Abstract
After vascular injury, a remodeling process occurs that features leukocyte migration and infiltration. Loss of endothelial integrity allows the leukocytes to interact with vascular smooth muscle cells (VSMCs) and to elicit “marching orders”; however, the signaling processes are poorly understood. We found that human monocytes inhibit VSMC proliferation and induce a migratory potential. The monocytes signal the VSMCs through the urokinase-type plasminogen activator (uPA). The VSMC uPA receptor (uPAR) receives the signal and activates the transcription factor Stat1 that, in turn, mediates the antiproliferative effects. These results provide the first evidence that monocytes signal VSMCs by mechanisms involving the fibrinolytic system, and they imply an important link between the uPA/uPAR-related signaling machinery and human vascular disease. (Blood. 2003;102: 4377-4383)
Introduction
Restenosis after percutaneous vascular therapeutic interventions remains unresolved.1,2 After vascular injury, the endothelial integrity is disturbed and monocytes infiltrate the injured vessels. The monocytes promote an inflammatory response by generating migratory and proliferative signals that converge on vascular smooth muscle cells (VSMCs). The VSMCs first migrate to the normally thin intimal layer and thereafter proliferate, causing neointimal formation and restenosis.3,4 Although VSMC proliferation was reported in most animal models of vascular remodeling, few proliferating VSMCs were detected in human lesions. This finding implies an antiproliferative mechanism for VSMC growth control, at least during the early phase of the remodeling process.5 We showed earlier that the urokinase-type plasminogen activator (uPA) and its receptor (uPAR) are up-regulated on VSMC-monocyte interaction in a coculture model and contribute to increased VSMC motility.6 uPA is an unusual molecule of dual function that switches from the proteolytic enzyme to the signal-inducing ligand, depending on external stimuli.7,8 The uPA/uPAR system has a nonproteolytic role in vitro and in vivo that extends beyond its role in fibrinolysis.9,10 We investigated the mechanisms underlying changes in VSMC behavior on interaction with monocytes. We found that the monocytes express uPA to convey an antiproliferative signal to the VSMCs through their uPAR receptor. This signaling is mediated by activation of the VSMC transcription factor signal transducer and activator of transcription 1 (Stat1). Our study may explain the increased VSMC migratory potential and the absence of VSMC proliferation observed at the early step of a remodeling process of the vessel wall after vascular injury in humans. Conceivably, a useful strategy for therapeutic intervention could ensue.
Materials and methods
Cell culture, monocyte isolation, coculture, and cell separation after coculture
Human vascular smooth muscle cells from coronary artery were obtained from Clonetics (San Diego, CA). Cells were grown in Smooth Muscle Medium-2 (SmGM2) medium (Clonetics) supplemented with 5% fetal bovine serum and were used between passages 4 and 6. Before coculture with monocytes or cytokine or antibody treatments, cells were serum starved for 8 to 12 hours unless otherwise indicated. For coculture, monocytes were isolated from human peripheral blood from healthy volunteers using Biocoll Separation Solution (Biochrom KG Seromed, Berlin, Germany) according to the standard protocol. Monocytes were stimulated with 100 ng/mL lipopolysaccharide (LPS) for 2 to 3 minutes, and, after centrifugation, the cell pellet was resuspended in SmGM2 medium without supplements. Then monocytes were directly added to VSMCs that were 60% to 80% confluent at a concentration of 1.9 × 105 monocytes/cm2 dish area so that the VSMC/monocyte ratio was approximately 1:3. In the following experiments, coculture was performed for 24 to 36 hours. Time-dependency experiments showed an increased activation of VSMCs up to 3 to 4 days of coculture. For further biochemical experiments, cocultured VSMCs were detached with 5 mM EDTA (ethylenediaminetetraacetic acid) and were separated from monocytes using the MACS cell separation system (Miltenyi Biotec, Bergisch Gladbach, Germany) using CD11b/CD14 microbeads according to the manufacturer's instructions. Separation efficiency for VSMCs was approximately 94% to 96%, as verified by fluorescence-activated cell sorter (FACS) analysis.
VSMCs from uPA, uPAR, and Stat1 knockout mice
uPA- and uPAR-deficient mice were kindly provided by Peter Carmeliet and Mieke Dewerchin (Leuven, Belgium). Stat1 knockout mice were purchased from Taconic M&B (Germantown, NY). VSMCs from the aorta were isolated as described here.11 Briefly, after the excision of the aorta from the mouse, the vessel was made free of the fatty tissues surrounding it and were cut longitudinally. Then the vessel was subjected to 2 steps of enzyme (collagenase + elastase) disaggregation. In the first step, the medial layer was separated from the adventitial layer by incubating the vessel for 8 to 10 minutes at 37°C in enzyme solution 1 (1.4 mg/mL collagenase and 0.5 mg/mL elastase). In the second step, the media tissue pieces were digested by incubation for 60 to 90 minutes in enzyme solution 2 (2 mg/mL collagenase and 0.5 mg/mL elastase) to obtain single VSMCs. The cell suspension was centrifuged to remove the enzymes, and the pellet was resuspended in SmGM2 medium supplemented with 5% fetal bovine serum (Clonetics) for culture. Passages 3 to 7 were used for the experiments. For coculture, the monocytes were prepared as described from the orbital vein blood of wild-type or transgene mice.
Plasmid construction, cell culture, transfection, and retroviral infection
pSUPER.retro was purchased from OligoEngine (Seattle, WA). To generate the pSUPER.retro-STAT1si, the pSUPER.retro vector was digested with BglII and HindIII, and the annealed oligos (5′-gat ccc cCA CGA GAC CAA TGG TGT GGt tca aga gaC CAC ACC ATT GGT CTC GTG ttt ttg gaa a; 5′-agc ttt tcc aaa aaC ACG AGA CCA ATG GTG TGG tct ctt gaa CCA CAC CAT TGG TCT CGT Ggg g) were ligated into the vector. The 19-nucleotide Stat1 target sequences are indicated in capitals in the oligonucleotide sequence.
Human embryonic kidney cells (293) were cultured in Dulbecco modified Eagle medium (DMEM) supplemented with 10% fetal calf serum. Pantropic retroviral supernatants were produced by transfection of these cells by calcium-phosphate precipitation. To produce high-titer virus transiently, 293T cells were cotransfected by pSUPER.retro-STAT1si, pVPack-VSV-G, and pVPack-GP (Stratagene, La Jolla, CA) in equivalent molar amounts. Sixty hours after transfection, the tissue culture medium was filtered through a 0.45-μm filter, and the viral supernatant was used to infect VSMCs. For control virus production, pSUPER.retro-STAT1si was substituted by LacZ control vector pQCLIN (BD Biosciences Clontech, Palo Alto, CA) with neomycin selection.
For retroviral infection, VSMCs were grown until 60% to 80% confluence in 6-well plates. Frozen viral supernatant was diluted 2-fold in SmGM2 medium with supplements and polybrene (final concentration, 8 μg/mL) and was added to the VSMCs. Plates were incubated for 20 minutes at 37°C, sealed with parafilm, and centrifuged for 30 minutes 1100g at 32°C. Cells were further incubated at 37°C for 12 hours before virus removal and then serum starved before experiments.
Stat1 messenger RNA (mRNA) level was determined by TaqMan analysis as described previously.6 The primers used for Stat1 were 5′-AGA CAG CCC TGC ATG CCA, 5′-AAC TGG ACC CCT GTC TTC AAG A, and TaqMan probe 5′-CGC ACC CTC AGA GGC CGC TG.
Immunofluorescence microscopy
Stat1 was detected using monoclonal Stat1 (C-terminus) and rabbit polyclonal Stat1 (N-terminus) (BD Transduction Laboratories, Lexington, KY) antibodies and Alexa 546-conjugated goat antimouse and antirabbit immunoglobulin G (IgG) (H+L) (Molecular Probes, Eugene, OR) as secondary antibodies. Fluorescence microscope studies were performed as described.12-14 For uPA and uPAR antibody treatments, the cells were incubated with uPA (25 μg/mL; American Diagnostica, Greenwich, CT) and uPAR R3 clone (100 ng/mL; Monozyme, Copenhagen, Denmark) antibody solutions. The antibody solution was prepared with serum-free medium and added 1 hour before the addition of monocytes.
Electromobility shift assay and Western blotting
Nuclear extracts were prepared from VSMCs that were either monocultured or directly cocultured with monocytes. For lysates from cells stimulated with interferon-γ (IFN-γ) (10 ng/mL; R&D Systems, Minneapolis, MN), cells were stimulated for 30 to 45 minutes before detachment with EDTA. After cell detachment and separation, the VSMC pellet was resuspended in buffer A (10 mM HEPES [N-2-hydroxyethylenepiperazine-N′-2-ethanesulfonic acid], pH 7.9, 1.5 mM MgCl2, 10 mM KCl, 0.5 mM dithiothreitol [DTT]), containing protease inhibitors (1 mM phenylmethylsulfonyl fluoride [PMSF], 5 mM iodoacetamide, 0.1 mM quercetin, 10 μg/mL aprotinin, 10 μg/mL leupeptin, 0.3 mM sodium vanadate) and incubated on ice for 15 minutes. After homogenization in a Wheaton 0.1-mL homogenizer, the nuclei were collected by centrifugation. The pellet was resuspended in buffer B (20 mM HEPES, pH 7.9, 25% glycerol, 1.5 mM MgCl2, 420 mM NaCl, 0.2 mM EDTA, 0.5 mM DTT) containing protease inhibitors and was incubated on ice for 30 minutes, followed by centrifugation at 13 000g (5 minutes, 4°C). The supernatant was dialyzed against buffer C (20 mM HEPES, 20% glycerol, 100 mM KCl, 0.2 mM EDTA, 0.5 mM DTT) containing protease inhibitors for 2 hours at 4°C, followed by centrifugation by 13 000g for 5 minutes at 4°C. The supernatant proteins were used immediately or aliquoted and stored at -80°C.
Binding reaction was performed for 30 minutes on ice in a volume of 20 μL, containing 4 μg nuclear protein extracts, 40 ng poly(dI-dC), 4 μL 5 × binding buffer (1 × binding buffer: 20 mM HEPES, pH 7.9, 50 mM KCl, 5 mM MgCl2, 1 mM EDTA, 1 mM DTT, 10% glycerol) with or without 20- to 50-fold excess of cold competitor or of unrelated competitor and a 32 P-labeled probe (3 × 104 cpm). In supershift electrophoretic mobility shift assay (EMSA), protein extracts were incubated with 6 μg Stat1 monoclonal antibody or isotype control before the addition of the 32P-labeled probe. DNA-protein complexes were separated on 5% polyacrylamide gel in Tris/glycine buffer at 4°C. The following double-stranded oligonucleotides purchased from Santa Cruz Biotechnology (Santa Cruz, CA) were used in this study: GAS/ISRE, 5′-AAGTACTTTCAGTTTCATATTACTCTA-3′, 27 base pair (bp) (sc-2537); AP-1, 5′-CGCTTGATGACTCAGCCGGAA-3′, 21 bp (sc-2501). Five end-labeled probes were prepared with 40 μCi (1480 MBq) [γ-32P] adenosine triphosphate (ATP) using T4 polynucleotide kinase and were gel-purified on NAP-5 Sephadex G-25 DNA-grade columns.
Nuclear and cytoplasmic fractions for Western blotting were prepared as described.15,16 Subcellular fraction proteins were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and were analyzed by rabbit polyclonal anti-pSer727 Stat1 and anti-pTyr701 Stat1 antibodies (BioSource International, Camarillo, CA) by Western blotting, as described elsewhere.13,14
Proliferation assay
VSMCs were cultured on coverslips to a density of 60% to 70% and serum starved for 24 hours and cocultured with freshly prepared monocytes. A set of controls was included, as follows: monoculture of VSMCs without supplements, with 5% FCS and coculture with 5% FCS. After incubation the cells were treated with 100 μM 5-bromodeoxyuridine (BrdU; Sigma-Aldrich, St. Louis, MO) and were further incubated for 8 to 12 hours at 37°C. Cells were washed, fixed in 4% paraformaldehyde, permeabilized with 0.5% Triton X-100 in phosphate-buffered saline (PBS), and blocked with 1% bovine serum albumin (BSA) in PBS for 30 minutes at room temperature. Cells were incubated in a cocktail containing anti-BrdU monoclonal antibody (Becton Dickinson, San Jose, CA), 1% BSA, 2 × DNAse buffer solution (1 M mercaptoethanol, 100 mM Tris-HCl, pH 8.1, 10 mM MgCl2), and DNAse I enzyme solution for 1 hour, washed, and incubated with Alexa 546-labeled goat antimouse IgG (Molecular Probes) for 1 hour at room temperature. After washing, DNA was counterstained with Hoechst 33258, washed with PBS, and mounted in Mowiol with 2.5% (wt/vol) DABCO (1,4-diazabicyclo[2.2.2]octane). Results were documented using a fluorescence microscope.
Microinjection assay
Serum-starved VSMCs (60%%-70% confluence) were microinjected with anti-pSer727 Stat1 rabbit polyclonal antibody (25 μg/mL in PBS with Mg2+) or nonspecific rabbit polyclonal IgG (25 μg/mL) using micromanipulator type MO-8 (Nikon Narishige; Nikon, Melville, NY) with microinjector PL-188 (Nikon) and were cocultured with monocytes for 24 hours. One hundred to 120 cells were microinjected in each experiment. Microinjected cells were used for Stat1 staining and proliferation assay, as indicated.
Results
VSMCs stop proliferating when cocultured with monocytes
We found unexpectedly that VSMCs stopped proliferating when monocytes were added to the coculture model, even if the cells were cultured in the presence of 5% fetal calf serum (FCS) (Figure 1). We followed 2 assumptions. First, we reasoned that VSMC growth inhibition might be mediated by transcription factor Stat1, a known powerful antiproliferative molecule.17 Second, we hypothesized that the required activation of Stat1 in VSMCs might be provided by the monocyte-expressed uPA. This idea was based on our previous studies demonstrating that Stat1 is activated in VSMCs in response to exogenous uPA12,14 and that the uPA expression in monocytes cocultured with VSMCs is strongly up-regulated.6
Inhibiting VSMC proliferation in coculture. VSMCs were cocultured with LPS-treated monocytes and were treated with BrdU to analyze DNA synthesis. Incorporation of BrdU was detected by immunofluorescence using monoclonal antibody against BrdU (bottom panels). DNA was counterstained with Hoechst 33258 (top panels). The above stainings were performed in serum-treated and nontreated samples, and VSMCs in monoculture was the control. Original magnification × 200.
Inhibiting VSMC proliferation in coculture. VSMCs were cocultured with LPS-treated monocytes and were treated with BrdU to analyze DNA synthesis. Incorporation of BrdU was detected by immunofluorescence using monoclonal antibody against BrdU (bottom panels). DNA was counterstained with Hoechst 33258 (top panels). The above stainings were performed in serum-treated and nontreated samples, and VSMCs in monoculture was the control. Original magnification × 200.
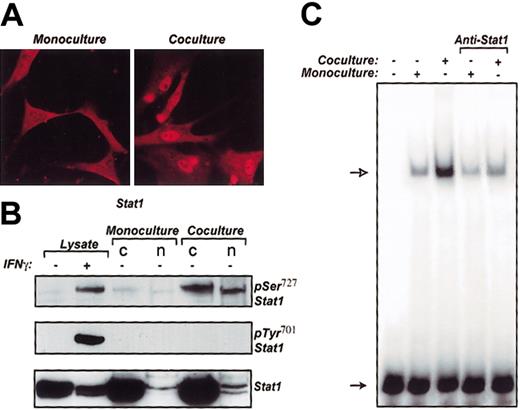
Stat1 is up-regulated in VSMCs cocultured with monocytes
To investigate whether VSMC Stat1 is affected after coculture with monocytes, we initially performed immunocytochemical studies to visualize the intracellular distribution of Stat1 (Figure 2A). A substantial portion of Stat1 molecules was activated after 24 hours of coculture and was translocated through the nuclear pores, leading to a striking accumulation (right panel). In contrast, no Stat1 nuclear translocation occurred when monocytes were absent (left panel). Reverse transcription-polymerase chain reaction (RT-PCR) confirmed Stat1 activation in cocultured VSMCs as long as Stat1 expression was not changed in these cells (data not shown). To assess the phosphorylation site of Stat1 affected in coculture, we isolated nuclear and cytosolic fractions from VSMCs cocultured with and then separated from monocytes and monitored the serine and tyrosine phosphorylation status of Stat1 by Western blotting (Figure 2B). The antibodies specifically recognized Stat1 phosphorylation on Ser727 and Tyr701. VSMCs in monoculture served as controls. As a positive control for Stat1 activation, cell lysate prepared from VSMCs stimulated with IFN-γ was used. Serine phosphorylation of Stat1 in cocultured VSMCs was promptly elevated (upper panel), whereas no activation of Stat1 tyrosine phosphorylation could be observed under the same experimental conditions (middle panel). In additional experimental settings, Stat1 phosphorylation on Tyr701 was identified only after longer exposure times than those used for Stat1-pSer727 phosphorylation (data not shown).
Stat1 in VSMCs is activated in coculture. (A) Stat1 immunofluorescence in VSMCs in coculture with monocytes and monoculture control. Original magnification × 400. (B) VSMCs after coculture were separated by MACS separating system and analyzed for pSer727 Stat1 and pTyr701 Stat1 in whole-cell lysate, cytosolic (c), and nuclear (n) fractions by subjection to SDS-PAGE and Western blotting with anti-pSer727 Stat1 and anti-pTyr701 Stat1 rabbit polyclonal antibodies, respectively. As a control for Stat1 amount, blots were analyzed for Stat1 using anti-Stat1 monoclonal antibody (bottom panel). (C) Gamma-activated site/interferon-stimulated response element (GAS-ISRE)-binding activity of nuclear extracts prepared from VSMCs cocultured with monocytes and from monoculture were analyzed by EMSA. Open arrow indicates the position of the protein-32P-GAS/ISRE complex, and the solid arrow indicates the position of the free probe.
Stat1 in VSMCs is activated in coculture. (A) Stat1 immunofluorescence in VSMCs in coculture with monocytes and monoculture control. Original magnification × 400. (B) VSMCs after coculture were separated by MACS separating system and analyzed for pSer727 Stat1 and pTyr701 Stat1 in whole-cell lysate, cytosolic (c), and nuclear (n) fractions by subjection to SDS-PAGE and Western blotting with anti-pSer727 Stat1 and anti-pTyr701 Stat1 rabbit polyclonal antibodies, respectively. As a control for Stat1 amount, blots were analyzed for Stat1 using anti-Stat1 monoclonal antibody (bottom panel). (C) Gamma-activated site/interferon-stimulated response element (GAS-ISRE)-binding activity of nuclear extracts prepared from VSMCs cocultured with monocytes and from monoculture were analyzed by EMSA. Open arrow indicates the position of the protein-32P-GAS/ISRE complex, and the solid arrow indicates the position of the free probe.
Although Stat1 phosphorylation on Ser727 was activated in cytosolic and nuclear fractions, the total amount of Stat1 protein in these fractions (lower panel) clearly demonstrated that activated Stat1 was mainly translocated to the nucleus. Additional experiments confirmed no activation of Stat1 in monocytes separated from VSMCs after coculture (data not shown).
We next examined the activation of Stat1 in VSMCs at the DNA-binding level. We monitored Stat1-DNA binding capacity in cocultured VSMCs by EMSA using nuclear extracts and a Stat1-specific GAS/ISRE oligonucleotide probe (Figure 2C). With coculture, the DNA-protein complex was increased, compared with monoculture controls. In gel supershift assay, anti-Stat1-specific antibody identified Stat1 in this prominent nuclear complex by reducing the intensity of the DNA binding.
VSMC Stat1 activation in coculture requires uPA/uPAR and is IFN-γ resistant
uPA expression by monocytes and uPAR expression on VSMCs are up-regulated in coculture.6 uPA is a known activator of Stat1 in these cells.12 We therefore reasoned that this interaction might explain the observed activation of Stat1. We treated cocultured cells with anti-uPA- and anti-uPAR-specific antibodies and examined Stat1 nuclear translocation in VSMCs with immunocytochemistry (Figure 3A). The antibody treatment abrogated Stat1 nuclear translocation in the coculture system. Consistent with these data, the EMSA showed that Stat1 activation was blocked in coculture when VSMCs were treated with the anti-uPA antibody (Figure 3B).
Stat1 activation in VSMCs depends on uPA/uPAR signaling.(A) VSMCs were treated with anti-uPA and anti-uPAR antibodies separately, before coculture with monocytes, and were analyzed for Stat1 by immunofluorescence. Total cell number and the number of cells with translocated Stat1 were counted for each view field. Nine to 10 view fields have been evaluated in each experiment. The result is a representative of 3 experiments (mean ± SD [n = 3]). (B) VSMCs were treated with anti-uPA antibody before the addition of monocytes; VSMCs in monoculture is control. GAS/ISRE binding activity of nuclear extracts prepared from the above anti-uPA antibody-treated VSMC was analyzed using EMSA. (C) Immunofluorescence staining for Stat1 in mouse VSMCs in coculture with monocytes from uPA-/- and in VSMCs from uPAR-/- mice cocultured with wild-type monocytes using anti-Stat1 rabbit polyclonal antibody. As controls, wild-type cells and VSMCs from uPA-/- mice are included.
Stat1 activation in VSMCs depends on uPA/uPAR signaling.(A) VSMCs were treated with anti-uPA and anti-uPAR antibodies separately, before coculture with monocytes, and were analyzed for Stat1 by immunofluorescence. Total cell number and the number of cells with translocated Stat1 were counted for each view field. Nine to 10 view fields have been evaluated in each experiment. The result is a representative of 3 experiments (mean ± SD [n = 3]). (B) VSMCs were treated with anti-uPA antibody before the addition of monocytes; VSMCs in monoculture is control. GAS/ISRE binding activity of nuclear extracts prepared from the above anti-uPA antibody-treated VSMC was analyzed using EMSA. (C) Immunofluorescence staining for Stat1 in mouse VSMCs in coculture with monocytes from uPA-/- and in VSMCs from uPAR-/- mice cocultured with wild-type monocytes using anti-Stat1 rabbit polyclonal antibody. As controls, wild-type cells and VSMCs from uPA-/- mice are included.
To verify these findings further, we made use of the uPA- and uPAR-deficient mice. We isolated monocytes from uPA-/- mice and VSMCs from uPAR-/- mice and used these cells for coculture (Figure 3C). In both cases, Stat1 was mainly localized in the cytoplasm and in the perinuclear space; nuclear translocation was minimal. In contrast, we again observed a pronounced Stat1 nuclear translocation when wild-type VSMCs and wild-type monocytes were used in the coculture system. To completely exclude the autocrine loop of Stat1 activation, a combination of VSMC from uPA-/- mice and wild-type monocytes was used for coculture setting. In agreement with other findings, Stat1 was still activated in VSMCs in this case (Figure 3C).
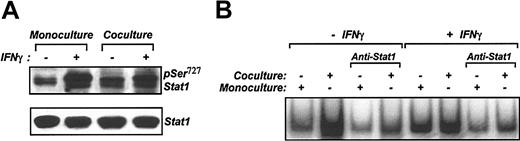
Because IFN-γ is a strong activator of Stat1,18,19 we used IFN-γ as a positive control. Surprisingly, IFN-γ-induced Stat1 activation was significantly reduced when VSMCs were cocultured with monocytes. Western blot analysis of proteins from cocultured VSMCs stimulated with IFN-γ shows only slight increase in Stat1 phosphorylation compared with the Stat1 phosphorylation profile in the IFN-γ-stimulated monocultured cells (Figure 4A). Consistent with these data, IFN-γ did not produce any significant increase in the intensity of Stat1-DNA binding in cocultured VSMCs, as monitored by EMSA (Figure 4B).
VSMCs do not respond to IFN-γ stimulation in coculture. (A) Lysates were prepared from MACS-separated VSMCs on coculture and were stimulated with IFN-γ. Proteins were separated on SDS-PAGE and analyzed by Western blotting with anti-pSer727 Stat1 rabbit polyclonal antibody. VSMCs in monoculture with and without stimulation are included. As a control for gel loading, anti-Stat1 antibody was used. (B) DNA binding activity of nuclear extracts prepared from VSMCs treated with or without IFN-γ from monoculture and coculture was analyzed by EMSA.
VSMCs do not respond to IFN-γ stimulation in coculture. (A) Lysates were prepared from MACS-separated VSMCs on coculture and were stimulated with IFN-γ. Proteins were separated on SDS-PAGE and analyzed by Western blotting with anti-pSer727 Stat1 rabbit polyclonal antibody. VSMCs in monoculture with and without stimulation are included. As a control for gel loading, anti-Stat1 antibody was used. (B) DNA binding activity of nuclear extracts prepared from VSMCs treated with or without IFN-γ from monoculture and coculture was analyzed by EMSA.
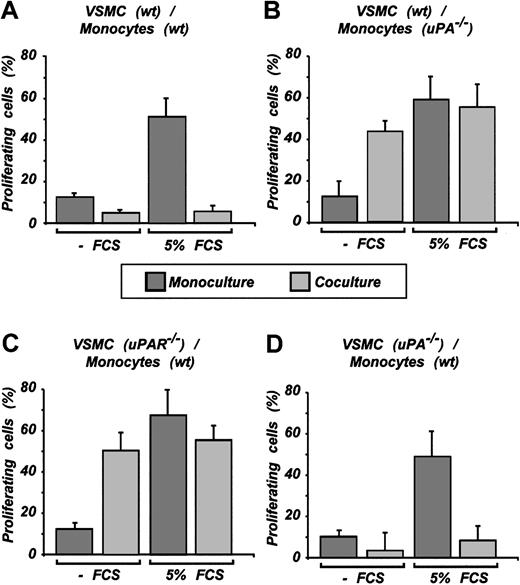
Stat1 elicits the antiproliferative effect that requires monocyte-expressed uPA and VSMC-expressed uPAR
To prove the hypothesis that monocyte-produced uPA locally binds to the VSMC cell-surface uPAR and induces Stat1 activation mediating antiproliferative effects, we again relied on monocytes from uPA-/- and VSMCs from uPAR-/- mice. Wild-type VSMCs in monoculture showed proliferation that was substantially increased when the FCS concentration was raised to 5%. This proliferation was sharply attenuated to low levels in the coculture system, regardless of the FCS concentration (Figure 5A). uPA-/- monocytes in coculture increased VSMC proliferation in the absence of FCS and had no effect in the presence of 5% FCS (Figure 5B). uPAR-/- VSMCs in coculture displayed no proliferation inhibition. The results were no different from those with uPA-/- monocytes (Figure 5C compared with Figure 5B). To completely exclude the autocrine loop of Stat1 activation, a combination of VSMCs from uPA-/- mice and wild-type monocytes was used. In agreement with other data, we observed the inhibition of VSMC growth in these experiments. Results were similar to those observed with wild-type VSMCs (Figure 5D compared with Figure 5A).
Inhibition of VSMC proliferation in coculture requires uPA/uPAR signaling. VSMCs on coculture with monocytes were treated with BrdU. Incorporation was detected by immunofluorescence using monoclonal antibody against BrdU. Cells were counted using Hoechst 33258 nuclear staining. Representative results of n experiments are shown. (A) Result (n = 4) using wild-type VSMCs and wild-type monocytes. (B) uPA-/- monocytes and wild-type VSMCs were used for coculture (n = 3). (C) uPAR-/- VSMCs and wild-type monocytes were used (n = 3). (D) uPA-/- VSMCs and wild-type monocytes were used (n = 3). The coverslips were counted in one given field for the number of cells in blue filter for Hoechst 33258 and for number of proliferating cells in red filter for BrdU (Alexa 546). Cells were counted (mean ± SD of 5-7 fields).
Inhibition of VSMC proliferation in coculture requires uPA/uPAR signaling. VSMCs on coculture with monocytes were treated with BrdU. Incorporation was detected by immunofluorescence using monoclonal antibody against BrdU. Cells were counted using Hoechst 33258 nuclear staining. Representative results of n experiments are shown. (A) Result (n = 4) using wild-type VSMCs and wild-type monocytes. (B) uPA-/- monocytes and wild-type VSMCs were used for coculture (n = 3). (C) uPAR-/- VSMCs and wild-type monocytes were used (n = 3). (D) uPA-/- VSMCs and wild-type monocytes were used (n = 3). The coverslips were counted in one given field for the number of cells in blue filter for Hoechst 33258 and for number of proliferating cells in red filter for BrdU (Alexa 546). Cells were counted (mean ± SD of 5-7 fields).
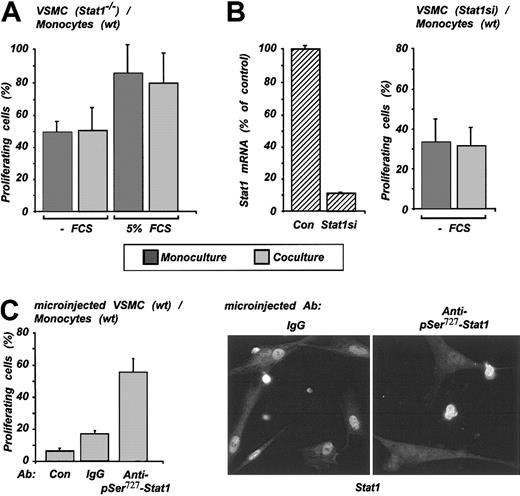
To prove that VSMC growth inhibition was mediated by the up-regulated Stat1, we turned to several experimental approaches. We isolated VSMCs from Stat1-deficient mice and used these cells for coculture with wild-type monocytes followed by proliferation assay, as described above. Monocultured Stat1-/- VSMCs showed a higher proliferation level compared with wild-type cells even in the absence of FCS. This proliferation was not inhibited further with coculture (Figure 6A). We next developed the RNA silencing technology for stable and specific Stat1 expression inhibition in VSMCs using a retroviral RNA interference vector.20 Figure 6B shows that Stat1 expression in these cells (Stat1si-VSMC) was abrogated, as verified by TaqMan RT-PCR (left panel). In proliferation assay, Stat1si-VSMCs behaved similarly to Stat1-/- VSMCs. The cells displayed an increased proliferation level in monoculture that was not affected by coculture with monocytes (right panel, shown for -FCS coculture). Finally, we neutralized Stat1 by microinjection in human VSMCs used for coculture-specific antibody blocking Stat1 Ser727 phosphorylation. The active antibody restored VSMC proliferation; control IgG did not (Figure 6C, left panel). Consistent with these data, no Stat1 nuclear translocation was observed in VSMCs microinjected with specific antibody, whereas microinjection of control antibody did not affect Stat1 nuclear accumulation (Figure 6C, right panel). Visible strong fluorescence of monocytes resulted from the unspecific binding of antibodies to Fc-γ receptors expressed on monocytes.
Stat1 mediates the antiproliferative effect in VSMCs in coculture. (A) VSMCs from Stat1-/- and monocytes from wild-type mice were used for coculture and proliferation assay, as indicated in the legend to Figure 5. (B) Stat1si-VSMC with down-regulated expression of Stat1 (left panel) were used for coculture setting and proliferation assay (right panel). (C) VSMCs were microinjected with pSer727 Stat1 antibody and then cocultured. One hundred to 120 cells were microinjected in each experiment,; results shown are representative of 4 experiments. As a control, nonmicroinjected and nonspecific IgG-injected VSMCs were included. Microinjected and control cells were used for proliferation assay (left panel) and for immunocytochemistry (right panel).
Stat1 mediates the antiproliferative effect in VSMCs in coculture. (A) VSMCs from Stat1-/- and monocytes from wild-type mice were used for coculture and proliferation assay, as indicated in the legend to Figure 5. (B) Stat1si-VSMC with down-regulated expression of Stat1 (left panel) were used for coculture setting and proliferation assay (right panel). (C) VSMCs were microinjected with pSer727 Stat1 antibody and then cocultured. One hundred to 120 cells were microinjected in each experiment,; results shown are representative of 4 experiments. As a control, nonmicroinjected and nonspecific IgG-injected VSMCs were included. Microinjected and control cells were used for proliferation assay (left panel) and for immunocytochemistry (right panel).
Discussion
We show that the interaction of human VSMCs with peripheral-blood-derived monocytes in a coculture model results in the inhibition of VSMC growth. The mechanism involves activation of the transcription factor Stat1 in VSMCs. This activation is mediated by uPA expressed on the monocytes, which signals the uPAR on the VSMCs. The observations imply that monocytes regulate VSMC responses through components of the fibrinolytic system and provide an important link between the uPA/uPAR signaling machinery and VSMC function. The findings may be relevant to VSMC behavior in the face of vascular injury.
The response-to-injury hypothesis proposes that increased endothelial permeability or frank disruption features leukocyte infiltration. By unknown mechanisms, VSMCs then migrate from the media into the intima. Here, they proliferate and synthesize an extracellular matrix to form an intimal lesion that may impede flow.21-23 Several in vivo and in vitro studies suggest that VSMC migration precedes proliferation and that the uPA/uPAR signaling system may be involved.24-26 There is also indirect evidence from humans that inhibition of these early processes can lead to long-term inhibition of the cellular changes.27 We asked whether VSMC migration is associated with feedback inhibition of cell proliferation and whether this balance might be controlled by cross-talk of the underlying signaling pathways. We used a coculture model in which human VSMCs were directly cocultured with human peripheral-blood-derived monocytes. Although such alternative in vitro models have obvious limitations, they may be helpful for studying communication between 2 or more cell types neighbored in the arterial vessel wall. Moreover, most data on cell regulation by uPA come from experiments on cultured cells exposed to exogenous purified or recombinant uPA or its peptides, sometimes at fairly high concentrations, making interpretations uncertain. A more specialized in vitro experimental system allowed us to overcome this problem.
Invasion of the vessel wall by activated monocytes is the first step in vascular remodeling after injury. Because monocyte infiltration appears to precede VSMC proliferation in the remodeling process, factors released by monocytes might contribute to the promotion of VSMC migration and the inhibition of its proliferation. Our present finding of the pronounced inhibition of VSMC growth in coculture is consistent with our earlier work showing that monocyte-expressed uPA stimulates VSMCs to migrate.6 The migration involves activation of Janus kinase Tyk2, phosphatidylinositol 3-kinase (PI3K) downstream of Tyk2,28 and small GTPases of the Rho family.29
One major pathway used by pleiotropic cytokines and growth factors to elicit a negative regulatory effect on cell growth of normal and malignant cells in vitro and in vivo involves the activation of Stat proteins.30-33 Previously we demonstrated that transcription factors Stat1, Stat2, and Stat4 are activated in VSMCs in response to exogenous uPA, though the functional purpose of this up-regulation remained unexplored.12,14
We provided several lines of evidence that the inhibition of VSMC growth was mediated by the transcription factor Stat1, which was activated by the monocyte-expressed uPA and required VSMC-expressed uPAR. We observed Stat1 activation in cocultured VSMCs at the level of its Ser727 phosphorylation, nuclear translocation, and DNA binding. This activation was uPA/uPAR-directed and might be abrogated by specific antibodies. We found neither Stat1 activation nor inhibition of VSMC growth in coculture settings in which uPA-/- monocytes or uPAR-/- VSMCs were used. Our experiments using Stat1-/- VSMCs provide direct evidence that this transcription factor was responsible for the growth inhibition observed in coculture. This conclusion was further confirmed in experimental settings in which human VSMCs with down-regulated Stat1 expression (Stat1si-VSMCs) were used. In agreement with these data, Stat1 inactivation by microinjected specific anti-Stat1-pSer727 antibody also resulted in the restoration of VSMC proliferation. Our experimental findings on Stat1 activation in coculture imply that Stat1 Ser727 phosphorylation might be particularly important. Although the enzyme responsible for Stat1 Ser727 phosphorylation in response to uPA remains to be identified, serine phosphorylation is believed to contribute to the specificity of the cellular responses through an intrinsically different requirement for serine phosphorylation at different target gene promoters.34-36
IFN-γ was an unreliable Stat1 activator in our coculture system, although clear-cut Stat1 activation was observed under the monoculture condition. One possible explanation for the observed decreased sensitivity to IFN-γ might be the desensitization of VSMCs induced by the overexpression of IFN-γ in coculture given that leukocytes are capable of synthesizing interferon. We observed a 2-fold increase in IFN-γ in our coculture system by enzyme-linked immunosorbent assay (ELISA) (not shown). We do not know whether this increase was sufficient for desensitization. The short period of cell stimulation with exogenous IFN-γ used in our experiments excludes uPA proteolysis reported in other systems.37 Although more work is needed to explore the possibility in greater detail, these data suggest that IFN-γ might use the Stat1-independent pathway in the physiologic situation in which Stat1 activation by uPA is predominant. Recent evidence suggests that non-Stat pathways are important in the generation of signals for interferons and may be primary mediators of their biologic consequences.38,39 Recently an unappreciated function of uPA was described involving different receptor complexes that mimic interferons in some of their effects.40,41
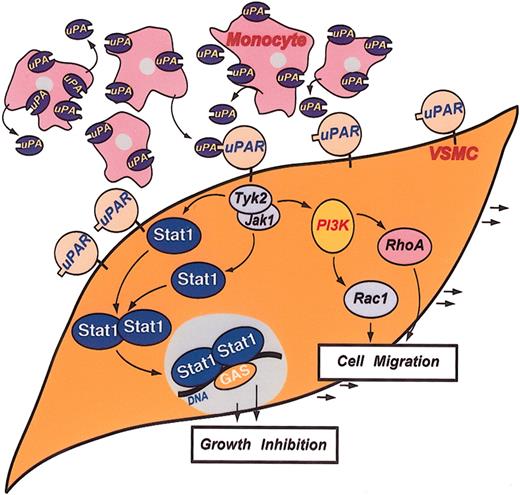
We suggest a new role for the nonproteolytic function of the fibrinolytic system in vascular remodeling after injury through the interplay of VSMCs with monocytes (Figure 7). The VSMCs receive their “marching orders” through their own uPAR. uPAR initiates a chain of events so that VSMCs cease their proliferative phenotype through Stat1. Instead, the uPAR signals a migratory behavior through Tyk2, Jak1, PI3K, and the Rho family of proteins.
Schematic representation of the uPA/uPAR-directed Stat1 signaling in coculture. The study proposes uPA/uPAR signaling at the cell surface results in the phosphorylation of Tyk2, Jak1, or both recruiting Stat1 to activate, dimerize, and translocate to the nucleus, leading to gene expression, inhibiting cell proliferation, and, through a different mechanism, to promote cell migration involving PI3K and small GTPases.
Schematic representation of the uPA/uPAR-directed Stat1 signaling in coculture. The study proposes uPA/uPAR signaling at the cell surface results in the phosphorylation of Tyk2, Jak1, or both recruiting Stat1 to activate, dimerize, and translocate to the nucleus, leading to gene expression, inhibiting cell proliferation, and, through a different mechanism, to promote cell migration involving PI3K and small GTPases.
The precise and rapid propagation of this signaling cascade demands strict and flexible negative regulatory processes that remain unexplored. The nature of the negative regulators involved may have therapeutic implications. Our schematic is based on the experimental coculture model and corresponds to in vivo data showing an increase in VSMC proliferation in uPA-deficient mice25 and the expression of uPA only in proliferating VSMCs in the injured rat carotid artery.24 Why VSMCs use the paracrine and not the autocrine loop for uPA-directed intracellular signaling and how this cell-cell cross-talk remains tightly regulated temporally and spatially in live cells in the face of vascular injury remain unresolved. Many factors other than uPA are involved in the development of vascular disease. However, we have identified a mechanism that suggests how the uPA/uPAR signaling system may contribute to this process through cell-cell communication.
Prepublished online as Blood First Edition Paper, August 14, 2003; DOI 10.1182/blood-2002-12-3872.
Supported in part by grants Du 344/1-3 and Du 379/S1-1 from the Deutsche Forschungsgemeinschaft, by grant C99/10 from the Clinical Research Cooperation Program of Max-Delbrück-Center for Molecular Medicine, and by grant CLG.978262 from North Atlantic Treaty Organization.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Steffen Lutter for technical assistance, Petra Quass for help in preparing mouse VSMCs, Dagmar Gerhard for obtaining blood from mice, Friedrich C. Luft for editing the manuscript, Peter Carmeliet and Mieke Dewerchin for providing uPA- and uPAR-deficient mice, and Michael Martin and Klaus Resch for the IFN-γ measurements.


![Figure 3. Stat1 activation in VSMCs depends on uPA/uPAR signaling.(A) VSMCs were treated with anti-uPA and anti-uPAR antibodies separately, before coculture with monocytes, and were analyzed for Stat1 by immunofluorescence. Total cell number and the number of cells with translocated Stat1 were counted for each view field. Nine to 10 view fields have been evaluated in each experiment. The result is a representative of 3 experiments (mean ± SD [n = 3]). (B) VSMCs were treated with anti-uPA antibody before the addition of monocytes; VSMCs in monoculture is control. GAS/ISRE binding activity of nuclear extracts prepared from the above anti-uPA antibody-treated VSMC was analyzed using EMSA. (C) Immunofluorescence staining for Stat1 in mouse VSMCs in coculture with monocytes from uPA-/- and in VSMCs from uPAR-/- mice cocultured with wild-type monocytes using anti-Stat1 rabbit polyclonal antibody. As controls, wild-type cells and VSMCs from uPA-/- mice are included.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/13/10.1182_blood-2002-12-3872/6/m_h82435333003.jpeg?Expires=1769114045&Signature=Pia~PR15hV-GeMqPxY3cy8UkShzJerDGPVm4lk1oV7dGJpStGbaxVwiYGc0H63Y~F4n1iyx5k3V8XVRxpL7VOzAL3JLGd0vO8XCfgQnI863QNfMwByOg~cSPqHZDv8AqMABUjDqRt7Vpk31wfr6lHNKGYuEu2BYt4Fj9gkFv0AuD~-OT0GySkkviTmFgOH874L0hKQEo1yMAhhCAoS3dv31jlr7Pc1q2ZSTXJcKKMBzJr3QuX5qDis81ouqPk9dm~HP03m~vjgJ-21M9CTE35QXIce0loojOvAo3PIm2SMWVGbOc6ThY~OIU~KJSMjhdiE-7IjC6AmC7V-qIWddCMA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal