Abstract
In chronic myelogenous leukemia (CML) imatinib mesylate has been shown to selectively inhibit the tyrosine kinase domain of the oncogenic bcr-abl fusion protein. Using this agent alone high rates of cytogenetic responses were recorded. However, several mechanisms of resistance have been described. In vitro studies examining the effects of imatinib mesylate plus cytarabine have shown synergistic antiproliferative effects of this combination. Thus, the CML French Group decided to perform a phase 2 trial testing a combination of imatinib mesylate and low-dose cytarabine in 30 previously untreated patients in chronic phase. Treatment was administered on 28-day cycles. Patients were treated continuously with imatinib mesylate orally at a dose of 400 mg daily. Cytarabine was given on days 15 to 28 of each cycle at an initial dose of 20 mg/m2/d via subcutaneous injection. Adverse events were frequently observed with grade 3 or 4 hematologic toxicities and nonhematologic toxicities in 53% (n = 16) and 23% (n = 7) of patients, respectively. The cumulative incidence of complete cytogenetic response (CCR) at 12 months was 83% and at 6 months 100% of the patients achieved complete hematologic response (CHR). We concluded that the combination was safe and promising given the rates of response. (Blood. 2003;102:4298-4305)
Introduction
For many years regimens based on interferon α (IFN-α) were offered to patients with chronic myelogenous leukemia (CML) in chronic phase. An improvement in survival was obtained in a minority of patients who achieved durable cytogenetic response.1 Imatinib mesylate has been shown to selectively inhibit the tyrosine kinase domain of the oncogenic bcr-abl fusion protein.2 Used alone, substantial and durable hematologic responses as well as cytogenetic responses were reported in most of the patients with chronic phase CML.3 In a large phase 3 randomized trial for newly diagnosed patients with chronic phase CML, imatinib mesylate produced significantly better results at 1 year as compared with the combination of IFN-α and cytarabine (Ara-C).4 Thus imatinib mesylate could be recommended as first-line therapy for chronic phase disease.
However, a concern with any single agent administered chronically is that resistant clones may emerge. Experimental studies suggest that imatinib mesylate as a single drug might not be sufficient to eradicate Philadelphia-positive (Ph+) stem cells.5 Thus, new therapeutic strategies taking into account experimental observations should be investigated in the clinical setting.
To achieve higher rates of cytogenetic response and to overcome these resistances, new strategies have emerged such as treating patients earlier and increasing the dose of imatinib or combining imatinib with other cytotoxic drugs. Drugs that are currently being tested are those that had been selected in the past for their high antileukemic activity. IFN-α, pegylated or nonpegylated forms, and cytarabine at various dosages are actively being tested.
Our decision to test the combination of imatinib mesylate with cytarabine was based on several observations. Cytarabine has well-described albeit modest activity as a single agent in CML.6-8 In combination with IFN-α, cytarabine significantly improved the rate of cytogenetic responses and survival in CML patients.9,10 An oral formulation of cytarabine (YNK01) has also been tested.11 In vitro studies examining the effects of imatinib mesylate plus cytarabine using CML cell lines and colony-forming assays of CML patient samples have shown synergistic and additive antiproliferative effects of this combination.12-15
Recently, a first phase 1 study of the combination of imatinib mesylate plus low-dose cytarabine was initiated in patients with CML in chronic phase in whom IFN-α therapy failed.16 In this study, 22 patients were enrolled into 4 cohorts. The preliminary conclusions were that the combination had an acceptable toxicity. It was also concluded that the maximum tolerated dose was 400 mg/d for imatinib and 20 mg/m2/d for 14 days for cytarabine, respectively.
Given these preliminary results, the CML French Group decided to perform a phase 2 trial testing a combination of imatinib mesylate and low-dose cytarabine in previously untreated patients with chronic phase CML.
Patients and methods
Aims of the study
The purpose of this phase 2 study was to determine the safety and tolerability of a combination of imatinib mesylate and cytarabine. Other end points were rate of complete hematologic response (CHR) at 6 months, rates and duration of major cytogenetic response (MCR) and complete cytogenetic response (CCR) at 6 and 12 months, level of molecular residual disease in patients in CCR after 6 and 12 months of treatment and thereafter, and overall survival.
Patients
To be eligible, patients were required to be 18 years or older with newly diagnosed Ph+ CML in chronic phase with no more than 5% of bone marrow blast cells. Patients were within 6 months of diagnosis with no prior therapy except hydroxyurea. Women who were pregnant or nursing a child were excluded from the trial and barrier contraceptive precautions had to be used throughout the trial in both sexes. Other exclusion criteria were patients with serum aspartate aminotransferase (AST) or alanine aminotransferase (ALT) concentrations equal to or more than 3 times the institutional upper limit of the normal range or serum bilirubin and creatinine levels higher than 1.5 times the upper limit of normal. Patients with Ph- CML or in accelerated phase or in blast crisis were also ineligible. Patients with identified sibling donors for whom allogeneic transplantation was elected as first-line treatment were not included in the trial.
Patients provided written informed consent before entry into the trial. The study protocol was reviewed and approved by the ethics committee of the Poitou-Charentes area and conducted in accordance with the Declaration of Helsinki.
Treatment and dose modification
Patients were treated continuously with imatinib mesylate taken orally at a daily dose of 400 mg. Cytarabine was given on days 15 to 28 of a cycle repeated every 28 days at an initial dose of 20 mg/m2/d administered subcutaneously; hydroxyurea was stopped at least 7 days before imatinib mesylate started.
The overriding approach to hematologic toxicity was that imatinib mesylate was considered the most active agent of the combination and that every effort should be directed at maintaining patients on continuous therapy with imatinib mesylate. Complete blood counts with differentials were checked prior to each cycle of therapy containing cytarabine. Each time cytarabine was held and restarted, a new 28-day cycle was to be initiated and the day of the first injection of cytarabine was considered as day 15 of the new cycle. If blood counts showed platelet counts less than 100 × 109/L or granulocyte counts less than 1.5 × 109/L, cytarabine was not administered; blood counts were checked weekly and cytarabine was resumed when these parameters were met. For platelet counts less than 75 × 109/L or granulocyte counts less than 1.25 × 109/L, cytarabine was not administered; blood counts were checked weekly and cytarabine was resumed 2 weeks after granulocytes counts were more than 1.5 × 109/L and platelet counts more than 100 × 109/L, the dose of cytarabine being reduced to 10 mg/m2 (ie, 50% dose reduction) for a first episode of toxicity or to 5 mg/m2/d if a second episode had occurred. If platelet counts were less than 50 × 109/L (ie, grade 3) and granulocyte counts less than 1 × 109/L (ie, grade 3), both imatinib mesylate and cytarabine were withheld; blood counts were checked weekly and imatinib mesylate was resumed when the granulocyte count was more than 1.5 × 109/L and platelet count more than 100 × 109/L, cytarabine being restarted 2 weeks later with the same dose reduction recommendation as mentioned in Table 2. If cytarabine could not be administered even at a dose of 5 mg/m2/d, then imatinib mesylate was reduced to a dose of 300 mg. If cytarabine could not be resumed at this reduced dose, cytarabine was permanently discontinued and imatinib mesylate was administered alone at a dose of 400 mg/d (or 300 mg/d if imatinib alone could not be maintained at 400 mg/d). Patients developing anemia received packed red cell transfusions and no dose reductions were foreseen for anemia. If a patient experienced persistent grade 2 nonhematologic toxicity unresponsive to symptomatic treatment, study drugs had to be withheld until the toxicity resolved to grade 1 or lower. Study drugs were then resumed at the previous doses. If the grade 2 toxicity recurred, study drugs had to be withheld until the toxicity resolved to grade 1 or lower. Imatinib mesylate was resumed at the same dose but the cytarabine dose was reduced as follows: 20 mg/m2 reduced to 10 mg/m2,10 mg/m2 reduced to 5 mg/m2. If the grade 2 toxicity occurred at dose levels of cytarabine of 5 mg/m2, study drugs had to be withheld until the toxicity had resolved to grade 1 or lower followed by dose reduction of imatinib mesylate to 300 mg. If the grade 2 toxicity recurred at these dosage levels, cytarabine was discontinued and therapy with imatinib mesylate continued as a single agent once the toxicity had resolved to grade 1 or lower. If the grade 2 toxicity recurred during imatinib mesylate therapy alone at the reduced dose of 300 mg, further dose reductions or discontinuation of all drugs had to be carried out after discussion with the principal investigator. Similar recommendations were followed in case of grade 3/4 nonhematologic toxicity. With the exception of those patients who experienced grade 3/4 nonhematologic toxicity, any patient receiving reduced dosages of study drugs who did not achieve or maintain a CHR after 84 days (3 cycles) at reduced dosages had the dose re-escalated to the preceding dosages, provided no grade 2 or higher toxicities were observed during cycles at the reduced dosages (Tables 1 and 2). In case of no cytogenetic response after 12 months, the dose of imatinib mesylate could be increased up to 800 mg. The dosage of imatinib mesylate could also be increased in case of loss of cytogenetic response defined as an increase of Ph+ cells by 20%. Cytarabine would be administered indefinitely in case of good tolerance and if the physician believed that the combination therapy was the best therapeutic option for the patient.
Dose modifications for hematologic toxicity
For Plt count < 100 × 109/L or ANC < 1.5 × 109/L | For Plt count < 75 × 109/L or ANC < 1.25 × 109/L | For Plt count < 50 × 109/L or ANC < 1.0 × 109/L |
| ↓ | ↓ | ↓ |
| Hold Ara-C | Hold Ara-C | Hold Ara-C |
| Continue imatinib mesylate | Continue imatinib mesylate | Hold imatinib mesylate |
| ↓ | ↓ | ↓ |
| Blood counts weekly | Blood counts weekly | Blood counts weekly |
| ↓ | ↓ | ↓ |
| Start next Ara-C cycle when | Start next Ara-C cycle | Start imatinib mesylate when |
| Plt count > 100 × 109/L and ANC > 1.5 × 109/L | 2 weeks after | Plt count > 100 × 109/L and ANC > 1.5 × 109/L |
| Plt count > 100 × 109/L and ANC > 1.5 × 109/L | Start next Ara-C cycle | |
| at a 50% dose reduction | 2 weeks later at a 50% dose reduction | |
| (to 10 mg/m2 with first occurrence, then 5 mg/m2) | (to 10 mg/m2 with first occurrence, then 5 mg/m2) |
For Plt count < 100 × 109/L or ANC < 1.5 × 109/L | For Plt count < 75 × 109/L or ANC < 1.25 × 109/L | For Plt count < 50 × 109/L or ANC < 1.0 × 109/L |
| ↓ | ↓ | ↓ |
| Hold Ara-C | Hold Ara-C | Hold Ara-C |
| Continue imatinib mesylate | Continue imatinib mesylate | Hold imatinib mesylate |
| ↓ | ↓ | ↓ |
| Blood counts weekly | Blood counts weekly | Blood counts weekly |
| ↓ | ↓ | ↓ |
| Start next Ara-C cycle when | Start next Ara-C cycle | Start imatinib mesylate when |
| Plt count > 100 × 109/L and ANC > 1.5 × 109/L | 2 weeks after | Plt count > 100 × 109/L and ANC > 1.5 × 109/L |
| Plt count > 100 × 109/L and ANC > 1.5 × 109/L | Start next Ara-C cycle | |
| at a 50% dose reduction | 2 weeks later at a 50% dose reduction | |
| (to 10 mg/m2 with first occurrence, then 5 mg/m2) | (to 10 mg/m2 with first occurrence, then 5 mg/m2) |
If Ara-C dose of 5 mg/m2 was unable to be administered, then imatinib mesylate was reduced to 300 mg/d. If Ara-C was unable to be administered at this dose, then imatinib mesylate was given at a dose of 300 mg/d and Ara-C was discontinued permanently.
Plt indicates platelets; ANC, absolute neutrophil count.
Dose modifications for nonhematologic toxicities
Grade 2 . | Grade 3/4 . |
|---|---|
| Interrupt treatment until recovery (≤ grade 1) | Interrupt treatment until recovery (≤ grade 1) |
| ↓ | ↓ |
| Resume at same doses | Resume full dose imatinib mesylate |
| ↓ | Reduce Ara-C to 10 mg/m2 |
| If grade 2 recurs | ↓ |
| ↓ | If grade 3/4 recurs |
| Interrupt treatment until recovery (≤ grade 1) | ↓ |
| ↓ | Resume full dose imatinib mesylate reduce Ara-C to 5 mg/m2 |
| Resume full dose imatinib mesylate | |
| Reduce Ara-C to 10 mg/m2 | ↓ |
| ↓ | If grade 3/4 recurs |
| If grade 2 recurs | ↓ |
| ↓ | Interrupt treatment until recovery (≤ grade 1) |
| Interrupt treatment until recovery (≤ grade 1) | ↓ |
| ↓ | Resume full dose imatinib mesylate |
| Resume full dose imatinib mesylate | STOP Ara-C |
| Reduce Ara-C to 5 mg/m2 | ↓ |
| ↓ | If grade 3/4 recurs |
| If grade 2 recurs | ↓ |
| ↓ | Interrupt treatment until recovery (≤ grade 1) |
| Interrupt treatment until recovery (≤ grade 1) | ↓ |
| ↓ | Reduce imatinib mesylate to 300 mg |
| Reduce dose imatinib mesylate to 300 mg | ↓ |
| Reduce Ara-C to 5 mg/m2 | If grade 3/4 recurs |
| ↓ | ↓ |
| If grade 2 recurs | Interrupt treatment until recovery (≤ grade 1) |
| ↓ | ↓ |
| Interrupt treatment until recovery (≤ grade 1) | Contact principal investigator to discuss further reduction of imatinib mesylate dose or discontinuation of all study drugs |
| ↓ | |
| Resume imatinib mesylate at 300 mg | |
| STOP Ara-C | |
| ↓ | |
| If grade 2 recurs | |
| ↓ | |
| Interrupt treatment until recovery (≤ grade 1) | |
| ↓ | |
| Contact principal investigator to discuss further reduction of imatinib mesylate dose or discontinuation of all study drugs |
Grade 2 . | Grade 3/4 . |
|---|---|
| Interrupt treatment until recovery (≤ grade 1) | Interrupt treatment until recovery (≤ grade 1) |
| ↓ | ↓ |
| Resume at same doses | Resume full dose imatinib mesylate |
| ↓ | Reduce Ara-C to 10 mg/m2 |
| If grade 2 recurs | ↓ |
| ↓ | If grade 3/4 recurs |
| Interrupt treatment until recovery (≤ grade 1) | ↓ |
| ↓ | Resume full dose imatinib mesylate reduce Ara-C to 5 mg/m2 |
| Resume full dose imatinib mesylate | |
| Reduce Ara-C to 10 mg/m2 | ↓ |
| ↓ | If grade 3/4 recurs |
| If grade 2 recurs | ↓ |
| ↓ | Interrupt treatment until recovery (≤ grade 1) |
| Interrupt treatment until recovery (≤ grade 1) | ↓ |
| ↓ | Resume full dose imatinib mesylate |
| Resume full dose imatinib mesylate | STOP Ara-C |
| Reduce Ara-C to 5 mg/m2 | ↓ |
| ↓ | If grade 3/4 recurs |
| If grade 2 recurs | ↓ |
| ↓ | Interrupt treatment until recovery (≤ grade 1) |
| Interrupt treatment until recovery (≤ grade 1) | ↓ |
| ↓ | Reduce imatinib mesylate to 300 mg |
| Reduce dose imatinib mesylate to 300 mg | ↓ |
| Reduce Ara-C to 5 mg/m2 | If grade 3/4 recurs |
| ↓ | ↓ |
| If grade 2 recurs | Interrupt treatment until recovery (≤ grade 1) |
| ↓ | ↓ |
| Interrupt treatment until recovery (≤ grade 1) | Contact principal investigator to discuss further reduction of imatinib mesylate dose or discontinuation of all study drugs |
| ↓ | |
| Resume imatinib mesylate at 300 mg | |
| STOP Ara-C | |
| ↓ | |
| If grade 2 recurs | |
| ↓ | |
| Interrupt treatment until recovery (≤ grade 1) | |
| ↓ | |
| Contact principal investigator to discuss further reduction of imatinib mesylate dose or discontinuation of all study drugs |
Cytarabine would be discontinued in patients who achieved a CCR that was confirmed on 2 successive bone marrow samples. Discontinuation of imatinib mesylate would be discussed if a complete molecular remission (ie, reverse transcription-polymerase chain reaction [RT-PCR] negativity) was achieved and maintained for 2 years.
Initial assessment and monitoring of the patients
The diagnosis of Ph+ chronic phase CML was confirmed in all patients clinically and biologically. Bone marrow aspiration was performed for cytogenetic or fluorescence in situ hybridization (FISH) and cytologic examination. FISH analysis used the Vysis BCR-ABL ES probe and was performed on 200 interphases. A complete blood count and a differential blood count were obtained weekly for the first 4 weeks and weekly for each cycle during which the 2 drugs were administered. Assessment at the end of the 3rd cycle, 6th cycle, 9th cycle, and 12th cycle included full clinical and laboratory evaluation including bone marrow cytogenetic analysis. Patients were then assessed every 4 months. Side effects were defined and graded according to World Health Organization (WHO) toxicity criteria.17
A CHR was defined as normalization of white blood cell (WBC) counts to less than 10 × 109/L with normal differential, normalization of platelet counts to less than 350 × 109/L, and disappearance of all signs and symptoms of the disease, for more than 4 weeks. Duration of CHR was defined as the time from first documentation of the CHR to the date of the loss of CHR. Cytogenetic response in terms of the percentage of Ph chromosome-positive metaphases in bone marrow was defined as follows (a minimum of 20 metaphases was required for analysis): complete (0% Ph+ cells), partial (1%-34%), minor (35%-94%), and none (95%-100%). Major cytogenetic response (MCR) included complete and partial cytogenetic responses. Duration of MCR was defined as the time from first documentation of the response to the date of the loss of such response. At diagnosis the Bcr-Abl transcript was determined by conventional PCR. Molecular response was assessed using real-time quantitative competitive PCR assays (TaqMan or LightCycler methodology) in blood samples collected at months 3, 6, 9, 12.18 Twenty-five patients were sequentially analyzed for minimal residual disease by qualitative or quantitative real-time RT-PCR in the blood or marrow of patients. In most laboratories total ABL and glucose-6-phosphate dehydrogenase (G6PD) transcripts were quantified as internal controls and results were expressed as the ratios BCR-ABL/ABL and BCR-ABL/G6PD, whereas other laboratories used the K562 cell line as the internal control.
Statistics
This phase 2 nonrandomized multicenter trial sought to evaluate the efficacy and tolerability of a combination of imatinib mesylate and intermittent subcutaneous administration of low-dose cytarabine. Thirty patients were included and observed for tolerability. The designated efficacy end points were the 6-month hematologic response rate and the 6- and 12-month MCR and CCR rates. Based on historical data, efficacy would be considered as acceptable if the 95% confidence interval (CI) of the CHR rate and MCR rate at 6 months contained 85% and 50%, respectively.19 Time to response was estimated by the cumulative incidence method. Analyses were conducted in an intention to treat basis; at the time of analysis no patients were lost to follow-up or censored. All statistical tests of significance were 2-tailed with a type I error of 0.05. Analyses were performed using SAS statistical software (SAS Institute, Cary, NC).
Results
Patients
From June to August 2001, 30 patients with newly diagnosed CML in chronic phase were recruited by 14 French centers. Main clinical and biologic characteristics are summarized in Table 3.
Clinical and biologic characteristics at diagnosis
Variables . | No. of patients (%) . | Mean (± SD) . | Median (range) . |
|---|---|---|---|
| Age, y | 48.00 (22-81) | ||
| Sex | |||
| Male | 20 (67) | ||
| Female | 10 (33) | ||
| Sokal risk group | |||
| Low | 11 (37) | ||
| Intermediate | 14 (47) | ||
| High | 5 (16) | ||
| Euro risk group | |||
| Low | 17 (57) | ||
| Intermediate | 12 (40) | ||
| High | 1 (3) | ||
| Spleen size, cm below costal margin | |||
| Normal | 17 (57) | 5.85 (± 3.70) | 6.00 (1-12) |
| Abnormal | 13 (43) | ||
| Hemoglobin, g/100 mL | 30 | 12.26 (± 2.18) | 12.85 (9.10-16.20) |
| Platelet count, × 109/L | 30 | 480.20 (± 321.97) | 433.00 (96.00-1499.00) |
| WBC count, × 109/L | 30 | 159.76 (± 238.43) | 66.05 (6.90-470.00) |
| Immature cells | |||
| Fewer than 5 | 4 (13) | — | — |
| 5 or more | 26 (87) | 27.80 (± 14.32) | 24.00 (6.00-58.00) |
| Blood blasts | |||
| 0% | 15 (50) | — | — |
| More than 0% | 15 (50) | 3.73 (± 3.04) | 3.00 (1.00-11.00) |
| Marrow blasts | |||
| 0% | 3 (10) | — | — |
| More than 0% | 17 (90) | 2.35 (± 1.63) | 2.00 (1.00-5.00) |
| Chromosomal anomalies | 6 (20) | ||
| Trisomy 8 | 1 (3) | ||
| Variant translocation involving chromosomes 7 and 20 | 2 (7) | ||
| Additional translocation (t9;14) | 1 (3) | ||
| Klinefelter syndrome | 1 (3) | ||
| Marked chromosome | 1 (3) |
Variables . | No. of patients (%) . | Mean (± SD) . | Median (range) . |
|---|---|---|---|
| Age, y | 48.00 (22-81) | ||
| Sex | |||
| Male | 20 (67) | ||
| Female | 10 (33) | ||
| Sokal risk group | |||
| Low | 11 (37) | ||
| Intermediate | 14 (47) | ||
| High | 5 (16) | ||
| Euro risk group | |||
| Low | 17 (57) | ||
| Intermediate | 12 (40) | ||
| High | 1 (3) | ||
| Spleen size, cm below costal margin | |||
| Normal | 17 (57) | 5.85 (± 3.70) | 6.00 (1-12) |
| Abnormal | 13 (43) | ||
| Hemoglobin, g/100 mL | 30 | 12.26 (± 2.18) | 12.85 (9.10-16.20) |
| Platelet count, × 109/L | 30 | 480.20 (± 321.97) | 433.00 (96.00-1499.00) |
| WBC count, × 109/L | 30 | 159.76 (± 238.43) | 66.05 (6.90-470.00) |
| Immature cells | |||
| Fewer than 5 | 4 (13) | — | — |
| 5 or more | 26 (87) | 27.80 (± 14.32) | 24.00 (6.00-58.00) |
| Blood blasts | |||
| 0% | 15 (50) | — | — |
| More than 0% | 15 (50) | 3.73 (± 3.04) | 3.00 (1.00-11.00) |
| Marrow blasts | |||
| 0% | 3 (10) | — | — |
| More than 0% | 17 (90) | 2.35 (± 1.63) | 2.00 (1.00-5.00) |
| Chromosomal anomalies | 6 (20) | ||
| Trisomy 8 | 1 (3) | ||
| Variant translocation involving chromosomes 7 and 20 | 2 (7) | ||
| Additional translocation (t9;14) | 1 (3) | ||
| Klinefelter syndrome | 1 (3) | ||
| Marked chromosome | 1 (3) |
Median time from diagnosis to imatinib mesylate therapy was 2.5 months (range, 2 weeks to 7 months). Hydroxyurea was administered according to the design to 28 patients for a median period of 2 months (range, 2 weeks to 5 months). Among them 2 patients were already in CHR at time of imatinib mesylate therapy. The European Cooperative Oncology Group (ECOG) performance status was grade 0 for 26 patients (87%) and grade 1 for 4 patients (14%).
Safety and tolerability
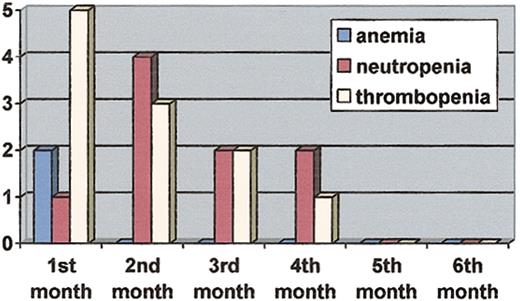
Grade 1 to 4 hematologic and nonhematologic adverse events are listed in Table 4. Grade 3 or 4 hematologic toxicities occurred in 53% (n = 16) of patients. Two patients had grade 3 anemia. Grade 3 or 4 neutropenia occurred in 8 patients (27%) and grade 3 or 4 thrombocytopenia in 11 patients (37%). No bleeding episodes or life-threatening infections were recorded during the trial. The median time to a first grade 3 or 4 episode of neutropenia was 51 days (range, 27-96 days) after the first administration of imatinib mesylate. The median time to a first episode of grade 3 or 4 thrombocytopenia was 32 days (range, 19-93 days). The median duration of grade 3 or 4 neutropenia was 8 days (range, 2-17 days) and the median duration of grade 3 or 4 thrombocytopenia was 6 days (range, 3-9 days). Five patients experienced more than one episode of severe neutropenia and 3 patients more than one episode of severe thrombocytopenia. Grade 3 or 4 neutropenia and thrombocytopenia were mainly observed during the first 2 months of the trial (Figure 1).
Adverse events
. | All grades, % (no.) . | Grades 3-4, % (no.) . |
|---|---|---|
| Nonhematologic toxicity* | ||
| Nausea | 83 (25) | 0 (0) |
| Asthenia | 70 (21) | 7 (2) |
| Vomiting | 63 (19) | 13 (4) |
| Abdominal pain | 53 (18) | 10 (3) |
| Musculoskeletal pain | 50 (15) | 0 (0) |
| Superficial edema | 50 (15) | 0 (0) |
| Diarrhea | 40 (12) | 0 (0) |
| Muscle cramps | 37 (11) | 0 (0) |
| Headache | 30 (9) | 0 (0) |
| Nasopharyngitis | 27 (8) | 0 (0) |
| Skin rash | 23 (7) | 0 (0) |
| Anorexia | 13 (4) | 0 (0) |
| Pruritus | 13 (4) | 0 (0) |
| Mucositis | 13 (4) | 0 (0) |
| Neurologic symptoms | 3 (1) | 0 (0) |
| Hematologic toxicity† | ||
| Anemia | 10 (3) | 7 (2) |
| Neutropenia | 53 (16) | 27 (8) |
| Thrombocytopenia | 50 (15) | 37 (11) |
. | All grades, % (no.) . | Grades 3-4, % (no.) . |
|---|---|---|
| Nonhematologic toxicity* | ||
| Nausea | 83 (25) | 0 (0) |
| Asthenia | 70 (21) | 7 (2) |
| Vomiting | 63 (19) | 13 (4) |
| Abdominal pain | 53 (18) | 10 (3) |
| Musculoskeletal pain | 50 (15) | 0 (0) |
| Superficial edema | 50 (15) | 0 (0) |
| Diarrhea | 40 (12) | 0 (0) |
| Muscle cramps | 37 (11) | 0 (0) |
| Headache | 30 (9) | 0 (0) |
| Nasopharyngitis | 27 (8) | 0 (0) |
| Skin rash | 23 (7) | 0 (0) |
| Anorexia | 13 (4) | 0 (0) |
| Pruritus | 13 (4) | 0 (0) |
| Mucositis | 13 (4) | 0 (0) |
| Neurologic symptoms | 3 (1) | 0 (0) |
| Hematologic toxicity† | ||
| Anemia | 10 (3) | 7 (2) |
| Neutropenia | 53 (16) | 27 (8) |
| Thrombocytopenia | 50 (15) | 37 (11) |
The total number of side effects exceeds the total number of patients because several side effects occurred in the same patients.
The total number of patients who experienced a nonhematologic grade 3 or 4 toxicity was 7.
The total number of patients who experienced a hematologic grade 3 or 4 toxicity was 16.
Number of patients who experienced a first episode of grade 3 or 4 hematologic toxicities during the first 6 months.
Number of patients who experienced a first episode of grade 3 or 4 hematologic toxicities during the first 6 months.
Granulocyte colony-stimulating factor (G-CSF) was not used during the trial; however, one patient received recombinant erythropoietin. This patient had experienced a grade 3 anemia and neutropenia, which resulted in cytarabine interruption after 11 days of cytarabine during cycle 1. Erythropoietin administered for 2 months resulted in normalization of the hemoglobin level. Cytarabine was resumed at the reduced dose of 10 mg/m2/d. Subsequently, 2 bone marrow aspirates at 3-month intervals showed a CCR leading to a permanent cessation of cytarabine according to the design. Another patient experienced a grade 2 anemia during cycle 2 and a grade 3 thrombocytopenia and neutropenia at cycle 3. Cytarabine was interrupted and then resumed at the reduced dose of 10 mg/m2. However, he permanently stopped at cycle 4 because of neutropenia and thrombocytopenia recurrence of grade 4, which required 2 red cell transfusions and one platelet transfusion. Still, a CCR was achieved at 12 months.
Grade 3 or 4 nonhematologic toxicities occurred in 23% (n = 7) of patients and consisted of abdominal pain (n = 3), vomiting or diarrhea (n = 4), and asthenia (n = 2).
Grade 1 to 4 frequent toxicities consisted of nausea (n = 25 patients), asthenia (n = 21), vomiting (n = 19), abdominal pain (n = 18), musculoskeletal pain (n = 15), fluid retention (n = 15), muscle cramps (n = 11), and skin rash (n = 7). Other adverse events are listed in Table 4. Adverse event such as mucositis, abdominal pain, and vomiting were considered in relationship with cytarabine, whereas other side effects were commonly those recently described with imatinib mesylate such as fluid retention or muscle cramps. Of note all patients were treated on an outpatient basis.
Doses and duration of treatment
All 30 patients started cycles of cytarabine. Of the 30 patients, 21 stopped cytarabine as per protocol, in 13 patients because of a sustained CCR (for these patients the median number of cycles with cytarabine was 6; range, 3-11 cycles). Other reasons for stopping cytarabine were hematologic toxicity in 4 patients (after 2, 3, 4, and 6 cycles of cytarabine, respectively) or nonhematologic toxicity in 4 additional patients (after 3, 3, 4, and 6 cycles of cytarabine, respectively). These toxicities were gastrointestinal in 3 patients and neurologic in one patient.
At the time of analysis (September 2002), 9 patients were still receiving both treatments with the median number of cycles of 10 (range, 7-14 cycles).
The median duration of time during which the 2 drugs were simultaneously administered to 30 patients was 8 months (range, 2-13 months). During this period the mean (± SD) dose of imatinib mesylate was 363 ± 52 mg (range, 167-400 mg), including 21 patients who temporarily stopped imatinib mesylate for a median duration of 12 days (range, 1-71 days). Of these 21 patients, 2 stopped imatinib, one for personal reasons and thereafter then went back to 400 mg and the other because of progression, but also went back to treatment at the dose of 400 mg after a course of high-dose chemotherapy. The remaining 18 patients had transient interruption of their treatment according to the rules of the trial at cycles 2 and 3. Of these, only 3 patients resumed imatinib at a dose of 300 mg, 1 during cycle 3 who then went back at 400 mg; the other 2 patients resumed imatinib at cycle 2 at a permanent dose of 300 mg. All other patients resumed imatinib after cycle 3 at a permanent dose of 400 mg. During the same period, the median number of cycles of cytarabine was 6 (range, 2-14 cycles) and the percentage of the planned dose of cytarabine ranged from 34% to 106% (median, 67%). The mean total dose (± SD) of cytarabine per day was 25 ± 9 mg (range, 11-40 mg) and the mean dose per m2/d was 13.5 mg. More than 50% of the planned dose of cytarabine was administered to 30 patients at cycle 1, 15 patients at cycle 3, 11 patients at cycles 6 and 9, and 6 patients at cycle 12, respectively.
During maintenance therapy without cytarabine the mean (± SD) daily dose of imatinib mesylate was 383 ± 59 mg (range, 125-400 mg). All 30 patients are still receiving imatinib mesylate.
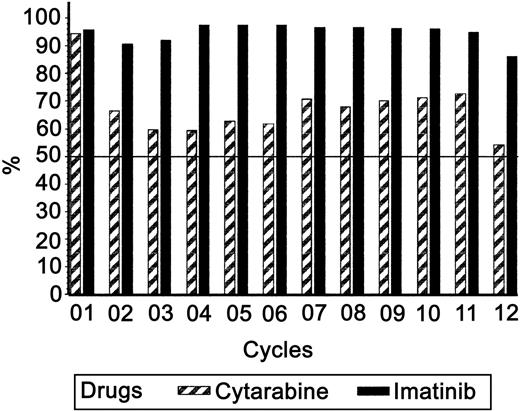
Overall, the addition of cytarabine to imatinib mesylate did not result in significant dose reduction for imatinib mesylate. As described in Figure 2 the mean dose of imatinib mesylate that the patients received is more than 90% of the scheduled dose during the first 11 cycles of the combination therapy.
Percentage of the expected drug dosage received by the patients for the first 12 months of treatment.
Percentage of the expected drug dosage received by the patients for the first 12 months of treatment.
Efficacy
The median duration of follow-up is 12 months (range, 12-13 months). All patients achieved CHR after a median time of treatment of 4 weeks (range, 0-12 weeks; Table 5). One patient progressed to myeloid blast crisis at 11 months despite achieving CCR at 9 months. At the time of relapse, a mutation (T315) was detected in the tyrosine kinase domain of the adenosine triphosphate (ATP)-binding site of Abl, which might explain this rapid progression. A second chronic phase was obtained after a course of high-dose cytarabine and daunorubicin. This patient is receiving imatinib alone in maintenance. The other 29 patients are in continuous CHR.
Hematologic and cytogenetic responses
. | 3 mo . | 6 mo . | 9 mo . | 12 mo . |
|---|---|---|---|---|
| No. of patients at risk | 30 | 30 | 30 | 30 |
| Complete hematologic response (95% CI) | 30* | 30 | 30 | 29 |
| 100% (88-100) | 100% (88-100) | 100% (88-100) | 97% (83-100) | |
| Cytogenetic response | ||||
| Major | 21 | 22 | 23 | 25 |
| 70% (51-85) | 73% (54-88) | 77% (58-90) | 83% (65-94) | |
| Complete | 7 | 17 | 16 | 21 |
| 23% (10-42) | 57% (37-75) | 53% (34-72) | 70% (51-85) | |
| Partial | 14 | 5 | 7 | 4 |
| 47% (28-66) | 17% (10-42) | 23% (10-42) | 13% (4-31) | |
| Minor | 2 | 3 | 2 | 2 |
| Failure | 2 | 2 | 1 | 1 |
| Not assessable† | 5 | 3 | 4 | 2 |
. | 3 mo . | 6 mo . | 9 mo . | 12 mo . |
|---|---|---|---|---|
| No. of patients at risk | 30 | 30 | 30 | 30 |
| Complete hematologic response (95% CI) | 30* | 30 | 30 | 29 |
| 100% (88-100) | 100% (88-100) | 100% (88-100) | 97% (83-100) | |
| Cytogenetic response | ||||
| Major | 21 | 22 | 23 | 25 |
| 70% (51-85) | 73% (54-88) | 77% (58-90) | 83% (65-94) | |
| Complete | 7 | 17 | 16 | 21 |
| 23% (10-42) | 57% (37-75) | 53% (34-72) | 70% (51-85) | |
| Partial | 14 | 5 | 7 | 4 |
| 47% (28-66) | 17% (10-42) | 23% (10-42) | 13% (4-31) | |
| Minor | 2 | 3 | 2 | 2 |
| Failure | 2 | 2 | 1 | 1 |
| Not assessable† | 5 | 3 | 4 | 2 |
Among the 30 patients, 2 were already in CHR at study treatment.
Not assessable patients: 12 for technical failure; 2 not done.
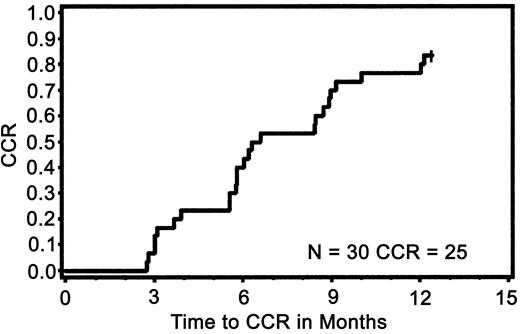
At 6 months MCR was achieved in 73% (95% CI, 54%-88%) of the patients (n = 22). The median period of time for achieving MCR was 3 months (range, 3-12 months). At 6 months, CCR was achieved in 57% of the patients (95% CI, 37%-75%). At 12 months, 83% (95% CI, 65%-94%) of the patients had MCR. At 12 months, CCR was achieved in 70% (95% CI, 51%-85%) of the patients (n = 21). The median period for achieving CCR was 6 months (range, 3-12 months). At 12 months the cumulative incidences of CCR (n = 25) and MCR (n = 27) were 83% (95% CI, 70%-97%) and 90% (95%CI, 79%-100%), respectively (Figure 3).
Cumulative incidence of complete cytogenetic response during the first 12 months of treatment.
Cumulative incidence of complete cytogenetic response during the first 12 months of treatment.
As mentioned, one patient had a cytogenetic relapse after achieving CCR and progressed to blast phase. One other patient had a cytogenetic relapse at 12 months (9% Ph+ cells). The other 21 patients are still in CCR at the last cytogenetic examination.
The outcome of 6 patients with unusual cytogenetic abnormalities is summarized in Table 6. The 2 cases with a variant translocation involving a third chromosome (7 or 20) achieved rapid CHR and were in sustained CCR at 12 months. The patient with the complex karyotype t (9;22) t(9;14) had no response after 1 year of treatment. One other patient lost his CCR with 9% Ph+ cells at 12 months. Of interest this patient had 3% metaphases with trisomy 8 at diagnosis.
Cytogenetic evolution of patients with unusual karyotypic abnormalities
UPN . | At diagnosis . | Mo 3 . | Mo 6 . | Mo 9 . | Mo 12 . |
|---|---|---|---|---|---|
| 423 | t(7,9,22): 100% | 5% | 0% | ND | 0% |
| 925 | t(9,20,22): 100% | 16% | 0% | 2% | 0% |
| 102 | +8: 3% Ph: 100% | 10% | 6% | 0% | 9% |
| 315 | t(9,22)t(9,14) 100% | NE | 100% | NE | 86% |
| 1504 | Masked Ph: FISH 98% | FISH 31% | NE | FISH 4% | FISH 5.7% |
| 1212 | Klinefelter 100% | NE | NE | FISH 5% | FISH 0% |
UPN . | At diagnosis . | Mo 3 . | Mo 6 . | Mo 9 . | Mo 12 . |
|---|---|---|---|---|---|
| 423 | t(7,9,22): 100% | 5% | 0% | ND | 0% |
| 925 | t(9,20,22): 100% | 16% | 0% | 2% | 0% |
| 102 | +8: 3% Ph: 100% | 10% | 6% | 0% | 9% |
| 315 | t(9,22)t(9,14) 100% | NE | 100% | NE | 86% |
| 1504 | Masked Ph: FISH 98% | FISH 31% | NE | FISH 4% | FISH 5.7% |
| 1212 | Klinefelter 100% | NE | NE | FISH 5% | FISH 0% |
UPN indicates unique patient number; ND, not determined; NE, not evaluable; and %, percent of Ph+ cells.
Finally, 1 patient, while achieving a CCR, had monosomy 7 in 19 of 21 mitoses with the bone marrow feature of myelodysplastic syndrome. No other additional cytogenetic abnormalities were recorded during the trial.
Of the 15 patients assessed using quantitative real-time RT-PCR, 10 had reduction of their minimal residual disease to less than 0.1% at month 9 or 12 and 2 patients had at least 2 consecutive PCRs with undetectable transcripts and are in continuous CCR after 9 months of treatment. These 12 patients are in CCR. The 3 patients with a PCR still more than 0.1% are Ph+ after 12 months of treatment (7%, 87%, 100% Ph+).
Discussion
Imatinib mesylate has emerged as a very powerful drug for Ph+ leukemias. When treated with imatinib alone, 95% of the patients achieved CHR and CCRs have been recorded in 41% of patients previously treated with IFN-α.19 In this group of pretreated patients, 10% had disease progression within 18 months, suggesting that a single drug might not be sufficient to totally eliminate the leukemic stem cells. Several mechanisms of resistance have been recently described, which might explain clinical relapse or progression. Kinase domain mutations, which modify the structure of the ATP-binding region of the protein, are the most frequently identified mechanisms associated with treatment failure.20-24 Thus, new strategies using a combination of cytotoxic drugs should be investigated to overcome at least some forms of cell resistance.
This phase 2 trial aimed to assess the safety and tolerability of combination therapy with imatinib mesylate and low-dose cytarabine. All the patients were in chronic phase and only a short period of pretreatment with hydroxyurea was permitted. All the patients received imatinib mesylate at the standard dose of 400 mg and cytarabine for a period of 14 out of 28 days. The rationale for the combination of imatinib mesylate with cytarabine was based on previous in vitro studies, suggesting synergistic antiproliferative effects of this combination.14 The dose and duration of cytarabine were selected on the basis of previous phase 1/2 studies.16 In the trial initiated by Druker and coworkers, imatinib mesylate was given daily and cytarabine was administered on days 14 to 28 with cycles repeated every 28 days.16 Three cohorts received imatinib mesylate at a dose of 400 mg with 3 different doses of cytarabine (5, 10, 20 mg/m2), and one cohort at a dose of 600 mg imatinib mesylate in combination with cytarabine at a dose of 20 mg/m2. In this study, the maximally tolerated dose was 400 mg imatinib mesylate daily with 20 mg/m2 cytarabine given for 2 of every 4 weeks. Myelosuppression at the highest dose level necessitated dose reductions in all patients such that no patient remained on treatment with 600 mg plus 20 mg/m2 cytarabine. Grade 3/4 hematologic toxicities occurred in 68% of patients. In our trial a rate of hematologic toxicities of 53% was recorded. Of note, toxicities in our trial were observed during the first 2 months of treatment and less frequently thereafter. Other nonhematologic toxicities in the U.S. trial included nausea (77%, grade 1/2; 14%, grade 3/4), diarrhea (36%, grade 1/2), fluid retention (73%, grade 1/2), and skin rashes (45%, grade 1/2; 9%, grade 3/4). Grade 3/4 fatigue and elevations in transaminase levels were seen in 2 of 22 patients (9%). In our trial, 27% of patients had nonhematologic toxicities of grade 3/4 consisting mainly of gut disorders. Overall rates of hematologic and nonhematologic toxicities were similar in our trial compared with the U.S. trial. However, our trial included patients with recently diagnosed CML, even though in the U.S. trial, patients enrolled in the study were previously treated with chemotherapy and IFN.
In our trial, the rates of grade 3 or 4 hematologic as well as nonhematologic toxicities were higher than previously described with imatinib mesylate alone. Adverse events in recently diagnosed patients treated with imatinib mesylate alone included grade 3/4 muscle cramps in 1.3%, abdominal pain in 2.4%, and vomiting in 1.5% of patients.4 Hematologic toxicities were less frequent with imatinib mesylate alone with anemia in 3.3%, neutropenia in 14.3%, and thrombocytopenia in 7.8% of patients compared with our combination. Thus, using combination therapies including cytarabine and imatinib mesylate requires closer complete blood count assessments.
It is widely accepted that treatment with IFN-α can induce a complete cytogenetic remission in 10% to 30% of cases. Patients who sustain a CCR are likely to survive longer. In a European collaborative study of 317 patients who became complete cytogenetic responders, the projected 10-year survival was 72% with 75% of the surviving patients still in CCR.1 Thus, cytogenetic response in CML is now considered as an important surrogate marker. It has been suggested that a trial that produces a high rate of response would be associated with a better survival. Thus, one of the objectives of our trial was also to demonstrate a high rate of response.
It is now clear that the cytogenetic response rate is significantly higher with imatinib mesylate compared with regimens based on IFN-α. This conclusion is based on the results of 2 international trials. In the first study, a phase 2 trial, CCR occurred at a rate of 41% in patients in whom previous IFN-α therapy had failed.19 In the second, more recent phase 3 trial comparing IFN-α plus cytarabine versus imatinib mesylate in previously untreated chronic phase patients (International Randomized Study of Interferon), the estimated rate of MCR at 18 months was 87.1% in the imatinib arm and 34.7% in the IFN-cytarabine arm.5 Despite these high rates of major or complete cytogenetic response with imatinib mesylate, data are too preliminary to draw conclusions on the durability of the response and impact on survival. In our trial, all patients achieved CHR and CCR was recorded in the majority of patients. These results are similar to those obtained with imatinib alone. However, large prospective randomized phase 3 trials are planned to adequately compare these 2 therapeutic approaches.
In some patients, resistance has been associated with a single amino acid substitution in a threonine residue of the Abl kinase domain.22 Further, other mutations were described by several groups.23,24 It was demonstrated that 3 mutations have a predictive role in abrogating imatinib mesylate binding (T315I, Y253H, F317L), whereas 3 other mutations do not (E255K, G250E, M351T). Using sensitive allele-specific oligonucleotide PCR assays, Roche-Lestienne et al demonstrated the presence of mutations prior to imatinib mesylate therapy.
Although most of the patients who experienced such resistance were patients in late chronic phase or patients who had been previously treated for myeloid or lymphoid blastic phase, this resistance might also occur in patients with recently diagnosed CML in chronic phase.24 Moreover, using a specific allele-specific oligonucleotide (ASO)-PCR on DNA for the T315L mutation, it was shown that rare mutated cells could be detected in the blood prior to imatinib mesylate therapy. In our trial, one of the patients who initially achieved a CCR subsequently had a relapse and progressed to a blastic phase. A T315I mutation was detected. This suggests that the combination of imatinib mesylate and cytarabine was not able to prevent expansion of mutated cells. Thus, for young patients, allogeneic bone marrow transplantation remains a treatment of choice for those with CML in chronic phase who are resistant to imatinib mesylate.
Recently, a population of primitive quiescent stem cells has been described from peripheral blood or bone marrow.6 These cells are Ph+, express high levels of CD34+ and can spontaneously exit G0 to enter a continuously proliferating state. In a series of experiments it was shown that in some patients a proportion of Ph+ CD34+ quiescent cells was highly insensitive to imatinib mesylate. In this in vitro study, analysis by FISH did not reveal gene amplification and drug efflux could be a reasonable explanation. These results raise again the critical question of cell dormancy. These cells might also enter in cell division. In this scenario, mutation would confer a growth advantage to the cells leading to clinical relapse.
Molecular responses were achieved in a reasonable number of our patients. However, the follow-up period is too short and a longer period is necessary to compare the molecular response achieved with the combination and the use of imatinib mesylate alone. Very high response rates are being achieved with the most recent therapeutic strategies; however, considerations mentioned in “Introduction” may suggest that combining drugs with distinct mechanisms of action could be useful to achieve evenly higher rates of sustained cytogenetic and molecular remission. Cytotoxic agents such as IFN or cytarabine or small molecules that target Ras administered in combination with imatinib mesylate might be synergistic in CML.26 Strategies for overcoming resistance are currently under study through exploitation of other molecular features of the BCR-ABL protein, such as its dependence on the molecular chaperone heat shock protein 90 (Hsp90), which affects the stability and function of the p210BCR-ABL.27 Our preliminary results using a combination of imatinib mesylate and cytarabine are encouraging. However, it is currently uncertain whether this combination will result in less cell resistance, more sustained cytogenetic response, and overall better survival.
Thus, combination of imatinib mesylate and cytarabine should be compared with imatinib mesylate alone at a standard or higher dosage28 or imatinib mesylate in combination with IFN in large phase 3 trials.
Appendix
Members of the the CML French Group (FIϕLMC): J. Reiffers, P. Cony-Makhoul, G. Marit, Pessac; A. P. Guerci, Vandoeuvre Les Nancy; C. Pautas-Chambon, Creteil; J. L. Harousseau, Nantes; R. Bouabdallah, Marseille; D. Guyotat, Saint Etienne; J. Briere, Clichy; and A. Najman and B. Rio, Paris.
Supported by grants from the Programme Hospitalier de Recherche Clinique and Novartis Pharma, France. These data were presented in part at the 2002 Annual Meeting of the American Society of Hematology (Philadelphia, PA, December 2002).
Prepublished online as Blood First Edition Paper, August 21, 2003; DOI 10.1182/blood-2003-04-1010.
M.G. and P.R. contributed equally to the study.
A complete list of the members of the CML French Group (FIϕLMC) appears in the “Appendix.”
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are indebted to Cécile Vignaud-Demer for technical assistance and to the clinical trial monitors, Vincent Bonnarme, Farid Guetarni, Alexandre Bernard, and Laurent Magaud for their contribution.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal