We read with interest Soverini et al's recent paper reporting that overexpression of cyclin D1 is a favorable prognostic factor for multiple myeloma (MM) patients.1 The authors used a real-time reverse transcription-polymerase chain reaction (RT-PCR) assay to quantify the expression of cyclin D1 in bone marrow samples. They concluded that patients with t(11;14)(q13;q32) or trisomy 11 significantly overexpressed cyclin D1 compared with patients without chromosome 11 abnormalities or healthy controls. These results are in good agreement with our previous studies and confirm that almost 50% of MM patients exhibit cyclin D1 expression and that t(11;14) as a CCND1 gene activation mechanism occurs in only half of the cases.2 Interestingly, the subset of patients with cyclin D1 expression has a prolonged duration of remission after autologous transplantation, confirming and completing previous results on the better survival and response to treatment of patients harboring t(11;14).3,4
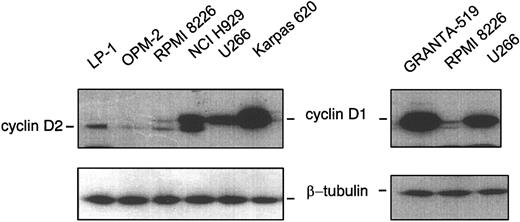
Of concern, however, is the claim that patients with trisomy 11 overexpressed cyclin D1. Although the authors unambiguously demonstrated the sensitivity and the reliability of the real-time RT-PCR technique, it was realized with RNA extracted from mononuclear cells obtained after Ficoll-Hypaque separation and not on a purified population of homogenous myeloma cells. We have set a real-time quantitative RT-PCR on myeloma cell lines displaying either t(11;14) (Karpas 620), a “switch” insertion (U266), trisomy 11 (NCI H929 and RPMI 8226), or no chromosome 11q abnormalities (LP1 and OPM-2), all described previously.2 Used as negative and positive controls were 2 other cell lines: Ramos, a Burkitt lymphoma without chromosome 11q abnormalities, and GRANTA-519, a mantle cell lymphoma (MCL) cell line with t(11;14) and overexpressing cyclin D1. Table 1 illustrates the ability of the assay to distinguish overexpressing cyclin D1 cells from other cells. Indeed, the distribution of ΔΔCt varied from values close to 0 (GRANTA-519: 0; Karpas 620: 0.48; and U266: 0.87); to intermediate values 3.49 and 7.48 for NCI H929 and RPMI 8226, respectively; to values close to 10 to 13 (Ramos: 13.11; LPI: 13.11; and OPM-2: 9.67). The calculation of N illustrates the heterogeneity of cyclin D1 mRNA level associated with trisomy 11 compared with the homogeneity of cyclin D1 level associated with t(11;14). Indeed, NCI H929 expressed 10 times less mRNA, and RPMI 8226 expressed 100 times less than GRANTA-519, U266, and Karpas 620. Using Western blotting, as depicted in Figure 1, GRANTA-519, Karpas 620, and U266 overexpressed cyclin D1. Cyclin D1 is moderately expressed in NCI H929, is at the limit of detection in RPMI 8226, and not detected in OPM-2, LP1, or Ramos, in good correlation with the results of real-time RT-PCR assay. Our findings demonstrate unambiguously that cyclin D1-expressing MM cells belong to 2 distinct groups according to the chromosome 11 abnormality and that the role of cyclin D1 in MM pathogenesis is not dependent on its protein level.
Real-time RT-PCR
Cell . | Pathology . | Chromosome 11 abnormality . | ΔΔCt . | N . |
|---|---|---|---|---|
| GRANTA-519 | MCL | t(11;14(q13;q32) | 0 | 1 |
| U266 | MM | Switch insertion | 0.87 | 0.546515 |
| Karpas 620 | MM | t(11;14(q13;q32) | 0.48 | 0.714497 |
| RPMI 8226 | MM | Trisomy | 7.48 | 0.005594 |
| NCI H929 | MM | Trisomy | 3.49 | 0.089312 |
| LP1 | MM | No | 13.11 | 0.000113 |
| OPM-2 | MM | No | 9.67 | 0.001226 |
| Ramos | Burkitt lymphoma | No | 13.11 | 0.000113 |
Cell . | Pathology . | Chromosome 11 abnormality . | ΔΔCt . | N . |
|---|---|---|---|---|
| GRANTA-519 | MCL | t(11;14(q13;q32) | 0 | 1 |
| U266 | MM | Switch insertion | 0.87 | 0.546515 |
| Karpas 620 | MM | t(11;14(q13;q32) | 0.48 | 0.714497 |
| RPMI 8226 | MM | Trisomy | 7.48 | 0.005594 |
| NCI H929 | MM | Trisomy | 3.49 | 0.089312 |
| LP1 | MM | No | 13.11 | 0.000113 |
| OPM-2 | MM | No | 9.67 | 0.001226 |
| Ramos | Burkitt lymphoma | No | 13.11 | 0.000113 |
Total RNA was extracted from cultured cells as described2 and reverse transcribed with the MMuLV-RT and random hexaprimers, according to the supplier's instructions (Promega, Charbonnières, France). Real-time quantitative PCR was performed on an ABI PRISM 7700 Sequence Detector (PE Applied Biosystems, Foster City, CA) with the SYBR Green PCR master mix (PE Applied Biosystems). The relative quantitation of cyclin D1 expression was normalized to 18S rRNA as internal standard. The primers were designed as follows: cyclin D1 sense, 5′-ACA AAC AGA TCA TCC GCA AAC AC-3′; cyclin D1 antisense, 5′-TGT TGG GGC TCC TCA GGT TC-3′, 18S rRNA sense, 5′-GCT GGA ATT ACC GCG GCT-3′; 18S rRNA antisense, 5′-CGG CTA CCA CAT CCA AGG AA-3′. PCR was performed in 25 μL final volume containing 10 ng cDNA templates, 300 nM of each primer. The quantitation of cyclin D1 compared with 18S rRNA was evaluated using the comparative threshold (Ct) method with the ABI PRISM 7000 SDS software.5 Triplicate experiments were done for each sample; for each sample, the average Ct value for the internal standard (18S rRNA) was subtracted from the average Ct value for cyclin D1 to yield ΔCt. ΔCt obtained for the calibrator cell line GRANTA-519 was then subtracted from the ΔCt of each cell line to give ΔΔCt. The relative amount of cyclin D1 compared with the control was calculated by the formula N = 2−ΔΔCt.
Western blot analysis. Procedures for protein extraction, sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and immunoblotting have been described in detail.6 The polyclonal anti-cyclin D1 and anti-β-tubulin antibodies, sc-718 and sc-9104, respectively, were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The lower bands observed are due to a cross-reactivity shown by anti-cyclin D1 antibody against cyclin D2.
Western blot analysis. Procedures for protein extraction, sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and immunoblotting have been described in detail.6 The polyclonal anti-cyclin D1 and anti-β-tubulin antibodies, sc-718 and sc-9104, respectively, were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The lower bands observed are due to a cross-reactivity shown by anti-cyclin D1 antibody against cyclin D2.
Cyclin D1 is probably not necessary to confer multiple myeloma because it is not observed in 100% of MM patients. The presence of cyclin D1 is a good prognostic factor in MM patients,1,3,4 which argues against the general admittance that cyclin D1 gives a proliferative advantage to plasmacytic cells. Alternatively, alteration of cyclin D1 could interfere with survival and/or apoptotic transduction pathways as suggested from previous results.7 Further studies are needed to clarify this point.
Cyclin D1 expression in multiple myeloma: a pathogenetic role besides the prognostic relevance?
Results reported in the preceding letter by Sola and Troussard using a real-time reverse transcription-polymerase chain reaction (RT-PCR) assay to quantify cyclin D1 mRNA levels in myeloma cell lines with either the t(11;14) or other chromosome 11q abnormalities were consistent with those found by us with a similar method in patients with newly diagnosed multiple myeloma (MM).1 In particular, Sola and Troussard observed that the highest expression of cyclin D1 was associated with the presence of the t(11;14) and that cyclin D1 mRNA levels were heterogeneous in cell lines with trisomy 11, but not in t(11;14)-positive cell lines. Notably, in our study a marked heterogeneity in cyclin D1 mRNA levels was detected also in MM patients with the t(11;14) (ΔΔCt values from 11.28 to 16.95) or who had no abnormalities of chromosome 11 (ΔΔCt values from 20.21 to 24.35), as well as in healthy individuals (ΔΔCt values from 20.85 to 24.10), suggesting that the different expression of cyclin D1 is not associated with a particular cytogenetic abnormality. The reasons for this heterogeneity, which was previously reported by other groups,2 are as yet not well defined. Regarding the issue raised by Sola and Troussard concerning the role of cyclin D1 for the pathogenesis of MM, an attractive hypothesis has recently been proposed on the basis of results of microarray expression analyses performed in a large series of MM patients.3 In this study, ectopic expression of a single cyclin D (D1, D2, or D3) secondary to primary immunoglobulin heavy chain locus (IgH) translocations (ie, 11q13, 6p21, 4p16, and 16q23) was observed in approximately 40% of patients. Remarkably, however, an additional 55% of patients overexpressed cyclin D1 (35%) or cyclin D2 (20%) in the absence of IgH translocations.3 Whether dysregulation of the cyclin D pathway may be an early, unifying event in the pathogenesis of MM and ectopic expression of cyclin D1 in patients lacking chromosome 11 abnormalities may result from tumor-specific interaction with bone marrow stromal cells3 is an intriguing hypothesis that deserves further confirmation in future studies.
Supported by Ministero dell'Università e Ricerca Scientifica (MIUR), progetti FIRB, RBAU012E9A_001 (M.C.); Università di Bologna, Progetti di Ricerca ex-60% (M.C.); and Fondazione Carisbo.
Correspondence: Michele Cavo, Institute of Hematology and Medical Oncology “Seràgnoli,” University of Bologna, Via Massarenti 9, 40138, Bologna, Italy; e-mail: mcavo@med.unibo.it

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal