Abstract
The embryonic origin and development of hematopoietic and endothelial cells is highly interdependent. We hypothesized that primary endothelial cells from murine yolk sac and para-aortic splanchnopleura (P-Sp) may possess the capacity to expand hematopoietic stem cells (HSCs) and progenitor cells ex vivo. Using Tie2-GFP transgenic mice in combination with fluorochrome-conjugated monoclonal antibodies to vascular endothelial growth factor receptor-2 (Flk1) and CD41, we have successfully isolated pure populations of primary endothelial cells from 9.5-days after coitus (dpc) yolk sac and P-Sp. Adult murine bone marrow Sca-1+c-Kit+lin- cells were cocultured with yolk sac or P-Sp Tie2-GFP+Flk-1+CD41- endothelial cell monolayers for 7 days and the total number of nonadherent cells increased 47- and 295-fold, respectively, and hematopoietic progenitor counts increased 9.4- and 11.4-fold, respectively. Both the yolk sac and P-Sp endothelial cell cocultures facilitated long-term (> 6 months) HSC competitive repopulating ability (2.8- to 9.8-fold increases, respectively). These data suggest that 9.5-dpc yolk sac- and P-Sp-derived primary Tie2-GFP+Flk-1+CD41- endothelial cells possess the capacity to expand adult bone marrow hematopoietic progenitor cell and HSC repopulating ability ex vivo. (Blood. 2003;102:4345-4353)
Introduction
The primary sites of hematopoiesis change during murine ontogeny. The murine yolk sac is well known as the first site of blood cell production.1 Primitive erythroid colony-forming cells (EryP) emerge at embryonic 7.0 days after coitus (dpc) along with minimal numbers of macrophage and megakaryocyte progenitors.2 On 8.25 dpc, definitive progenitor cells also emerge in the yolk sac but not the embryo.3,4 By 8.5 dpc, the frequency of EryP diminishes and EryP are absent in the 9.0-dpc yolk sac; however, the frequency of definitive progenitor cells increases substantially.5 On 10.0 dpc, adult repopulating hematopoietic stem cells (HSCs) emerge in the aorta-gonad-mesonephros region (AGM) of the embryo proper.6 Soon after, the liver becomes populated with yolk sac-derived definitive progenitor cells and circulating stem cells from the yolk sac or AGM or both. HSCs are present in the circulation, liver, AGM, and yolk sac of 11- to 13-dpc embryos, but thereafter the fetal liver serves as the primary site of HSC proliferation and differentiation.7 At 16 to 18 dpc, liver HSCs enter the circulation and seed the nascent spleen and bone marrow compartments. Postnatally, the liver remains hematopoietically active for several weeks, but activity then subsides and the spleen and marrow serve as the lifelong sites of steady-state adult hematopoiesis.8
Yolk sac-derived hematopoietic cells obtained prior to 10 dpc fail to repopulate lethally irradiated adult recipient mice on transplantation.9 Explant cultures of precirculation yolk sac and para-aortic splanchnopleura (P-Sp), the precursor to the AGM region, have demonstrated that the P-Sp possesses intrinsic adult repopulating HSC activity that has not been demonstrable under the same culture conditions with yolk sac cells.10 However, coculture of precirculation yolk sac cells with an AGM-derived stromal cell line results in a gain of function of the yolk sac cells that now long-term repopulate lethally irradiated adult recipient mice.11 A similar gain of function to repopulate adult recipient mice can be obtained by infecting yolk sac cells with a retroviral construct encoding for the transcription factor HoxB4.12 It remains unclear if the lack of stem cell repopulating ability of the less than 10-dpc yolk sac is due to an absence of cells with this potential or rather to lack of an appropriate environment to support the proliferation or survival of adult repopulating HSCs.
Recently, Kumaravelu et al have reported that adult repopulating HSCs are present in the murine AGM, circulation, liver, and yolk sac of 11- to 13-dpc embryos.7 A demonstrable increase in HSC frequency in the yolk sac occurs during this time, suggesting that the yolk sac microenvironment does play a role in HSC expansion in vivo. At present it is unclear which cells of the yolk sac microenvironment stimulate the HSC expansion.
Several groups have isolated yolk sac and AGM endothelial cell lines and reported that these cell lines stimulate HSC and progenitor cell proliferation and differentiation in vitro.13-16 Primary AGM endothelial cells have also been demonstrated to support hematopoiesis in vitro.17 These data suggest that endothelial cells from the yolk sac and P-Sp may play an important role in HSC development. However, the ability of less than 10-dpc primary yolk sac or P-Sp endothelial cells to support hematopoiesis ex vivo has not been reported.
Hematopoietic and endothelial cells share a number of cell surface markers in the developing yolk sac and embryo. Likewise, genetic disruption of numerous genes affect both hematopoietic and endothelial development. In fact, these lineages are believed to be derived from a common precursor, the hemangioblast.18-20 Thus, isolation of pure endothelial populations from the early embryo has been problematic.
We report that endothelial and hematopoietic cell lineages can be discriminated in the 9.5-dpc yolk sac and P-Sp using mice expressing an endothelial restricted green fluorescence protein transgene (Tie2-GFP), and differential expression of vascular endothelial growth factor receptor-2 (Flk-1) and CD41. Whereas Tie2-GFP+ endothelial cells from yolk sac and P-Sp possess some hematopoietic and endothelial potential, Tie2-GFP+Flk-1+CD41- cells lack hematopoietic potential in vitro and in vivo and display morphologic and gene expression patterns of vascular endothelial cells. Yolk sac and P-Sp endothelial cells (Tie2-GFP+Flk-1+CD41-) were grown as monolayers, and adult marrow Sca-1+c-Kit+lin- cells were plated in coculture for 7 days. The total number of nonadherent cells and hematopoietic progenitor cells increased significantly in the yolk sac and P-Sp endothelial cell cocultures. Both yolk sac and P-Sp Tie2-GFP+Flk-1+CD41- endothelial cell cocultures supported increases in adult marrow HSC short-term and long-term competitive repopulating unit (CRU) activity compared with freshly isolated and transplanted adult marrow Sca-1+c-Kit+lin- cells. These data suggest that 9.5 dpc yolk sac and P-Sp-derived primary Tie2-GFP+Flk-1+CD41- endothelial cells possess the capacity to expand adult marrow progenitor cells and HSC CRUs ex vivo.
Materials and methods
Mice
FVB/N-TgN (Tie2-GFP) transgenic mice and C57BL/6J (CD45.2) mice were obtained from commercial stocks at the Jackson Laboratory (Bar Harbor, ME). Homozygous glucose phosphate isomerase-1a (Gpi-1a) mice (derived from B.6CAST-Gpi-1a/Gpi-1a mice; gift of Dr David Harrison, Jackson Laboratory) were bred with B6.SJL-PtprcaPep3b/BoyJ (Jackson Laboratory) to obtain homozygous Gpi-1a mice that were also homozygous for CD45.1 expression (Gpi-1a/BoyJ). The Gpi-1a/BoyJ mice have been inbred more than 12 generations and are maintained in the animal facility of Indiana University School of Medicine, Indianapolis. In all mating procedures, female mice were exposed to male mice overnight. Identification of a vaginal plug the next morning was used to determine the pregnant stage as 0.5 days after coitus (dpc). The Indiana University Institutional Animal Care and Use Committee approved all protocols.
Isolation of primary yolk sac and P-Sp endothelial cells and BM HSCs
Embryonic yolk sac or P-Sp at 9.5 dpc was carefully dissected from pregnant Tie2-GFP transgenic mice in α-minimal essential medium (α-MEM) containing 10% fetal bovine serum (FBS). Yolk sac and P-Sp were digested with 0.1% collagenase/dispase (Roche Applied Science, Indianapolis, IN) plus 20% FBS (Hyclone, Logan, UT) in phosphate-buffered saline (PBS) for 45 minutes at 37°C with gentle trituration at 15-minute intervals. Cells were washed twice with α-MEM containing 10% FBS. Cell clumps were removed by pouring cells through a 70-μm nylon mesh (BD Biosciences, Discovery Labware, Bedford, MA), then cells were centrifuged, washed, and resuspended in 200 μL Fc-blocking antibody conditioned medium produced by the 2.4G2 cell line (American Type Culture Collection, Manassas, VA).21 Washed cells were then incubated with murine monoclonal antibodies (mAbs) to Flk-1 and CD41 (BD Pharmingen, San Diego, CA) on ice for 30 minutes. Isolation and preparation of adult FVB wild-type control cell suspensions and use of appropriate isotype antibody controls for all studies were conducted as previously described.22 For yolk sac and P-Sp endothelial cell isolation via flow cytometry, cells were washed, spun, and resuspended in α-MEM media containing 10% FBS. Tie2-GFP+, Tie2-GFP+Flk-1+CD41-, and Tie2-GFP+Flk-1+CD41+ subpopulations were collected using a macrosort nozzle.
Donor bone marrow (BM) cells were isolated from C57BL/6J mice. BM cells were suspended in Fc-blocking solution followed sequentially on ice by fluorescein isothiocyanate (FITC)-conjugated lineage-specific rat anti-mouse mAbs (anti-B220, anti-CD11b, anti-CD4, anti-CD8a, anti-Gr-1), and phycoerythrin (PE)-conjugated rat antimouse Sca-1 and allophycocyanin (APC)-conjugated rat antimouse c-Kit (BD Pharmingen). Appropriate isotype antibody controls were used as previously described.22 Sca-1+c-Kit+lin- cells were isolated using a FACStar instrument (BD Pharmingen).
Capillary-like tube formation on Matrigel and Dil-Ac-LDL uptake and CD31 staining
Aliquots (200 μL) of 0.1% Matrigel (BD Bioscience, Discovery Labware) were added to each precooled well of 24-well culture plates and incubated at 37°C until gelation occurred. To each well, sorted cell populations from 9.5-dpc yolk sac and P-Sp (10 000/well) were suspended in 200 μL α-MEM containing 15% FBS, 4.5 × 10-5 M 2-mercaptoethanol, 2 mM l-glutamine, 5 ng/mL recombinant murine vascular endothelial growth factor (VEGF), 100 ng/mL recombinant human β-fibroblast growth factor (βFGF), and 50 μg/mL endothelial cell growth supplement (ECGS). The cells were incubated at 37°C in a 5% CO2, 3% O2, and 92% N2 humidified environment and capillary tube formation was assessed via phase contrast microscopy.
Capillary-like tube structures were incubated with 10 μg/mL Dil-Ac-LDL (Molecular Probes, Eugene, OR) for 4 hours at 37°C in normal medium. The cells were washed with medium for 10 minutes, rinsed with PBS, and then fixed with 1% paraformaldehyde in PBS for 1 hour at 4°C and washed with PBS. The cells were then immersed in PBS containing 3% milk, 0.025% Triton X-100 (PMT solution) for 30 minutes to block nonspecific protein interactions and permeabilize the cell membranes. The CD31 rat antimouse mAb was conjugated to Alexa Fluor 488 using the manufacturer's protocol (Molecular Probes). The cells were labeled with conjugated CD31 mAb in PMT solution and incubated at 4°C overnight. In the morning the cells were washed and images of the labeled cells were obtained using an MRC1024 laser scanning confocal microscope (Bio-Rad Microscopy Division, Cambridge, MA). Optical sectioning was performed along the xyz-axis. Projections of the z-stacks were generated at 512 × 512 pixel resolution with a × 60 water-immersion objective (1-μm intervals). Composite images were assembled in MetaMorph imaging series 5.0 software (Universal Imaging, Downingtown, PA).
Coculture assay
Purified primary Tie2-GFP+Flk-1+CD41- endothelial cells (10 000/well) from 9.5 yolk sac and P-Sp were plated on 0.1% Matrigel-coated 24-well dishes (Becton Dickinson, Bedford, MA) in 100 μL α-MEM containing 15% FBS, 4.5 × 10-5 M 2-mercaptoethanol, 2 mM l-glutamine, 5 ng/mL VEGF, 100 ng/mL βFGF, and 50 μg/mL ECGS. Cells were incubated for 7 days at 37°C in a 5% CO2, 3% O2, and 92% N2 humidified environment. These conditions permitted endothelial cells to grow to 70% to 80% confluence. To these endothelial monolayers, 1000 BM Sca-1+c-Kit+lin- cells were added and the cocultures continued at 37°C in a 5% CO2, 5% O2, and 90% N2 humidified environment for 7 days with or without addition of 50 ng/mL stem cell factor (SCF), 50 ng/mL thrombopoietin (TPO), and 10 ng/mL interleukin 6 (IL-6). Adult marrow Sca-1+c-Kit+lin- cells cultured on Matrigel in the presence or absence of growth factors but without any endothelial cells were used as controls.
Assays for hematopoietic colony-forming cells and CFU-Ss
The entire cell contents of the test and control wells were harvested by addition of trypsin-EDTA (ethylenediaminetetraacetic acid; Invitrogen, Grand Island, NY) followed by 2 rinses with medium. Cells were plated in triplicate in 0.9% methylcellulose culture with Iscove modified Dulbecco medium (IMDM) containing 15% FBS, 100 ng/mL SCF, 200 U/mL IL-3, 4 U/mL recombinant human erythropoietin (Epogen, Amgen, CA), hemin (100 μM), 4.5 × 10-5 M 2-mercaptoethanol (Sigma, St Louis, MO), and 2 mM l-glutamine (Invitrogen). Cultures were incubated in a humidified incubator at 37°C in 5% CO2 and colonies were counted on day 7. For the spleen colony-forming units day 8 (CFU-S8) assay, the entire contents of each coculture or control well were injected into lethally irradiated (11 Gy administered in divided doses 4 hours apart using a 137Cs irradiator) recipient mice (3-6 C57 BL/6 recipients per cell dose per experiment). The recipient mice were killed on day 8 after transplantation of the cells, spleens were removed, and visible surface colonies were counted under a dissecting microscope (Leica Microsystem, Bannockburn, IL).
Transplantation
Gpi-1a/BoyJ recipient mice were lethally irradiated 1 day before transplantation as described (“Assays for hematopoietic colony-forming cells and CFU-Ss”). Control donor cell injections included freshly sorted BM Sca-1+c-Kit+lin- cells (1000) plus 10 000 freshly isolated adult Tie2-GFP+ heart endothelial cells (serve as a control for any contaminating endothelial cells carried over from the cocultures) and freshly isolated 9.5-dpc yolk sac or P-Sp Tie2-GFP+Flk-1+CD41- endothelial cells without donor marrow cells to determine whether the endothelial cells alone possessed competitive repopulating ability in vivo. In each control and in all test injections, 1.5 × 105 competitor cells (Gpi-1a/BoyJ low-density mononuclear cells) were coinjected. In all studies, 6 to 10 recipient mice were injected for each test or control sample. The recipient animals were maintained in a pathogen-free environment. At various times after transplantation, peripheral blood was collected from the tail vein of each mouse in heparinized tubes and assessed for the presence of CD45.2 (donor cell derived) expressing cells. Red blood cells were lysed with red cell lysis buffer (Sigma), and the nucleated cells were labeled with FITC-conjugated anti-CD45.2 mAb for 30 minutes on ice. The cells were then washed and analyzed on a FACSCalibur machine (Becton Dickinson) for enumeration of donor-derived cells. All recipient mice with more than or equal to 1% CD45.2-expressing cells in the peripheral blood were considered reconstituted. At 6 months after transplantation, the recipient mice were killed, and donor-derived CD45.2+ cells with lineage-specific markers were identified in the BM and peripheral blood.
CRU activity was determined as previously described.23,24 One CRU represents the repopulating ability shown by a standard number of fresh competitor cells (150 000 adult low-density BM cells in present experiments). To calculate the number of CRUs present in an unknown donor HSC sample, the percentage of donor type cells in the peripheral blood of the recipient called Pd and the number of competitor cells (× 104) termed C are used to solve the equation: CRU = Pd × C/(100-Pd). Differences in CRU values were computed using the Wilcoxon signed ranks test for paired data and Mann-Whitney U test for unpaired data. The competitor cells were used as a standard of repopulating potential to which the other donor activities in all independent experiments were compared. This permitted quantitative comparisons of repopulating ability by different donors in the same experiment or different groups of donors in independent experiments in this study.
RT-PCR analysis
Sorted 9.5-dpc Tie2-GFP+, Tie2-GFP+Flk-1+CD41-, Tie2-GFP+Flk-1+CD41+ and adult marrow Sca-1+c-Kit+lin- cells were isolated and total RNA harvested for interrogation by reverse transcription-polymerase chain reaction (RT-PCR). Total RNA from sorted cells was obtained using a standard TRIzol extraction procedure as outlined in the manufacturer's protocol (Invitrogen). The resulting material was treated using DNase I (8.5 μL total RNA, 0.5 U DNase I, 1 μL DNase I buffer, at 37°C for 30 minutes; Invitrogen), and phenol/chloroform extracted twice.25 We attempted to screen for gene products that are typically enriched in endothelial or hematopoietic cells in an effort to discriminate between these 2 lineages. The 15 gene product mRNAs selected for study included the endothelial-“specific” genes,26,27 vascular endothelial-cadherin (CD144), CD31, insulin-like growth factor-binding protein (ilgfbp), Tie2, von Willebrand factor (VWF), collagen type 4, hevin, Flk-1, and vascular endothelial zinc finger 1 (vezf1) or hematopoietic-“specific” genes,18,19,28-30 including βH1 (embryonic hemoglobin), βmajor (adult hemoglobin), GATA1, PU.1, SCL, and erythroid Kruppel-like factor (EKLF). Oligonucleotide primer sequences include CD144-5′: 5′-ggatgcagaggctcacagag-3′, CD144-3′: 5′-ctggcggttcacgttggact-3′; CD31-5′: 5′-gtcatggccatggtcgagta-3′; CD31-3′: 5′-ctcctcggcatcttgctgaa-3′; ilgfbp-5′: 5′-tgcacagcttgcacctggagca-3′, ilgfbp-3′: 5′-ggttctgtagggcaaagtcacg-3′; Tie2-5′: 5′-ccttcctacctgcta-3′, Tie2-3′: 5′-ccactacacctttctttaca-3′; VWF-5′: 5′-tgtgacaccacatagtgtgga-3′, VWF-3′: 5′-tgaagtcgtaggatctccact-3′; collagen-5′:5′-acgacaagtcctactggctct-3′, collagen-3′: 5′-atggtgtgctctggaagttctg-3′; hevin-5′: 5′-aacctcactgtgtttgccaag-3′, hevin-3′: 5′-tcaaggtgatgtgcttatcct-3′; Flk-1-5′: 5′-tctgtggttctgcgtggaga-3′, Flk-1-3′: 5′-gtatcatttccaaccaccct-3′; vezf1-5′: 5′-atgtgacaagctggccagggaa-3′, vezf1-3′: 5′-cagggtgtgctatattcactgg-3′; βH1-5′: 5′-agttccccatggagtcaaaga-3′, βH1-3′: 5′-ctcaaggagacctttgctca-3′; βmajor-5′: 5′-ctgacagatgctctcttggg-3′, βmajor-3′: 5′-cacaaaccccagaaacagaca-3′; GATA1-5′: 5′-actcgtcataccactaaggt-3′, GATA 1-3′: 5′-agtgtctgtaggcctcagct-3′; PU.1-5′:5′-acagatgcacgtcctcgatact-3′, PU.1-3′:5′-tccttgtgcttcgacgagaact-3′; SCL-5′:5′-tagccttagccagccgctcg-3′, SCL-3′:5′-gcggaggatctcattcttgc-3′; EKLF-5′: 5′-tcgccggagacgcaggct-3′, and EKLF-3′: 5′-cccagtccttgtgcagga-3′. Samples were denatured at 95°C for 5 minutes, followed by 35 cycles of amplification consisting of 94°C for 30 seconds (denaturing), 55°C for 30 seconds (annealing), and 72°C for 2 minutes (extension), and 72°C for 10 minutes (extension). The PCR products were visualized with ethidium bromide in 2% agarose gels as previously described.22
Yolk sac whole-mount immunolabeling
The 9.5-dpc embryonic yolk sacs from Tie2-GFP transgenic mice were dissected, washed with PBS, fixed for 1 hour in 4% paraformaldehyde/PBS at 4°C, and washed 3 times with PBS. The yolk sacs were then immersed in PBS containing PMT solution for 30 minutes to block nonspecific protein interactions and to permeabilize the cell membranes. The Flk-1 and CD41 rat antimouse mAbs were conjugated to Alexa Fluor 546 and 647, respectively, using the manufacturer's protocol (Molecular Probes). The yolk sacs were labeled with conjugated Flk-1 and CD41 mAb in PMT solution, and incubated at 4°C overnight. In the morning the tissues were washed and mounted onto glass slides. Images were obtained using an MRC1024 laser scanning confocal microscope. FVB wild-type tissues and appropriate isotype antibodies were used as controls. Optical sectioning was performed along the xyz-axis. Projections of the z-stacks were generated at 512 × 512 pixel resolution with a 20 × water-immersion objective (2-μm intervals). Composite images were assembled in MetaMorph imaging series 5.0 software (Universal Imaging, Downingtown, PA) and Adobe Photoshop 5.0 (Adobe Systems, San Jose, CA).
Results
Identification of 9.5-dpc yolk sac- and P-Sp-derived primary endothelial cells
One-step isolation of GFP-expressing cells from Tie2-GFP transgenic mice has previously been reported to identify primary organ endothelial cells.27 Using this transgenic mouse strain, we performed a series of experiments to test whether GFP+ cells sorted from embryos represent purified primary embryonic endothelial cells. Confocal image analysis for GFP expression was restricted to capillary and large vessel endothelial cells in 9.5-dpc yolk sac (Figure 1) and embryo proper (data not shown). GFP expression was present throughout the cell cytoplasm, but the most intense GFP signal was displayed over endothelial nuclei. Rare GFP expression was apparent on round cells; some in contact with the endothelium and some round cells within the vessel lumen. The round cells within the lumen suggested that some hematopoietic elements might be expressing GFP. Recently, CD41 has been identified as a cell surface antigen that permits discrimination of embryonic stem cell-derived hematopoietic and endothelial cells.28-30 Double immunofluorescence images demonstrated that Tie2-GFP and CD41 were coexpressed on some round cells (Figure 1C-F, white arrowhead). Tie2-GFP and Flk-1 were coexpressed in many cells (Figure 1C-E, white arrows). Round cells expressing only CD41 were also present (yellow arrow). Confocal analysis did not permit us to determine whether these round cells were endothelial cells or hematopoietic cells and led us to conduct additional assays.
Confocal images of Flk1- and CD41-expressing cells detected in 9.5-dpc Tie2-GFP transgenic yolk sac vessels. (A) Image of a 9.5-dpc embryo proper (EP) and yolk sac (YS) with an insert panel to indicate the area depicted in panel B. (B) Tie2-GFP- (green), Flk1- (red), and CD41- (blue) expressing cells are seen in this composite image of day-9.5 YS tissue. The inset panel depicts the area visualized at higher magnification in panels C to F. (C) A higher power image of panel B that represents a composite of cells expressing Tie-2GFP (green), Flk1 (red), and CD41 (blue). Tie2-GFP expression (green) is primarily restricted to endothelial cells that coexpress Flk1 (red) as evidenced by cells highlighted by white arrows (C-E). Heterogeneity in the expression of Tie2-GFP and Flk1 leads to variation in the composite coexpression of these antigens leading to the appearance of bright yellow to orange aggregates (illustrated in the 2 cells indicated with white arrows in panel C). Few cells coexpress Tie2-GFP (D), Flk-1 (E), and CD41 (F), but 2 illustrative cells are indicated by white arrowheads in panels C to F. CD41 (blue) expression alone is restricted to round cells present within the lumen of vessels in the yolk sac (yellow arrow in panels C-F). Images were generated at projections of the z-stacks at 512 × 512 pixels. Panel A viewed at 4× with phase contrast. Bar in panel B represents 100 μm; bar in panels C-F is 50 μm.
Confocal images of Flk1- and CD41-expressing cells detected in 9.5-dpc Tie2-GFP transgenic yolk sac vessels. (A) Image of a 9.5-dpc embryo proper (EP) and yolk sac (YS) with an insert panel to indicate the area depicted in panel B. (B) Tie2-GFP- (green), Flk1- (red), and CD41- (blue) expressing cells are seen in this composite image of day-9.5 YS tissue. The inset panel depicts the area visualized at higher magnification in panels C to F. (C) A higher power image of panel B that represents a composite of cells expressing Tie-2GFP (green), Flk1 (red), and CD41 (blue). Tie2-GFP expression (green) is primarily restricted to endothelial cells that coexpress Flk1 (red) as evidenced by cells highlighted by white arrows (C-E). Heterogeneity in the expression of Tie2-GFP and Flk1 leads to variation in the composite coexpression of these antigens leading to the appearance of bright yellow to orange aggregates (illustrated in the 2 cells indicated with white arrows in panel C). Few cells coexpress Tie2-GFP (D), Flk-1 (E), and CD41 (F), but 2 illustrative cells are indicated by white arrowheads in panels C to F. CD41 (blue) expression alone is restricted to round cells present within the lumen of vessels in the yolk sac (yellow arrow in panels C-F). Images were generated at projections of the z-stacks at 512 × 512 pixels. Panel A viewed at 4× with phase contrast. Bar in panel B represents 100 μm; bar in panels C-F is 50 μm.
By FACS analysis, we detected that 7.8% of yolk sac cells and 1.4% of P-Sp cells expressed Tie2-GFP (Figure 2A,E). No GFP expression was detected in wild-type mice, indicating the specificity of GFP expression by FACS in the transgenic animals (indicated in Figure 2A,E as faint outlined histograms). We examined yolk sac and P-Sp cells for CD41 expression and observed that 3.0% of the yolk sac and 0.5% P-Sp cells expressed CD41 (Figure 2B,F). Approximately 2.0% of the yolk sac and 1.2% of P-Sp cells coexpressed Tie2-GFP and Flk-1 (Figure 2C,G), and 0.7% of yolk sac and 0.07% of P-Sp cells also coexpressed CD41 (Figure 2D,H). We interpreted these results to indicate that the Tie2-GFP+ and Tie2-GFP+Flk-1+ cells either contained 2 populations of cells, one with hematopoietic and the other with endothelial potential, or that the coexpressing cells may represent hemogenic endothelium.
CD41 and Flk1 are expressed in Tie2-GFP+cells in the 9.5-dpc yolk sac and P-Sp. Flow cytometric histograms depicting Tie2-GFP+ cells from 9.5-dpc yolk sac (A) and P-Sp (E) tissues isolated from transgenic mice. Autofluorescence of the cells is illustrated by the faint line; GFP expression is indicated by the bold line. The percentage of GFP-expressing cells (of the total for each tissue) is indicated in the upper right corner of the histogram for each population (A,E). CD41 expression in yolk sac (B) and P-Sp cells (F) is illustrated by the bold histograms, whereas isotype-binding control cells are indicated by the faint line. Dot plots of cells coexpressing Tie2-GFP+ and Flk1 in 9.5-dpc yolk sac (C) and P-Sp (G) indicate that the percent of total cells simultaneously expressing these fluorochromes is 2% or less. Further analysis of the yolk sac (D) and P-Sp (H) cells coexpressing Tie2-GFP, Flk-1, and CD41 revealed a 10-fold greater frequency of coexpressing cells in the yolk sac-derived cells (D) compared to P-Sp cells (H). These results are representative data of 10 experiments where 30 to 40 yolk sac or P-Sp tissues were analyzed in each experiment.
CD41 and Flk1 are expressed in Tie2-GFP+cells in the 9.5-dpc yolk sac and P-Sp. Flow cytometric histograms depicting Tie2-GFP+ cells from 9.5-dpc yolk sac (A) and P-Sp (E) tissues isolated from transgenic mice. Autofluorescence of the cells is illustrated by the faint line; GFP expression is indicated by the bold line. The percentage of GFP-expressing cells (of the total for each tissue) is indicated in the upper right corner of the histogram for each population (A,E). CD41 expression in yolk sac (B) and P-Sp cells (F) is illustrated by the bold histograms, whereas isotype-binding control cells are indicated by the faint line. Dot plots of cells coexpressing Tie2-GFP+ and Flk1 in 9.5-dpc yolk sac (C) and P-Sp (G) indicate that the percent of total cells simultaneously expressing these fluorochromes is 2% or less. Further analysis of the yolk sac (D) and P-Sp (H) cells coexpressing Tie2-GFP, Flk-1, and CD41 revealed a 10-fold greater frequency of coexpressing cells in the yolk sac-derived cells (D) compared to P-Sp cells (H). These results are representative data of 10 experiments where 30 to 40 yolk sac or P-Sp tissues were analyzed in each experiment.
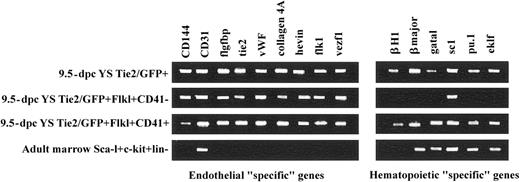
To further characterize the sorted cell populations, we interrogated the total RNA isolated from each population for evidence of genes typically expressed by hematopoietic or endothelial cells. Representative results of endothelial-“specific” and hematopoietic-“specific” gene expression profiles of fractionated yolk sac cells and adult control tissues are depicted in Figure 3. The Tie2-GFP+Flk-1+CD41- yolk sac 9.5-dpc cells expressed endothelial-“specific” mRNA, such as CD144, CD31, ilgfbp, Tie2, VWF, collagen, hevin, Flk-1, and vezf1. These gene products have previously been used to identify cells of the endothelial lineage. In addition, Tie2-GFP+Flk-1+CD41- yolk sac 9.5-dpc cells also expressed the transcription factor SCL that is required for both hematopoietic and endothelial cell development. In contrast, the yolk sac Tie2-GFP+ cells not only expressed endothelial-“specific” mRNA but also hematopoietic-“specific” mRNA (such as βH1, βmajor, GATA1, PU.1, SCL, and EKLF). A similar gene expression pattern was observed in the Tie2-GFP+Flk-1+CD41+ yolk sac 9.5-dpc population. Adult marrow Sca-1+c-Kit+lin- cells expressed hematopoietic-“specific” mRNA, and CD31, which has previously been reported to be present on marrow repopulating cells. These data suggested that the Tie2-GFP+Flk-1+CD41- cells display a gene expression profile like adult organ endothelial cells and not HSCs.
Endothelial and hematopoietic-“specific” gene expression profiles of fractioned 9.5-dpc yolk sac and BM cells. RT-PCR analysis revealed the gene expression profile of cells sorted from 9.5-dpc yolk sac and adult BM. The 9.5-dpc Tie2-GFP+ and Tie2-GFP+Flk1+CD41+ yolk sac cells express both endothelial and hematopoietic-“specific” gene transcripts. The Tie2-GFP+Flk1+CD41-yolk sac cells express endothelial-“specific” gene products, whereas BM Sca-1+ckit+lin-cells express hematopoietic-“specific” genes only. These data are representative of 3 independent analyses.
Endothelial and hematopoietic-“specific” gene expression profiles of fractioned 9.5-dpc yolk sac and BM cells. RT-PCR analysis revealed the gene expression profile of cells sorted from 9.5-dpc yolk sac and adult BM. The 9.5-dpc Tie2-GFP+ and Tie2-GFP+Flk1+CD41+ yolk sac cells express both endothelial and hematopoietic-“specific” gene transcripts. The Tie2-GFP+Flk1+CD41-yolk sac cells express endothelial-“specific” gene products, whereas BM Sca-1+ckit+lin-cells express hematopoietic-“specific” genes only. These data are representative of 3 independent analyses.
The accumulating data suggested that FACS sorting of the embryonic cells might permit separation of cells with hematopoietic potential from endothelial cells. Therefore, we plated the various Tie2-GFP-sorted cell populations into hematopoietic progenitor colony-forming cell (CFC) assays. Tie2-GFP+ cells sorted from 9.5-dpc yolk sac and P-Sp contained hematopoietic progenitor activity (Table 1). Similarly, Tie2-GFP+Flk-1+CD41+ yolk sac and P-Sp cells gave rise to CFCs. However, Tie2-GFP+Flk-1+CD41- cells were depleted of hematopoietic progenitor activity (Table 1) even if plated at 10-fold higher concentrations. These data support the identification of Tie2-GFP+Flk-1+CD41- cells as an endothelial-enriched population lacking in hematopoietic activity.
Frequency of CFC from sorted 9.5-dpc yolk sac and P-Sp subpopulations
Cell subpopulations . | No. of CFCs . |
|---|---|
| YS Tie2-GFP+ | 4.8 ± 0.7 |
| YS Tie2-GFP+Flk-1+ | 0.9 ± 0.2 |
| YS Tie2-GFP+Flk-1+CD41− | 0 ± 0 |
| YS Tie2-GFP+Flk-1+CD41+ | 1.4 ± 0.7 |
| P-Sp Tie2-GFP+ | 1.3 ± 0.3 |
| P-Sp Tie2-GFP+Flk-1+ | 1.2 ± 0.9 |
| P-Sp Tie2-GFP+Flk-1+CD41− | 0 ± 0 |
| P-Sp Tie2-GFP+Flk-1+CD41+ | 1.8 ± 0.4 |
Cell subpopulations . | No. of CFCs . |
|---|---|
| YS Tie2-GFP+ | 4.8 ± 0.7 |
| YS Tie2-GFP+Flk-1+ | 0.9 ± 0.2 |
| YS Tie2-GFP+Flk-1+CD41− | 0 ± 0 |
| YS Tie2-GFP+Flk-1+CD41+ | 1.4 ± 0.7 |
| P-Sp Tie2-GFP+ | 1.3 ± 0.3 |
| P-Sp Tie2-GFP+Flk-1+ | 1.2 ± 0.9 |
| P-Sp Tie2-GFP+Flk-1+CD41− | 0 ± 0 |
| P-Sp Tie2-GFP+Flk-1+CD41+ | 1.8 ± 0.4 |
The number of CFCs indicates the mean ± SD of triplicate cultures of 3 experiments initiated with 1250 cells/plate (12 500 YS or P-Sp Tie2-GFP+Flk-1+CD41−cells/plate).
YS indicates yolk sac.
Endothelial cells will form capillary-like structures when placed in culture in Matrigel, an extracellular matrix basement membrane material. Tie2-GFP+Flk-1+CD41- cells sorted from 9.5-dpc yolk sac cells formed the characteristic capillary-like tube morphology of mature endothelial cells (Figure 4A). Capillary-like tube structures also formed from plated 9.5-dpc P-Sp Tie2-GFP+Flk-1+CD41- cells (Figure 4B) and Tie2-GFP-expressing endothelial cells from adult heart tissue (Figure 4C). Yolk sac-derived Tie2-GFP+Flk-1+CD41- cells plated on Matrigel displayed the ability to take up Dil-Ac-LDL and coexpressed CD31 (Figure 4D-F). Based on cumulative data, we have defined the Tie2-GFP+Flk-1+CD41- cells sorted from 9.5-dpc yolk sac and P-Sp tissues as primary endothelial cells.
The 9.5-dpc cells sorted from yolk sac and P-Sp formed endothelial capillary-like tubes on Matrigel. Sorted Tie2-GFP+Flk1+CD41-cells from 9.5-dpc yolk sac and P-Sp and Tie2-GFP+ cells from adult heart were seeded on Matrigel-coated dishes. Capillary tube formation was induced in the presence of βFGF, VEGF, and ECGS (see “Materials and methods”) in 2% O2, 5% CO2 over a 7-day culture period. (A) Yolk sac-derived, (B) P-Sp-derived, and (C) adult heart-derived capillary-like tubes are depicted. Representative endothelial cells forming capillary-like tubes are highlighted in the photomicrographs by white arrowheads (A-C) at an original magnification of × 10. (D) Sorted Tie2-GFP+Flk1+CD41-yolk sac cells take up soluble Dil-Ac-LDL and coexpress CD31 in this composite confocal image as highlighted in 2 representative cells by white arrows (D-F). Panels E and F depict individual CD31 and Dil-Ac-LDL expression patterns. Bar represents 50 μm.
The 9.5-dpc cells sorted from yolk sac and P-Sp formed endothelial capillary-like tubes on Matrigel. Sorted Tie2-GFP+Flk1+CD41-cells from 9.5-dpc yolk sac and P-Sp and Tie2-GFP+ cells from adult heart were seeded on Matrigel-coated dishes. Capillary tube formation was induced in the presence of βFGF, VEGF, and ECGS (see “Materials and methods”) in 2% O2, 5% CO2 over a 7-day culture period. (A) Yolk sac-derived, (B) P-Sp-derived, and (C) adult heart-derived capillary-like tubes are depicted. Representative endothelial cells forming capillary-like tubes are highlighted in the photomicrographs by white arrowheads (A-C) at an original magnification of × 10. (D) Sorted Tie2-GFP+Flk1+CD41-yolk sac cells take up soluble Dil-Ac-LDL and coexpress CD31 in this composite confocal image as highlighted in 2 representative cells by white arrows (D-F). Panels E and F depict individual CD31 and Dil-Ac-LDL expression patterns. Bar represents 50 μm.
Primary endothelial cells derived from 9.5-dpc yolk sac and P-Sp efficiently supported the expansion of marrow nucleated cells and CFCs
Isolation of pure populations of endothelial cells (Tie2-GFP+Flk-1+CD41-) permitted us to determine whether 9.5-dpc yolk sac or P-Sp endothelial cocultures support expansion of adult marrow HSCs and CFCs in vitro. Proliferation of adult BM nucleated nonadherent cells in the yolk sac and P-Sp cocultures occurred in the presence (47.3 ± 15.3-fold and 295.1 ± 44.9-fold increases, respectively) or absence of added growth factors (29.0 ± 3.1-fold and 59.0 ± 6.7-fold increases, respectively), whereas endothelial cell-free culture conditions resulted in a 24.0 ± 7.7-fold increase (in the presence of growth factors) and less than a 10.0-fold increase (in the absence of growth factors). Thus, 9.5-dpc yolk sac or P-Sp Tie2-GFP+Flk-1+CD41- endothelial cocultures supported hematopoietic cell proliferation particularly in the presence of added growth factors and more so in the P-Sp-derived cocultures than the yolk sac-derived cocultures.
Yolk sac and P-Sp endothelial cell cocultures also supported an increase in Sca-1+c-Kit+lin--derived hematopoietic progenitors in the presence of growth factors (9.4- and 11.4-fold increases, respectively) compared with endothelial cell-free conditions (Table 2). In the absence of growth factor addition, yolk sac and P-Sp maintained some cells with CFC potential; however, the frequency of CFC at the end of the 7 days of coculture was significantly lower than the input number of CFCs (Table 2). These data indicate that yolk sac and P-Sp Tie2-GFP+Flk-1+CD41- endothelial cells promote increases in adult marrow CFC formation in vitro in the presence of added SCF, IL-6, and TPO. The amount of CFC support provided by the sorted yolk sac and P-Sp endothelial cells alone in coculture equaled that demonstrated by addition of SCF, IL-6, and TPO alone in endothelial cell-free conditions (Table 2).
The 9.5-dpc YS and P-Sp EC cocultures support the expansion of marrow CFCs
Cell groups . | CFU-GM . | BFU-E . | CFU-Mix . | Total . |
|---|---|---|---|---|
| With growth factors | ||||
| Fresh Sca-1+ ckit+lin− | (194.0 ± 36.0) | (22.0 ± 6.7) | (20.0 ± 5.4) | (235.0 ± 35.8) |
| EC free | 35.0 ± 7.0 | 8.8 ± 3.1 | 6.9 ± 2.9 | 50.9 ± 9.8 |
| 9.5-dpc YS | 413.0 ± 21.9 | 44.0 ± 11.0 | 30.0 ± 8.4 | 487.0 ± 25.5*† |
| 9.5-dpc P-Sp | 491.0 ± 47.8 | 51.0 ± 15.0 | 39.1 ± 10.8 | 582.0 ± 65.2*† |
| Without growth factors | ||||
| EC free | 0 | 0 | 0 | 0 |
| 9.5-dpc YS | 28.1 ± 6.6 | 4.2 ± 3.5 | 3.0 ± 1.6 | 35.3 ± 6.4 |
| 9.5-dpc P-Sp | 34.9 ± 8.2 | 8.9 ± 4.2 | 4.9 ± 1.4 | 48.7 ± 7.5 |
Cell groups . | CFU-GM . | BFU-E . | CFU-Mix . | Total . |
|---|---|---|---|---|
| With growth factors | ||||
| Fresh Sca-1+ ckit+lin− | (194.0 ± 36.0) | (22.0 ± 6.7) | (20.0 ± 5.4) | (235.0 ± 35.8) |
| EC free | 35.0 ± 7.0 | 8.8 ± 3.1 | 6.9 ± 2.9 | 50.9 ± 9.8 |
| 9.5-dpc YS | 413.0 ± 21.9 | 44.0 ± 11.0 | 30.0 ± 8.4 | 487.0 ± 25.5*† |
| 9.5-dpc P-Sp | 491.0 ± 47.8 | 51.0 ± 15.0 | 39.1 ± 10.8 | 582.0 ± 65.2*† |
| Without growth factors | ||||
| EC free | 0 | 0 | 0 | 0 |
| 9.5-dpc YS | 28.1 ± 6.6 | 4.2 ± 3.5 | 3.0 ± 1.6 | 35.3 ± 6.4 |
| 9.5-dpc P-Sp | 34.9 ± 8.2 | 8.9 ± 4.2 | 4.9 ± 1.4 | 48.7 ± 7.5 |
One thousand BM Sca-1+c-kit+lin− cells were cocultured with 10 000 ECs derived from 9.5-dpc YS and P-Sp. The CFC counts derived from freshly isolated and plated sorted cells reflect the input counts and are enclosed in parentheses. Growth factors used included 50 ng/mL SCF, 50 ng/mL TPO, and 10 ng/mL IL-6. The total number of hematopoietic CFCs indicates the mean ± SD of triplicate cultures of 3 independent experiments.
P <.05 YS and P-Sp coculture cells compared with fresh sorted BM cells.
P <.01 YS and P-Sp coculture cells compared with EC-free cultures.
The 9.5-dpc yolk sac- and P-Sp-derived endothelial cells promote CFU-S expansion
Sca-1+c-Kit+lin- cells are enriched in HSCs and multipotent progenitor cells. To assay for CFU-S survival and proliferation in yolk sac and P-Sp Tie2-GFP+Flk-1+CD41- endothelial cell cocultures, we isolated the entire contents of each coculture after 7 days in vitro and injected the expanded Sca-1+c-Kit+lin- cells into lethally irradiated recipient mice. Yolk sac and P-Sp Tie2-GFP+Flk-1+CD41- endothelial cell cocultures significantly increased CFU-S8 counts (18.7 ± 2.0 and 26.0 ± 2.6, respectively, P < .01) relative to the input number of CFU-S8 present in the fresh Sca-1+c-Kit+lin- cells (10.2 ± 1.2; Table 3). CFU-S8 counts did not change significantly in the growth factor-supplemented endothelial cell-free coculture conditions (Table 3). The culture of Sca-1+c-Kit+lin- cells with yolk sac and P-Sp Tie2-GFP+Flk-1+CD41- endothelial cells but without growth factors failed to support input numbers of CFU-S8 (Table 3). In control experiments, no CFU-S8 were detected in any mice receiving transplants of fresh yolk sac or P-Sp Tie2-GFP+Flk-1+CD41- endothelial cells (Table 3) clearly supporting the previous evidence (Table 1; Figure 3) that these endothelial cell populations are devoid of hematopoietic potential. These results suggest that the yolk sac and P-Sp Tie2-GFP+Flk-1+CD41- endothelial cells are capable of promoting progenitor cell expansion but only in the presence of added SCF, IL-6, and TPO.
9.5-dpc YS and P-Sp EC cocultures support the generation of CFU-S8
Cell groups . | CFU-S . | Cocultures with growth factors CFU-S . | Cocultures without growth factors CFU-S . |
|---|---|---|---|
| Fresh BM Sca-1+ ckit+lin− cells | 10.0 ± 1.2 | — | — |
| Fresh YS ECs | 0 ± 0 | — | — |
| Fresh P-Sp ECs | 0 ± 0 | — | — |
| EC-free | — | 6.0 ± 0.8 | 0 ± 0 |
| 9.5-dpc YS ECs | — | 18.7 ± 2.0† | 3.0 ± 1.0*† |
| 9.5-dpc P-Sp ECs | — | 26.0 ± 2.6† | 4.0 ± 1.0*† |
Cell groups . | CFU-S . | Cocultures with growth factors CFU-S . | Cocultures without growth factors CFU-S . |
|---|---|---|---|
| Fresh BM Sca-1+ ckit+lin− cells | 10.0 ± 1.2 | — | — |
| Fresh YS ECs | 0 ± 0 | — | — |
| Fresh P-Sp ECs | 0 ± 0 | — | — |
| EC-free | — | 6.0 ± 0.8 | 0 ± 0 |
| 9.5-dpc YS ECs | — | 18.7 ± 2.0† | 3.0 ± 1.0*† |
| 9.5-dpc P-Sp ECs | — | 26.0 ± 2.6† | 4.0 ± 1.0*† |
One thousand bone marrow Sca-1+c-kit+lin− cells were cocultured with 10 000 ECs derived from 9.5-dpc YS and P-Sp. Growth factors included 50 ng/mL SCF, 50 ng/mL TPO, and 10 ng/mL IL-6. The total number of CFU-S8 indicates the mean ± SD of 3 to 6 mice in 3 independent experiments.—indicates no data.
P <.05 YS and P-Sp EC cocultures compared with EC-free.
P <.01 YS and P-Sp EC cocultures compared with EC-free and sorted BM cells.
Yolk sac- and P-Sp-derived endothelial cells facilitate marrow HSC competitive repopulating ability
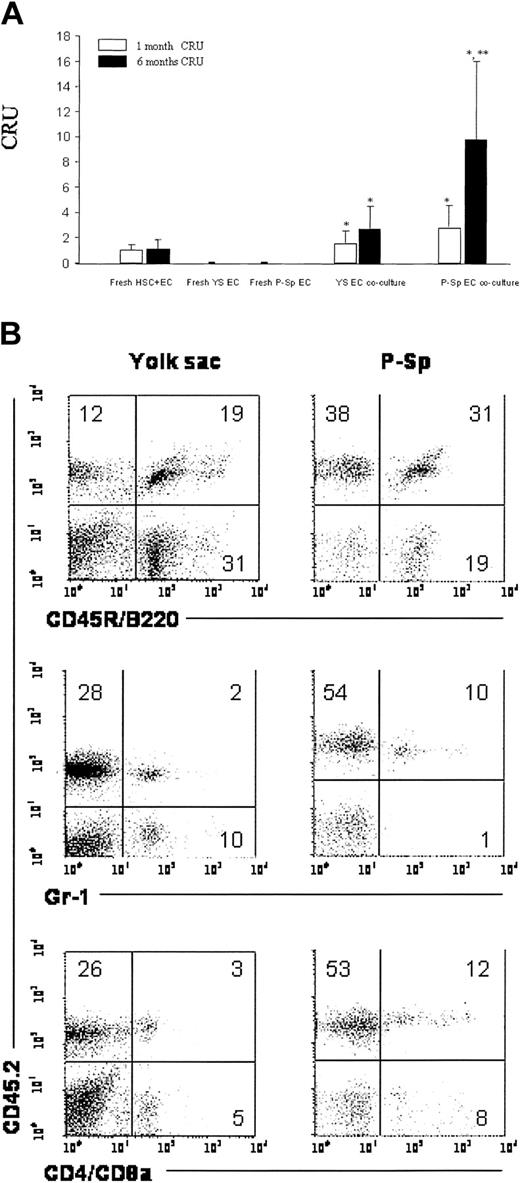
The gold standard for assaying HSC function is to transplant a donor cell population into a conditioned recipient animal and to determine the ability of the donor cells to repopulate the ablated host lineages long term. To determine this ability, we transplanted the entire cell contents of each coculture (along with fresh BM competitor cells) into lethally irradiated recipient mice. As initial controls, we transplanted freshly isolated yolk sac and P-Sp Tie2-GFP+Flk-1+CD41- endothelial cells (5000-80 000 cells/mouse) and saw no evidence (121 recipient animals) in donor-derived CD45.2-expressing cells in the peripheral blood (data not shown). Because we were culturing bone marrow cells with Tie2-GFP+Flk-1+CD41- endothelial cells in vitro and knew that harvesting of all the cells in the cocultures would include endothelial cells, fresh sorted bone marrow Sca-1+c-Kit+lin- cells (1 × 103) plus fresh adult heart endothelial cells (1 × 104) were transplanted into lethally irradiated congenic (Gpi-1a/BoyJ) mice with 1.5 × 105 marrow (competitor) cells from CD45.1-expressing Gpi-1a/BoyJ mice as the input source for CRU determination. The CRU contributions of the yolk sac and P-Sp Tie2-GFP+Flk-1+CD41- endothelial cell cocultures are depicted in Figure 5. The Tie2-GFP+Flk-1+CD41- yolk sac and P-Sp endothelial cell monolayers supported a 1.6-fold (1.6 ± 1.0) and 2.8-fold (2.8 ± 1.7), respectively, short-term (1 month after transplantation) CRU increase relative to fresh control CRUs (0.9 ± 0.1; P < .05). Both yolk sac and P-Sp Tie2-GFP+Flk-1+CD41- endothelial cells promoted increases in long-term (≥ 6 months following transplantation) HSC CRU 2.8-fold (2.8 ± 1.7) and 9.8-fold (9.8 ± 6.2) increase, respectively, compared with the fresh control CRUs (1.0 ± 0.2; Figure 5A). Tie2-GFP+Flk-1+CD41- yolk sac- and P-Sp endothelial cell-facilitated HSC engraftment was demonstrated to encompass multiple lineages (B and T lymphocytes and granulocytes) and to be maintained long term (Figure 5B). These results demonstrate that HSC competitive repopulating ability is promoted in ex vivo coculture with primary endothelial cells from 9.5-dpc yolk sac and P-Sp in the presence of SCF, IL-6, and TPO.
Comparison of BM CRU ability with multilineage reconstitution supported by 9.5-dpc yolk sac- and P-Sp-derived endothelial cell cocultures. (A) The CRU activity in peripheral blood of recipient mice 1 and 6 months after transplantation with fresh or cultured donor stem cells. Freshly isolated Tie2-GFP+Flk1+CD41-yolk sac and P-Sp endothelial cells failed to result in any donor evidence of hematopoietic cell repopulation in mice that received competitive transplants. The 9.5-dpc Tie2-GFP+Flk1+CD41-yolk sac and P-Sp endothelial cell cocultures facilitated both short-term (1 month) and long-term (6 month) donor stem cell CRUs compared with fresh control CRUs (*P < .05). The P-Sp endothelial cell coculture conditions markedly increased CRU activity of the donor stem cells as evidenced in the peripheral blood chimerism of recipient mice at 6 months after transplantation compared with donor stem cell Sca-1+c-Kit+lin-cell CRUs harvested from yolk sac endothelial coculture conditions (**P < .05). Data are presented as the mean ± SD of 6 animals at each time point in each group. Representative peripheral blood analysis (B) of reconstitution of recipient mice receiving transplants with Sca-1+c-Kit+lin-cells following 7 days of coculture with Tie2-GFP+Flk1+CD41-yolk sac or P-Sp endothelial cells performed 6 months after the donor cell transplantation. Percent donor type CD45.2-expressing cells (y-axis) in recipient mice given transplants of 9.5-dpc Tie2-GFP+Flk1+CD41-yolk sac endothelial cell/Sca-1+c-Kit+lin-cocultures or 9.5-dpc P-Sp Tie2-GFP+Flk1+CD41-endothelial cell/Sca-1+c-Kit+lin-cocultures are depicted with B lymphocyte (CD45R/B220), granulocyte (Gr-1), and T-lymphocyte markers (CD4 and CD8) depicted on the x-axis.
Comparison of BM CRU ability with multilineage reconstitution supported by 9.5-dpc yolk sac- and P-Sp-derived endothelial cell cocultures. (A) The CRU activity in peripheral blood of recipient mice 1 and 6 months after transplantation with fresh or cultured donor stem cells. Freshly isolated Tie2-GFP+Flk1+CD41-yolk sac and P-Sp endothelial cells failed to result in any donor evidence of hematopoietic cell repopulation in mice that received competitive transplants. The 9.5-dpc Tie2-GFP+Flk1+CD41-yolk sac and P-Sp endothelial cell cocultures facilitated both short-term (1 month) and long-term (6 month) donor stem cell CRUs compared with fresh control CRUs (*P < .05). The P-Sp endothelial cell coculture conditions markedly increased CRU activity of the donor stem cells as evidenced in the peripheral blood chimerism of recipient mice at 6 months after transplantation compared with donor stem cell Sca-1+c-Kit+lin-cell CRUs harvested from yolk sac endothelial coculture conditions (**P < .05). Data are presented as the mean ± SD of 6 animals at each time point in each group. Representative peripheral blood analysis (B) of reconstitution of recipient mice receiving transplants with Sca-1+c-Kit+lin-cells following 7 days of coculture with Tie2-GFP+Flk1+CD41-yolk sac or P-Sp endothelial cells performed 6 months after the donor cell transplantation. Percent donor type CD45.2-expressing cells (y-axis) in recipient mice given transplants of 9.5-dpc Tie2-GFP+Flk1+CD41-yolk sac endothelial cell/Sca-1+c-Kit+lin-cocultures or 9.5-dpc P-Sp Tie2-GFP+Flk1+CD41-endothelial cell/Sca-1+c-Kit+lin-cocultures are depicted with B lymphocyte (CD45R/B220), granulocyte (Gr-1), and T-lymphocyte markers (CD4 and CD8) depicted on the x-axis.
Discussion
We report that purified murine primary endothelial cells can be derived from 9.5-dpc embryonic yolk sac and P-Sp cells using a combination of cell surface and transgene expressed markers. Plating of the purified Tie2-GFP+Flk-1+CD41- populations on Matrigel in vitro and then adding adult murine marrow Sca-1+c-Kit+lin- cells for 7 days led to demonstrable increases in nonadherent cell, hematopoietic CFC, and CFU-S counts. Transplantation analysis revealed that both the yolk sac and P-Sp Tie2-GFP+Flk-1+CD41- endothelial cell monolayers support HSC short-term CRU (1.6-fold and 2.8-fold increases, respectively) and HSC long-term CRUs (2.8- and 9.8-fold increase) relative to fresh HSC control grafts. These results support our primary hypothesis that endothelial cells derived from embryonic tissues promote HSC expansion.
The greatest obstacle to completing this study was the difficulty in isolating a pure population of primary endothelial cells from the yolk sac and P-Sp. The Tie2-GFP transgenic mouse we used in the present studies had previously been characterized and expression of the transgene was thought to be specifically restricted to endothelial cells from 8.5 dpc throughout adulthood.27 We anticipated that use of this reporter mouse would permit us to isolate pure endothelial cell populations from the embryonic tissues. GFP+ cells sorted from 9.5-dpc yolk sac and P-Sp exhibited classical endothelial features such as the formation of capillary-like tubes on plating in Matrigel-containing cultures and expression of endothelial-“specific” mRNA. However, these GFP+ cells also displayed hematopoietic CFC activity and expressed a variety of hematopoietic-“specific” mRNA. To purify embryonic endothelial cells, we used CD41 expression as a negative selector (to remove hematopoietic cells) based on our recent observations that CD41 expression defines the onset of primitive and definitive hematopoiesis in the murine embryo.30 The results indicated that the Tie-GFP+Flk-1+CD41- cells formed capillary-like tubes in vitro, expressed only endothelial-specific RNA transcripts by RT-PCR, failed to form hematopoietic CFCs in progenitor assays, and possessed no stem cell repopulating ability in vivo. Thus, we have determined a method for purifying primary endothelial cells from murine embryonic tissues.
Strategies for isolating endothelial and hematopoietic cells from embryonic stem (ES) cell-derived cells and primary yolk sac and P-Sp cells have been previously published.31,32 Ogawa et al reported that as ES cell-derived mesoderm precursors differentiated in vitro, cells specified to become endothelium expressed CD144 but not VLA4, whereas the first hematopoietic cells were CD144+VLA4+.33 Using the same system, hematopoietic cells have also been discriminated from endothelial cells via CD45 expression (a cell surface phosphatase expressed on all nucleated blood cells).34 Flk-1 is expressed by ES cell-derived hemangioblast precursors and continues to be expressed in endothelium, whereas cell surface Flk-1 expression is gradually extinguished in hematopoietic progenitors. In preliminary studies we have determined that essentially all of the 9.5-dpc yolk sac and P-Sp Tie-GFP+Flk-1+CD41- cells coexpress CD144, CD34, CD31, and fail to express CD45 (W.L., unpublished data, December 2002). Thus, we have identified additional antigens that permit isolation of endothelium from hematopoietic cells in the murine embryo and have advanced the field to demonstrate that the primary endothelial cells serve to support HSC function ex vivo.
In the process of identifying pure endothelial populations, we also isolated cells that coexpressed a variety of endothelial and hematopoietic lineage gene products and possessed hematopoietic CFC activity. Others have identified endothelial cells with hematopoietic potential as both Runx1- and Sca-1-expressing cells.35,36 As noted above, ES cell-derived hemangioblast precursors of the endothelial and hematopoietic lineages express Flk-1. We have identified 9.5-dpc yolk sac and P-Sp Tie-GFP+Flk-1+CD41+ cells as a population that either represents hemogenic endothelium or is composed of a mixture of endothelial and hematopoietic cells. At present we cannot state whether these sorted cells represent hemogenic endothelium. Studies designed to examine the clonal differentiation potential of this population are under way.
The P-Sp region serves as a precursor of the AGM, the first site of adult engrafting HSCs. Explant cultures of yolk sac and P-Sp have demonstrated that the P-Sp possesses intrinsic adult repopulating stem cell activity that has not been demonstrable under the same culture conditions with yolk sac cells.10 It remains unclear if the lack of intrinsic stem cell repopulating activity in the explanted yolk sac is due to an absence of cells with this potential or rather to lack of an appropriate environment to support the proliferation or survival of adult repopulating stem cells. Our results demonstrated that 9.5-dpc primary endothelial cells isolated from yolk sac and P-Sp facilitate adult marrow HSC competitive repopulating ability. Moreover, P-Sp-derived Tie2-GFP+Flk-1+CD41- endothelial cells displayed more potential for supporting HSC repopulating ability than yolk sac-derived Tie2-GFP+Flk-1+CD41- endothelial cells. These results are consistent with the tissue explant studies and suggest that the P-Sp microenvironment may be intrinsically more supportive of HSC development and expansion than the early yolk sac microenvironment. Our results suggest that primary endothelial cells from these embryonic sites play an important and perhaps significant role in determining the level of HSC support displayed by the entire tissue.
Recently, Chute et al have reported that human brain vascular endothelial cells promote the expansion of human BM NOD/SCID engrafting cells in ex vivo culture.37 Similar results were obtained when transplantable nonhuman primate HSCs were expanded on porcine microvascular endothelial cells ex vivo.38 Our observations that yolk sac- and P-Sp-derived Tie2-GFP+Flk-1+CD41- endothelial cells promote expansion of adult marrow HSC competitive repopulating ability ex vivo in the presence of SCF, IL-6, and TPO, provide additional support for considering the use of endothelial monolayers in HSC and progenitor cell ex vivo expansion protocols.
Prepublished online as Blood First Edition Paper, July 31, 2003; DOI 10.1182/blood-2003-03-0729.
Supported in part by grant R01 HL63169 (M.C.Y.) from the National Institutes of Health.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We would like to thank Dr David Ingram for critical reading of the manuscript and Pat Fox for editorial assistance.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal