.
Two-signal requirement for expansion and effector function of NK cells in response to melanoma B16 variants
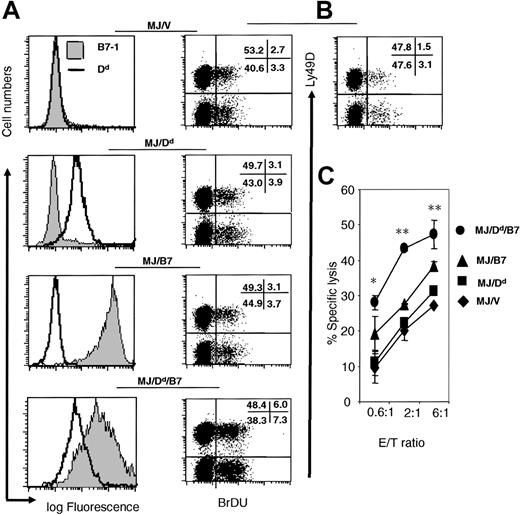
To verify the function of H-2Dd and B7-1 in promoting NK expansion and expansion in conjunction with B7-1, we used B78H1, a variant of B16 melanoma that lacked MHC class I heavy chains and TAP-2. Chiang et al recently transfected the B78H1 with TAP-2.36 The TAP-2 transfectant is hereby called MJ. For the current study, the MJ were further transfected with either vector alone (MJ/V) or H-2Dd (MJ/Dd), H-2Dd + B7-1 (MJ/Dd/B7), or B7-1 alone (Figure 7A). To test the effect of H-2Dd and B7-1 on NK cell proliferation in vivo, we injected either PBS or the 4 variants of the MJ tumor cells into the RAG-1(-/-) C57BL/6 mice intravenously and pulsed with recipient mice with BrdU. On day 2, when the maximal proliferation of NK cells were observed (J.X.G., unpublished observations, 2002), we analyzed the division of NK cells by flow cytometry. As shown in Figure 7B, while tumor challenge results in an increase of NK cell division over the PBS control, tumor cells expressing either B7-1 or H-2Dd had essentially the same level of NK cell division. Thus, either molecule alone did not lead to increased NK cell expansion. In contrast, coexpression of B7-1 and H-2Dd results in substantially higher expansion of NK cells. Surprisingly, we did not observe preferential expansion of H-2Dd-specific Ly49D+ NK cells in multiple experiments. This could be due to nonspecific NK response subsequently to H-2Dd-triggered IFNγ production, as has been reported for Ly49H+ NK cells on day 2 of MCMV infection.13
Coexpression of H-2Dd and B7-1 on a B16 melanoma variant B78H1 promotes expansion and effector function of NK cells. (A) Profile of H-2Dd (open histograms) and B7-1 (shaded histograms) on 4 transfectants of MJ, the TAP-2-transfected B78H1 cells. (B) Proliferation of NK cells in RAG-1(-/-) C57BL/6j mice at 2 days after challenge with PBS or 4 transfectants depicted in (A). Data shown were representative dot plots of gated NK1.1+ cells from an experiment involving 2-3 mice per group. Numbers in the panels show the percent of cells in each quadrant. The enhanced proliferation of NK cells induced by MJ/B7/Dd tumor cells has been reproduced with 2 independent clones. (C) Coexpression of B7-1 and H-2Dd enhanced cytolysis of IL-15-activated NK cells. Note that a significant difference was observed between the lysis of M/J/Dd/B7 target and the next most susceptible target (M/J/B7). The results have been repeated 3 times. Data are means ± SEM. *P < 0.05. **P < 0.01.
Coexpression of H-2Dd and B7-1 on a B16 melanoma variant B78H1 promotes expansion and effector function of NK cells. (A) Profile of H-2Dd (open histograms) and B7-1 (shaded histograms) on 4 transfectants of MJ, the TAP-2-transfected B78H1 cells. (B) Proliferation of NK cells in RAG-1(-/-) C57BL/6j mice at 2 days after challenge with PBS or 4 transfectants depicted in (A). Data shown were representative dot plots of gated NK1.1+ cells from an experiment involving 2-3 mice per group. Numbers in the panels show the percent of cells in each quadrant. The enhanced proliferation of NK cells induced by MJ/B7/Dd tumor cells has been reproduced with 2 independent clones. (C) Coexpression of B7-1 and H-2Dd enhanced cytolysis of IL-15-activated NK cells. Note that a significant difference was observed between the lysis of M/J/Dd/B7 target and the next most susceptible target (M/J/B7). The results have been repeated 3 times. Data are means ± SEM. *P < 0.05. **P < 0.01.
We used the IL-15-activated spleen NK cells to test if expression of H-2Dd and B7-1 promote NK lysis in vitro. As shown in Figure 7C, although a slight increase of susceptibility was conveyed by either B7-1 or H-2Dd, substantial increase in NK lysis was achieved only if both MHC class I and B7-1 were codelivered to NK cells.
Discussion
Recent studies revealed that the function of NK cells is regulated by both activating and inhibitory receptors.5,41 It is clear that different NK cells can display a distinct pattern of inhibitory receptors. The existence of NK subsets with different specificities raised the issue of whether NK cells with desired specificity expand in response to infection or malignancy. However, of all the activating receptors identified, only 2 of them, Ly49D and Ly49H, are not present on all NK cells. Ly49H is involved in the control of MCMV infection,42,43 and recent studies have identified its target as an MCMV-encoded MHC-like molecule.14,15 Ly49D has a known ligand, H-2Dd.33
We have investigated the contribution of B7-1 and MHC class I on activation and effector function of NK cells in vivo using tumor variants that lack either MHC or B7-1. We found that tumor rejection is achieved only if the tumor cells express both MHC class I and the costimulatory molecule B7-1. The requirement for both an activating ligand and the costimulatory molecule B7-1 in NK-mediated tumor rejection reveals a striking similarity between the T-cell-mediated tumor immunity and NK-cell-mediated tumor immunity.
A hallmark of T-cell immune response is the extensive clonal expansion of antigen-reactive T cells.1 Recent work from Dokun et al revealed selective expansion of NK cells bearing a specific receptor for the MCMV-infected cells.13 Here we showed that tumor cells also triggered DNA synthesis of NK cells in vivo. More importantly, the extent of NK cell activation can be controlled by expression of both antigen and costimulatory molecule B7-1 on the tumor cells, with optimal activation depending on codelivery of both activating ligand and costimulator. Moreover, in the plasmocytoma model, these 2 signals promote preferential activation of NK cells bearing Ly49D, an activating receptor for H-2Dd on the tumor cell cells. The requirement for both triggering allogeneic MHC (H-2Dd) and costimulatory molecule B7-1 for optimal NK activation and effector function of NK cells also have been demonstrated in variants of B16 melanoma, although we did not observe selective expansion of Ly49D+ NK cells in vivo. This can be due to nonspecific interferon (IFN) response, as has been reported by others.13 In addition, it is also possible that B16 may have other aNKL capable of triggering Ly49D- NK cells in conjunction with H-2Dd. Moreover, the Ly49D+ NK cells may have vanished as a result of activation-induced cell death. Regardless of which interpretation is correct, our results indicate that the 2 signals that control T-cell activation also regulate NK cell function.
The need to study specific interaction between an aNKRL and its receptor led us to study the NK function in the allogeneic model. The results from the allogeneic model have direct implications for regulating the alloreactivity of NK cells in bone marrow transplantation and cancer therapy.44 Moreover, our conclusions are consistent with a host of observations, including a recently reported selective expansion of Ly49H+ NK cells in the course of MCMV infection.13 Although the requirement for B7-1 and B7-2 was not addressed in the latter study, B7-1 and B7-2 may well play an important role in the process, as they are expressed on host antigen-presenting cells (APCs),45,46 and NK cells express CD28 on the cell surface.28 Second, several studies have indicated a critical role of B7-1 and B7-2 in NK-mediated tumor rejection,31,32 although it has been difficult to address whether B7-1/2 can work in concert with an aNKRL to induce a seemingly adaptive NK response, perhaps due to the lack of known aNKRLs and their receptors in those models. Third, dendritic cells, which are critically important in regulating T-cell activation, also guide NK cell differentiation.47
Although our data showed parallel functions for both antigenic and costimulatory signals in activation and effector function of NK and T cells, several interesting differences between T-cell activation and NK activation deserve discussion. First, specificity in the expansion of NK cells documented here was much less refined than that of T cells. The bystander activation of T-cell response during an immune response is generally considered to be rare.48,49 However, here we showed that BrdU incorporation also increased among Ly49D- NK cells in mice that received tumor cells expressing both B7-1 and H-2d on the cell surface. In case of H-2Dd+ and B7-1+ melanoma B78H1, the increase was equal in Ly49D+ and Ly49D- NK cells. This can be attributed either to an unidentified MHC receptor on the Ly49D- NK cells or to higher levels of bystander activation. The apparent bystander activation also was observed by Dokun et al early in viral infection,13 although it remains possible that the cells that incorporate BrdU were responding to viral (or virus-induced) molecules. Second, although rapid incorporation of BrdU into NK cells can be easily demonstrated, a change in overall NK subset presentation was not significant in the first 4 days analyzed (unpublished observation). Over the course of 2-3 weeks, we did observe significant increases of NK cells with desired specificity in the mice that received MHC+B7+ tumor cells. However, this increase was less than what one would expect based on the rate of BrdU incorporation. Our analysis of DNA contents and cell membrane alterations revealed high proportions of activated NK cells undergoing apoptosis. Interestingly, essentially all of the cells with subdiploid DNA contents had incorporated BrdU. This argues for the notion that death of NK cells followed activation. In this regard, the apparent 2-signal-dependent cell death observed in our study can either be simply a consequence of increased activation or a reflection of direct roles for the 2 signals in triggering cell death. Third, a critical issue is the biochemical basis for the requirement of codelivery of costimulatory molecules and aNKL for NK activation, as demonstrated here. In the T cells, it has been demonstrated that activation of JNK can serve as a point for integration of signals 1 and 2.50 However, our extensive analysis did not show activation of JNK among NK cells by any of the tumor lines studied here (unpublished observation). Moreover, it has been demonstrated that CD28-mediated phosphorylation of phosphatidylinositol-specific phospholipase Cγ151 can be modulated by killer immunoglobulin-like receptor,52 although we have so far failed to reveal any effect of codelivery of B7-1 and MHC class I on accumulation of phosphorylation of phosphatidylinositol-specific phospholipase Cγ1 (J.X.G., unpublished observations, 2002). Finally, previous studies have shown clear NK function in mice with targeted mutation.53 Thus, much like what was reported in T-cell activation,54-56 the requirement for B7-CD28 costimulation is unlikely to be universal.
Although the cell death prevented immediate accumulation of NK cells, rapid and selective division of NK cells with specificity for the tumor cells may provide a large number of innate defense forces that can participate and die in battles against malignant cells. The increased turnover of fresh NK cells may explain the enhanced NK-mediated resistance to tumor cells expressing both MHC and B7-1, as demonstrated here. On the other hand, limited accumulation of NK cells of given specificity suggests that NK cells may retain short-term memory after a recent insult.
It is well established that the innate immune responses lead to the induction of costimulatory molecules.1,57,58 These costimulatory molecules may not only pave the way for adaptive immunity, as previously envisaged,1,57,58 but also promote the innate immunity, as we demonstrated here. The fact that both T cells and NK cell response can be modulated by both antigen (activating ligand) and costimulators implies that the 2-signal-based immune regulation may have evolved prior to adaptive T-cell responses.
Prepublished online as Blood First Edition Paper, August 21, 2003; DOI 10.1182/blood-2003-07-2480.
Supported by a program project from the National Cancer Institute (P01CA954201) and grants from the National Institutes of Health (CA58033, CA69091, AI32981, CA82355, and AI19624).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Jennifer Kiel and Lynde Shaw for editorial assistance.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal