Abstract
Platelet transfusions are a crucial component of support for patients with severe thrombocytopenia. Storage of platelet concentrates, however, is associated with a reduction in platelet posttransfusion recovery and hemostatic function. In this study, we established a model of mitochondrial injury that resembles platelet storage lesion. Mitochondrial injury, provoked by incubation of platelets with carbonyl cyanide m-chlorophenylhydrazone (CCCP), led to reduced posttransfusion recovery in mice, an effect that directly correlated with the duration of treatment. Damaged platelets were characterized by shape change, disruption of membrane asymmetry, surface expression of P-selectin, and profound proteolysis of GPIbα. Using our model, we identified a key role for endogenous metalloproteinase(s) in platelet clearance, as their inhibition markedly improved posttransfusion recovery of both the mitochondria-injured and in vitro-aged mouse platelets. Metalloproteinase inhibition also prevented proteolysis of GPIbα on damaged platelets, thereby improving the hemostatic function of these cells in vivo. We propose that inhibition of metalloproteinase activity during storage could significantly improve the effectiveness of platelet transfusions. Surface expression of GPIbα might be a powerful marker to determine the quality of platelet concentrates, because it reflects metalloproteinase activity in vitro. (Blood. 2003;102: 4229-4235)
Introduction
Platelet concentrates (PCs) are widely used to support patients who receive intensive therapies for hematologic malignancies and solid tumors.1 Platelets in PCs undergo a number of events during collection, processing, and storage that adversely affect their structure and function, resulting in reduced posttransfusion recovery.2 The observed changes are summarized as platelet storage lesion (PSL) and are indicative of partial platelet activation, including the rearrangement of the platelet cytoskeleton, microvesiculation, translocation of phosphatidyl serine (PS) to the outer leaflet of the plasma membrane, and surface expression of P-selectin.3 GPIb-V-IX, a surface receptor mediating primary adhesion of circulating platelets to a thrombogenic surface,4 is also affected during PSL. As shown by various groups, long-term storage induces proteolysis of an approximately 130-kDa fragment (glycocalicin) of the GPIbα subunit from the receptor complex.5-7 The protease(s) involved in the cleavage and their relevance to platelet survival in vivo, however, remain elusive. The shelf-life of PCs is currently limited to 5 days by the risk of bacterial contamination rather than the quality of the stored platelets. However, as new methods of pathogen inactivation are currently tested for clinical use,8,9 the potential of prolonged PC storage raises questions about what determines the survival of platelets in vivo and how PC quality can be accurately assessed in vitro.
Microvesiculation and PS exposure are also hallmark characteristics of apoptosis, a physiologic program for the safe elimination of cells. In most pathways leading to apoptosis, permeabilization of the inner and outer mitochondrial membranes is critical, resulting in the uncoupling of the respiratory chain with collapse of the electrochemical gradient Δψm.10,11 This transmembrane potential is essential for various cellular functions, including the production of adenosine triphosphate via oxidative phosphorylation. Platelets have been shown to contain many components of the apoptotic machinery.12,13 A recent study describes a complete apoptotic program in human platelets aged in vitro for up to 24 hours at 37°C in the presence and absence of plasma. Apoptosis in platelets leads to cytoplasmic condensation, retention of plasma membrane integrity, cell-surface exposure of PS and P-selectin, and clearance of still-functional platelets by phagocytes.14 Interestingly, apoptosis under such experimental conditions seems to be caspase independent, as it was not affected by caspase inhibitors nor was there evidence for caspase-3 activation. Matrix metalloproteinases (MMPs) were also implicated in apoptosis of different cell types, including endothelial cells15 and neuronal cells.16 To date, 4 metalloproteinases have been identified in human platelets: MMP-1, MMP-2, MMP-3, and MMP-9. MMP-117 and MMP-218-20 were shown to prime platelets for adhesion and aggregation, whereas MMP-9 inhibited platelet activation.19,20 However, a possible role of MMPs in PSL or the related cleavage of GPIbα has not yet been investigated.
In this study, we show that specific mitochondrial damage induces morphologic and functional changes in platelets that resemble those of PSL. We further demonstrate that inhibition of platelet-derived metalloproteinase(s) during platelet in vitro aging or mitochondrial damage markedly improves the recovery of these cells in mice and significantly improves the adhesive function of the platelets under physiologic flow conditions.
Materials and methods
Animals
C57BL/6 WT mice (Jackson Laboratory, Bar Harbor, ME) were used for all experiments throughout the study. Experimental procedures were approved by the Animal Care and Use Committee of the CBR Institute for Biomedical Research, Inc., Boston, MA.
Reagents and antibodies
Lovenox (enoxaparin sodium) (Aventis Pharmaceuticals Products, Bridgewater, NJ), heparin-coated microcapillaries (VWR Scientific, West Chester, PA), collagen reagent Horm (NYCOMED, Munich, Germany), bovine serum albumin (BSA; Chrono-log, Havertown, PA), carbonyl cyanide m-chlorophenylhydrazone (CCCP), prostacyclin (PGI2), human thrombin, A23187, ferric chloride (FeCl3) (all from Sigma, St Louis, MO), annexin V-fluorescein isothiocyanate (FITC), JC-1, calcein acetoxymethyl ester (calcein) (all from Molecular Probes, Eugene, OR), and GM6001 (Calbiochem, San Diego, CA) were purchased. Monoclonal antibodies against mouse CD62P, human GPIbα, and human GPIX were purchased from BD Pharmingen (San Diego, CA), antibodies against tubulin-α, -β1, and -β2 were purchased from Sigma. All other antibodies (p0p1-anti-mGPIbβ, p0p3/4/5-anti-mGPIbα, JON1-anti-mGPIIb/IIIa) were generated and described by Dr B. Nieswandt.21,22
Platelet preparation and counting
Mouse platelets. Mice were bled under isoflurane anesthesia (IsoFlo; Abbott Laboratories, North Chicago, IL) from the retro-orbital plexus. Blood (7 volumes) was collected into a tube containing 30 U/mL heparin in phosphate-buffered saline (PBS), pH 7.4 (3 volumes). Platelet-rich plasma (PRP) was obtained by centrifugation at 300g for 10 minutes. PRP was centrifuged at 1000g in the presence of PGI2 (0.1 μg/mL) for 7 minutes at room temperature (RT). After one washing step, pelleted platelets were resuspended in modified Tyrode-HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) buffer (137 mM NaCl, 0.3 mM Na2HPO4, 2 mM KCL, 12 mM NaHCO3, 5 mM HEPES, 5 mM glucose, pH 7.3) containing 0.35% bovine serum albumin (BSA) and PGI2. Human platelets. Blood (9 volumes) was collected in acid citrate dextrose (ACD) (1 volume) and PRP was obtained by centrifugation at 300g for 10 minutes. PRP was centrifuged at 1000g in the presence of PGI2 (0.1 μg/mL) and 2 mM EDTA (ethylenediaminetetraacetic acid) for 7 minutes at RT. After one washing step, pelleted platelets were resuspended in modified Tyrode-HEPES buffer containing 1 mM MgCl2 and CaCl2. We obtained informed consent from all donors and approval from the Institutional Review Board of the CBR Institute for Biomedical Research, Inc.
Mitochondrial damage and in vitro aging of platelets
Washed platelets resuspended at a concentration of approximately 1.5 × 109 platelets/mL were treated for the times indicated in the respective figures at 37°C with 100 μM CCCP in the presence or absence of GM6001 (100 μM). PRP was incubated for 16 hours at 37°C under agitation, washed once, and resuspended in modified Tyrode-HEPES buffer at a concentration of 1.5 × 109 platelets/mL.
Flow cytometry
Platelets (1 × 106) were stained at RT for 10 minutes with saturating amounts of fluorophore-conjugated antibodies or 1 μM JC-1 and immediately analyzed on a FACScalibur (BD Biosciences, San Diego, CA). Flow cytometric analysis of annexin V-binding to the washed platelets preparation was measured according to the instructions of the manufacturer. Platelets were gated by forward scatter (FSC) and side scatter (SSC) characteristics.
Aggregometry
To determine platelet aggregation, light transmission was measured using washed platelets adjusted to a platelet concentration of 3 × 108 platelets/mL with modified Tyrode buffer containing 1 mM CaCl2 (thrombin) or plasma (U46619). Agonists were added as 100-fold concentrates and transmission was recorded over 14 minutes on a 4-channel optical aggregation system (Chrono-log).
Immunoblotting
Washed platelets, treated with CCCP in the presence or absence of GM6001, were lysed in 2 × sodium dodecyl sulfate (SDS) sample buffer. After lysis, samples were analyzed on an SDS-polyacrylamide gel electrophoresis (PAGE) gel under nonreducing conditions and transferred to a polyvinylidene difluoride membrane. The membrane was first incubated with 5 μg/mL p0p5 antibody followed by rabbit antirat-horseradish peroxidase (1 μg/mL). Proteins were visualized by enhanced chemiluminescence (ECL).
Immunofluorescence
Washed platelets were treated for 60 minutes with dimethyl sulfoxide (DMSO) or CCCP, immediately fixed in 2% formaldehyde, and permeabilized in 0.1% Triton X-100, 0.1 mM EGTA (ethylene glycol tetraacetic acid). Platelets were incubated with a mixture of antibodies against tubulin-α (1 μg/mL), tubulin-β1, and tubulin-β2 (5 μg/mL) for 2 hours and secondary antibody (5 μg/mL) for 1 hour, with extensive washes with PBS between and after the incubations. Images were obtained using a Zeiss Axiovert S100 microscope (Thornwood, NY) equipped with a 100 × differential interference contrast (DIC) oil immersion objective.
Electron microscopy
Mouse platelets treated with CCCP or GM6001/CCCP for 60 minutes were applied to glass coverslips by centrifugation in PBS containing 0.05% glutaraldehyde, fixed with 0.5% glutaraldehyde in PBS for 10 minutes, quenched with 0.1% sodium borohydride in PBS, and washed with PBS containing 1% BSA. GPIbα was labeled with a mix of 3 rat anti-mouse GPIbα monoclonal antibodies, each at 10 μg/mL for 1 hour, followed by incubation with 10 nm gold-conjugated goat anti-rat immunoglobulin G (IgG). The coverslips were washed, fixed with 1% glutaraldehyde, and prepared for electron microscopy as described.23
Platelet recovery and survival in mice
Platelets were labeled with 1 μg/mL calcein for 15 minutes at RT, washed once, resuspended in modified Tyrode-HEPES buffer, and intravenously infused into the retro-orbital plexus of mice (approximately 1.5 × 108 platelets per 15 g body weight). For determination of the in vivo recovery and survival of transfused platelets, blood samples were collected at various time points after transfusion using heparin-coated microcapillaries. Diluted whole-blood samples (1:20 in PBS containing 10 U/mL heparin) were stained with JON1-phycoerythrin (PE) and analyzed by flow cytometry to determine the percentage of calcein-positive platelets. Platelets were identified by FSC and fluorescence 2 (Fl2).
Flow chamber studies
Washed platelets were treated with CCCP in the presence or absence of GM6001, labeled with 2.5 μg/mL calcein, and washed once in modified Tyrode-HEPES buffer. Platelet-poor whole blood was reconstituted with 0.5 × 109 labeled platelets/mL immediately before perfusion in a parallel-plate flow chamber system. A silicone gasket with a flow path height of 127 μm was placed between a flat perfusion chamber (Glycotech, Rockville, MD) and a 35-mm tissue-culture dish coated with 50 μg/mL collagen Horm for 1 hour at RT. Perfusion was carried out at a wall shear rate of 1000 s-1 for 2 minutes. Platelet adhesion was visualized with an Axiovert 135 inverted microscope (Zeiss) equipped with a 100-W HBO fluorescent lamp source (Optiquip, Highland Mills, NY) and a silicon-intensified tube camera (C 2400; Hamamatsu, Middlesex, NJ) connected to an S-VHS video recorder (AG-6730; Panasonic, Matsushita Electric, Osaka, Japan). Images were analyzed using NIH Image 1.61 software (NIH, Bethesda, MD).
In vivo thrombosis model
Male mice (3-4 weeks old) were infused intravenously with calcein-labeled platelets of the same genotype, as described previously,24 anesthetized, and the mesentery was exposed through a midline abdominal incision. Vessels were monitored for 50 minutes after FeCl3 treatment or until cessation of blood flow lasted longer than 10 seconds (occlusive thrombi).
Statistical analysis
If not stated otherwise, data are presented as mean plus or minus SEM.
Results
Effect of mitochondrial damage on platelet function and posttransfusion recovery
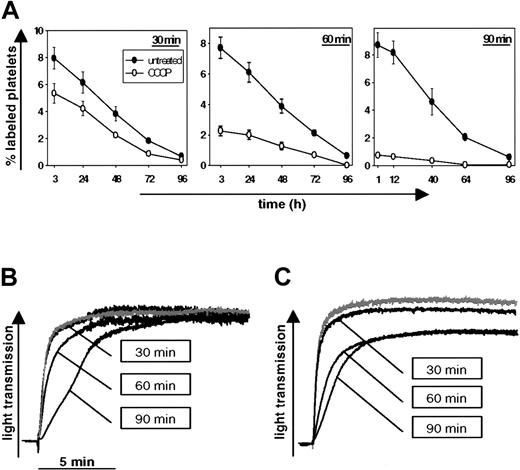
To evaluate the effect of mitochondrial dysfunction on platelet recovery, we treated mouse platelets with CCCP, a lipid-soluble amphipathic molecule that uncouples oxidative phosphorylation.25 The platelets were then labeled with calcein-am and transfused into mice of the same genotype. CCCP treatment markedly reduced the recovery of infused platelets, an effect that directly correlated with the duration of treatment (Figure 1A). However, we did not observe significant differences in the lifespan of the surviving circulating untreated and CCCP-treated platelets. As shown in standard aggregometry, platelets treated with CCCP for up to 90 minutes responded almost normally to thrombin (Figure 1B) and the thromboxane A2 analog U46619 (Figure 1C), indicating that mitochondrial damage induces rapid clearance of aggregation-competent platelets in vivo.
CCCP treatment decreases posttransfusion recovery of functional mouse platelets. (A) Washed platelets were treated for 30 minutes, 60 minutes, or 90 minutes with 100 μM CCCP, labeled with calcein, and infused into mice. Blood was drawn at different time points after infusion and stained for GPIIβ/IIIα. Platelets were identified by PE-fluorescence and forward scatter. Results are shown as percent calcein-labeled platelets ± SEM, n = 5. Similar results were obtained with platelets labeled by biotinylation (not shown). (B, C) Washed platelets were treated with CCCP for the indicated times and platelet responses were tested in standard aggregometry by adding 0.5 U/mL thrombin (B) or 10 μM thromboxane A2 analog U46619 (C). The gray line represents DMSO-treated platelets (90 minutes) activated with the respective agonist. The bar indicates 5 minutes along the x axis. Results are representative of 3 separate experiments.
CCCP treatment decreases posttransfusion recovery of functional mouse platelets. (A) Washed platelets were treated for 30 minutes, 60 minutes, or 90 minutes with 100 μM CCCP, labeled with calcein, and infused into mice. Blood was drawn at different time points after infusion and stained for GPIIβ/IIIα. Platelets were identified by PE-fluorescence and forward scatter. Results are shown as percent calcein-labeled platelets ± SEM, n = 5. Similar results were obtained with platelets labeled by biotinylation (not shown). (B, C) Washed platelets were treated with CCCP for the indicated times and platelet responses were tested in standard aggregometry by adding 0.5 U/mL thrombin (B) or 10 μM thromboxane A2 analog U46619 (C). The gray line represents DMSO-treated platelets (90 minutes) activated with the respective agonist. The bar indicates 5 minutes along the x axis. Results are representative of 3 separate experiments.
Effects of CCCP on platelet morphology, membrane asymmetry, and surface expression of P-selectin and GPIbα
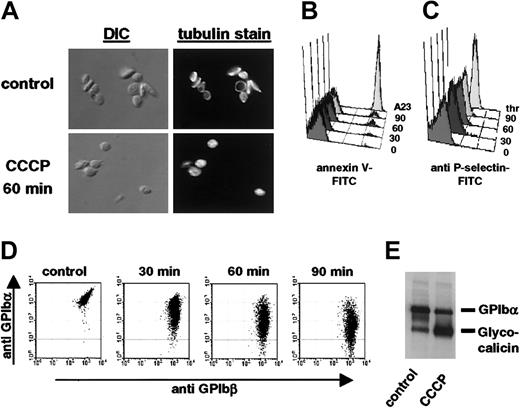
To investigate whether CCCP-treated mouse platelets are characterized by changes similar to those observed in PSL, we studied their shape, membrane asymmetry, and surface expression of various glycoproteins. Whereas the majority of control platelets were discoid in shape, platelets treated with CCCP appeared spherical (Figure 2A, differential interference contrast images [DIC]). This change in shape was also indicated by fluorescence microscopy showing the disassembly of the marginal microtubulin ring characteristic for resting platelets (Figure 2A, tubulin stain).
Functional and morphological changes observed on CCCP-treated platelets resemble platelet storage lesion. (A) Washed mouse platelets were treated with DMSO (control) or CCCP for 60 minutes. Platelets were immediately fixed, permeabilized, and stained for tubulin-β1 and tubulin-β2, and tubulin-α. DIC indicates differential interference contrast images; tubulin stain, immunofluorescence images. Original magnification, × 1000. (B,C) Platelets were stained with annexin V-FITC or RB40.34-FITC (anti-P-selectin) and immediately analyzed on a FACScalibur. 0 indicates DMSO; 30,60,90, platelets treated with CCCP for 30, 60, or 90 minutes; A23, platelets activated for 10 minutes with 50 μg/mL A23187; thr, platelets activated for 10 minutes with 0.5 U/mL thrombin. (D) Dual-color analysis of control (DMSO) and CCCP-treated platelets stained for GPIbα and GPIbβ. (E) Immunoblot analysis of platelet lysates. Cells were treated for 60 minutes with DMSO (control) or CCCP, lysed by addition of 2 × SDS sample buffer, and proteins were separated by 10% SDS-PAGE. Results are representative of 5 individual experiments.
Functional and morphological changes observed on CCCP-treated platelets resemble platelet storage lesion. (A) Washed mouse platelets were treated with DMSO (control) or CCCP for 60 minutes. Platelets were immediately fixed, permeabilized, and stained for tubulin-β1 and tubulin-β2, and tubulin-α. DIC indicates differential interference contrast images; tubulin stain, immunofluorescence images. Original magnification, × 1000. (B,C) Platelets were stained with annexin V-FITC or RB40.34-FITC (anti-P-selectin) and immediately analyzed on a FACScalibur. 0 indicates DMSO; 30,60,90, platelets treated with CCCP for 30, 60, or 90 minutes; A23, platelets activated for 10 minutes with 50 μg/mL A23187; thr, platelets activated for 10 minutes with 0.5 U/mL thrombin. (D) Dual-color analysis of control (DMSO) and CCCP-treated platelets stained for GPIbα and GPIbβ. (E) Immunoblot analysis of platelet lysates. Cells were treated for 60 minutes with DMSO (control) or CCCP, lysed by addition of 2 × SDS sample buffer, and proteins were separated by 10% SDS-PAGE. Results are representative of 5 individual experiments.
Incubation of isolated platelets with CCCP for 30, 60, and 90 minutes induced PS exposure, as measured by binding of annexin V-FITC, on approximately 2.4%, 5.2%, and 10.0% of the platelets, respectively. Annexin V-positive platelets were smaller in size, indicating that they might have released microparticles into the supernatant (not shown).26 Activation of platelets with the ionophore A23187 has been shown to induce PS exposure and microvesiculation on platelets and was therefore used as a positive control.27 P-selectin expression on CCCP-treated platelets also significantly increased over time (Figure 2C), but even after 90 minutes P-selectin levels were low when compared with those observed on platelets activated with thrombin (33 ± 7 SD vs 160 ± 12 SD).
The most striking phenotype of CCCP-treated platelets was observed by dual-color flow cytometry measuring the surface expression of GPIbα and GPIbβ, 2 subunits of the von Willebrand factor receptor complex. Compared with untreated control platelets, the surface expression of GPIbα on platelets treated with CCCP for 90 minutes was reduced by 95.2% (Figure 2D). In contrast, the surface expression of GPIbβ remained unchanged, indicating that CCCP treatment specifically induced proteolysis of the GPIbα subunit. The latter was verified by immunoblot analysis, showing the appearance of an approximately 130-kDa fragment of GPIbα (glycocalicin) upon treatment of platelets with CCCP (Figure 2E).
Taken together, these studies demonstrate that mitochondrial damage rapidly induces a phenotype in mouse platelets that is comparable to that reported for human platelets stored under blood-banking conditions.2
Morphologic and functional changes of in vitro-aged mouse platelets
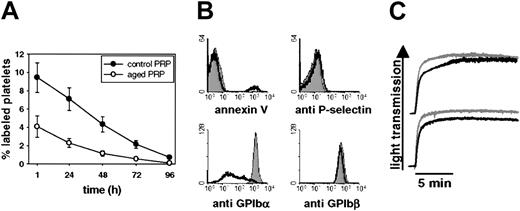
To further correlate CCCP-induced changes in platelet function and morphology to those observed in stored platelets, we analyzed mouse platelets, which we kept in plasma for an extended period of time (16 hours, 37°C, agitation). Posttransfusion recovery of platelets aged under such conditions was less than 50% compared with fresh PRP (Figure 3A), the survival curves being similar to those observed for platelets treated with CCCP for 60 minutes. Aged platelets underwent shape change (not shown), partially expressed PS, and lost GPIbα but not GPIbβ staining from their surfaces (Figure 3B). The increase in the surface expression of P-selectin, however, was consistently small. Like CCCP-treated platelets, aged platelets showed only slightly reduced responses to thrombin and thromboxane A2 analog U46619 in standard aggregometry (Figure 3C).
In vitro aging of mouse platelets induces profound shedding of GPIbα and platelet clearance. (A) Freshly isolated (0 h) or in vitro-aged (16 h) PRP was washed twice and platelets were labeled with calcein. Platelets (1.5 × 108 per 15 g body weight) were infused intravenously into mice and blood was drawn at the indicated time points. Blood platelets were stained for GPIIbIIIa and analyzed by flow cytometry. Results are shown as percent calcein-labeled platelets ± SEM, n = 5. (B) Surface expression of P-selectin, PS (annexin V), GPIbα, and GPIbβ was determined by flow cytometry on fresh PRP (shaded area) and PRP aged for 16 hours at 37°C (black curve). Results are representative of 5 experiments. (C) Platelets in PRP were aged for 16 hours, washed once, and resuspended in modified Tyrode buffer containing 1 mM CaCl2 (thrombin) or plasma (U46619). Platelet responses were tested in standard aggregometry by adding 0.5 U/mL thrombin (upper panel) or 10 μM thromboxane A2 analog U46619 (lower panel). The gray line represents platelets from fresh PRP, the black line platelets from aged PRP. The bar indicates 5 minutes along the x axis. Results are representative of 3 separate experiments.
In vitro aging of mouse platelets induces profound shedding of GPIbα and platelet clearance. (A) Freshly isolated (0 h) or in vitro-aged (16 h) PRP was washed twice and platelets were labeled with calcein. Platelets (1.5 × 108 per 15 g body weight) were infused intravenously into mice and blood was drawn at the indicated time points. Blood platelets were stained for GPIIbIIIa and analyzed by flow cytometry. Results are shown as percent calcein-labeled platelets ± SEM, n = 5. (B) Surface expression of P-selectin, PS (annexin V), GPIbα, and GPIbβ was determined by flow cytometry on fresh PRP (shaded area) and PRP aged for 16 hours at 37°C (black curve). Results are representative of 5 experiments. (C) Platelets in PRP were aged for 16 hours, washed once, and resuspended in modified Tyrode buffer containing 1 mM CaCl2 (thrombin) or plasma (U46619). Platelet responses were tested in standard aggregometry by adding 0.5 U/mL thrombin (upper panel) or 10 μM thromboxane A2 analog U46619 (lower panel). The gray line represents platelets from fresh PRP, the black line platelets from aged PRP. The bar indicates 5 minutes along the x axis. Results are representative of 3 separate experiments.
Role of metalloproteinases in platelet clearance and proteolysis of GPIbα
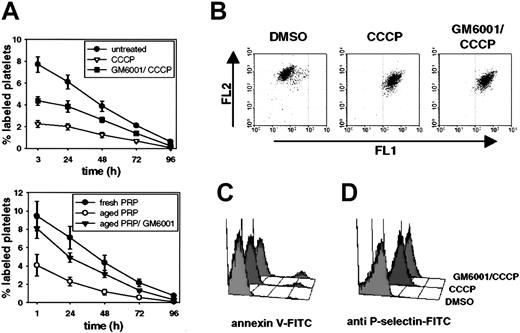
To address whether platelet-derived metalloproteinase(s) are important for the development of platelet damage and therefore in vivo clearance, we studied how inhibition of metalloproteinase activity affects the recovery of transfused damaged platelets in mice. As shown in Figure 4A, we observed a markedly improved recovery of both CCCP-treated platelets (60 minutes; Figure 4A, upper panel) and aged platelets (16 hours; Figure 4A, lower panel), when the cells were coincubated with the broad-spectrum metalloproteinase inhibitor GM6001.28 These findings indicate that both mitochondrial injury and aging induce the activation of endogenous metalloproteinase(s) that are detrimental to platelet survival. GM6001 did not correct the CCCP-induced reduction of the mitochondrial transmembrane potential (Figure 4B), shape change (not shown), or surface PS and P-selectin expression (Figure 4C-D), demonstrating that GM6001 does not inhibit CCCP action. However, as shown by flow cytometry and electron microscopy, GM6001 almost completely inhibited proteolysis of GPIbα induced by CCCP or by platelet in vitro aging (Figure 5A). Inhibition of metalloproteinase activity not only prevented GPIbα cleavage in CCCP-treated platelets but also preserved the normal topologic arrangement of GPIbα in linear arrays, as documented by electron microscopy (Figure 5B). Inhibition of calpain, a calcium-dependent platelet protease known to cleave isolated GPIbα,29 by the cell-permeable inhibitor calpeptin30 did not prevent CCCP-induced cleavage of GPIbα (Figure 5A, lower panel). These data suggest that metalloproteinase but not calpain activity is involved in the proteolysis of GPIbα observed on damaged platelets.
Inhibition of metalloproteinase activity improves posttransfusion recovery of CCCP-treated and aged platelets. (A) Upper panel: survival of washed platelets incubated for 60 minutes with CCCP in the presence or absence of GM6001. Lower panel: survival of platelets aged in plasma in the presence or absence of GM6001. Upon treatment, platelets were labeled with calcein and infused into mice. Blood was drawn at different time points and analyzed by flow cytometry. Results are shown as percent calcein-labeled platelets ± SEM, n = 5. (B) Washed platelets were incubated for 60 minutes with DMSO, CCCP, or GM6001/CCCP, stained for their mitochondrial potential with JC-1,49 and analyzed immediately. JC-1 is a carbocyanine that, in its monomeric form, exhibits an emission maximum at 527 nm (Fl1). High mitochondrial potential promotes a directional uptake of JC-1 into the matrix and subsequent formation of J-aggregates (emission maximum: 590 nm, Fl2). Results are representative of 3 separate experiments. (C, D) Platelets were treated as indicated, stained with annexin V-FITC or RB40.34-FITC (anti-P-selectin) and analyzed on a FACScalibur. Results are representative of 5 experiments.
Inhibition of metalloproteinase activity improves posttransfusion recovery of CCCP-treated and aged platelets. (A) Upper panel: survival of washed platelets incubated for 60 minutes with CCCP in the presence or absence of GM6001. Lower panel: survival of platelets aged in plasma in the presence or absence of GM6001. Upon treatment, platelets were labeled with calcein and infused into mice. Blood was drawn at different time points and analyzed by flow cytometry. Results are shown as percent calcein-labeled platelets ± SEM, n = 5. (B) Washed platelets were incubated for 60 minutes with DMSO, CCCP, or GM6001/CCCP, stained for their mitochondrial potential with JC-1,49 and analyzed immediately. JC-1 is a carbocyanine that, in its monomeric form, exhibits an emission maximum at 527 nm (Fl1). High mitochondrial potential promotes a directional uptake of JC-1 into the matrix and subsequent formation of J-aggregates (emission maximum: 590 nm, Fl2). Results are representative of 3 separate experiments. (C, D) Platelets were treated as indicated, stained with annexin V-FITC or RB40.34-FITC (anti-P-selectin) and analyzed on a FACScalibur. Results are representative of 5 experiments.
Inhibition of metalloproteinase activity prevents cleavage of GPIbα on damaged platelets. (A) Surface expression of GPIbα and GPIbβ as determined by flow cytometry. Upper panel: washed platelets treated for 60 minutes with DMSO, CCCP, or GM6001/CCCP. Middle panel: PRP aged for 16 hours at 37°C in the presence (GM6001/16 h) or absence (16 h) of GM6001. Lower panel: washed platelets treated for 60 minutes with calpeptin (25 μM), CCCP, or calpeptin/CCCP. Results are representative of 5 experiments. (B) Washed platelets treated for 60 minutes with DMSO, CCCP, or GM6001/CCCP were surface labeled by 10 nm anti-GPIbα immunogold. As previously reported, anti-GPIbα immunogold is found in linear arrays on the surface of the resting mouse platelet.48 This topology is preserved when platelets are treated with CCCP in the presence of GM6001. Anti-GPIbα immunogold does not label the surface of CCCP-treated platelets. Shown are representative electron micrographs; the bar is 100 nm; arrows point to gold particles bound to surface-expressed GPIbα.
Inhibition of metalloproteinase activity prevents cleavage of GPIbα on damaged platelets. (A) Surface expression of GPIbα and GPIbβ as determined by flow cytometry. Upper panel: washed platelets treated for 60 minutes with DMSO, CCCP, or GM6001/CCCP. Middle panel: PRP aged for 16 hours at 37°C in the presence (GM6001/16 h) or absence (16 h) of GM6001. Lower panel: washed platelets treated for 60 minutes with calpeptin (25 μM), CCCP, or calpeptin/CCCP. Results are representative of 5 experiments. (B) Washed platelets treated for 60 minutes with DMSO, CCCP, or GM6001/CCCP were surface labeled by 10 nm anti-GPIbα immunogold. As previously reported, anti-GPIbα immunogold is found in linear arrays on the surface of the resting mouse platelet.48 This topology is preserved when platelets are treated with CCCP in the presence of GM6001. Anti-GPIbα immunogold does not label the surface of CCCP-treated platelets. Shown are representative electron micrographs; the bar is 100 nm; arrows point to gold particles bound to surface-expressed GPIbα.
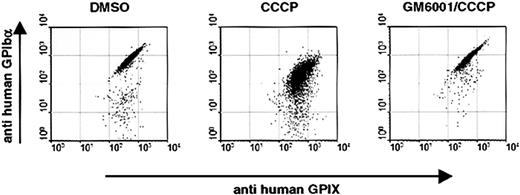
Similar results were obtained when we studied CCCP-induced changes in the surface expression of human GPIbα. As shown by flow cytometry, CCCP treatment of human platelets led to a marked loss of GPIbα on the cell surface, whereas GPIX levels were virtually unaffected (Figure 6). GM6001 almost completely inhibited CCCP-induced cleavage of GPIbα, confirming that metalloproteinase(s) play a key role in this process.
Inhibition of metalloproteinase activity prevents cleavage of GPIbα on CCCP-treated human platelets. Washed human platelets were treated for 8 hours at 37°C with DMSO, CCCP, or GM6001/CCCP. Cells were stained with monoclonal antibodies against GPIbα and GPIX and analyzed by flow cytometry. Results are representative of 5 experiments.
Inhibition of metalloproteinase activity prevents cleavage of GPIbα on CCCP-treated human platelets. Washed human platelets were treated for 8 hours at 37°C with DMSO, CCCP, or GM6001/CCCP. Cells were stained with monoclonal antibodies against GPIbα and GPIX and analyzed by flow cytometry. Results are representative of 5 experiments.
Effect of metalloproteinase inhibition on platelet function
Since GM6001 had such profound effects on posttransfusion recovery of CCCP-treated and aged platelets in mice, we tested whether the inhibitor affects platelet function. GM6001 did not inhibit platelet aggregation in response to the strong agonists thrombin (0.5 U/mL) and thromboxane A2 analog U46619 (10 μM) as well as the weak agonist adenosine diphosphate (10 μM and 1 μM; not shown). We also did not observe a significant reduction in platelet adhesion and thrombus formation on a collagen-coated surface under arterial flow conditions (not shown).
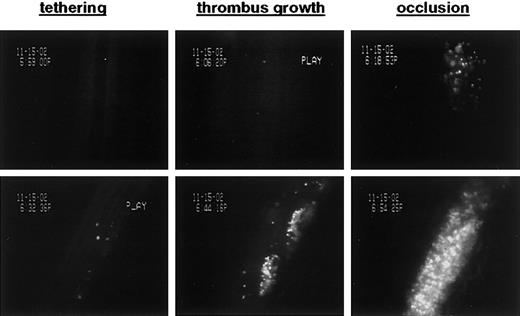
To test whether platelets injured in the presence of GM6001 are functional in vivo, we performed intravital microscopy studies in a model of arterial thrombosis. In this model, calcein-labeled platelets were infused into mice and their adhesion to the FeCl3-injured vessel wall as well as their incorporation into a growing thrombus was monitored. As shown in Figure 7, GM6001/CCCP-treated platelets were fully functional in both adhesion and thrombus formation. In contrast, only a few CCCP-treated platelets were detectable shortly after infusion or were found in the growing thrombus. Thus, GM6001 not only prevented platelets from rapid clearance but also rescued their adhesive function. The latter was confirmed in flow chamber studies where whole-blood samples containing equal numbers of platelets were perfused over a collagen surface at a shear rate of 1000 s-1. Under these conditions, the surface area covered by CCCP-treated platelets was markedly decreased when compared with untreated platelets (7.9 ± 0.4% vs 36.0 ± 0.8%, respectively), whereas adhesion was almost completely restored when platelets were treated with CCCP in the presence of GM6001 (27.3 ± 2.8%). A similar improvement by GM6001 was found for platelets aged in vitro when studied for their adhesion to a collagen surface under flow (not shown).
Inhibition of metalloproteinase activity during mitochondrial injury improves hemostatic function of platelets in arterial thrombosis. Washed platelets were treated for 60 minutes with CCCP (top row) or GM6001/CCCP (bottom row), labeled with calcein, and infused into anesthetized mice. Vascular injury was induced by superfusion with FeCl3; thrombus growth was monitored until blood flow stopped. Images were selected to visualize the ability of treated platelets to adhere to the damaged vessel wall (tethering) and incorporate into the growing thrombus. GM6001/CCCP-treated platelets were incorporated into the growing thrombus to an extent similar to that seen with untreated platelets.24
Inhibition of metalloproteinase activity during mitochondrial injury improves hemostatic function of platelets in arterial thrombosis. Washed platelets were treated for 60 minutes with CCCP (top row) or GM6001/CCCP (bottom row), labeled with calcein, and infused into anesthetized mice. Vascular injury was induced by superfusion with FeCl3; thrombus growth was monitored until blood flow stopped. Images were selected to visualize the ability of treated platelets to adhere to the damaged vessel wall (tethering) and incorporate into the growing thrombus. GM6001/CCCP-treated platelets were incorporated into the growing thrombus to an extent similar to that seen with untreated platelets.24
Discussion
PSL leads to platelet activation and microvesiculation with expression of PS on the outer layer of the membrane, a phenotype that closely resembles apoptosis. The collapse of the inner transmembrane potential (Δψm) in mitochondria is accepted as a major pathway of apoptosis,10,11 but its role in PSL remains elusive. In the present study, we propose mitochondrial damage as a model for PSL. Uncoupling of the mitochondrial oxidative phosphorylation by the protonophore CCCP induced platelet shape change, disruption of membrane asymmetry and microvesiculation, surface expression of P-selectin, and proteolysis of GPIbα (Figure 2). Furthermore, CCCP treatment of platelets resulted in profound clearance of these cells in mice (Figure 1A). Protonophores like CCCP have been shown to induce mitochondrial permeability transition and reduction of Δψm in various cell types.31-33 Depending on the time of incubation in the protonophore, these changes are fully reversible and do not necessarily lead to cell death.34 Two of our results confirm these findings: (1) a normal lifespan was observed for those platelets treated with CCCP that remained in circulation upon transfusion (Figure 1A), and (2) CCCP-treated platelets showed almost normal activation in response to soluble agonists such as thrombin or the thromboxane A2 analog U46619 (Figure 1B-C).
A recent study by Clarke et al demonstrated that mitochondrial damage in human platelets, induced by long-term storage or use of CCCP, results in reduced Δψm and release of cytochrome c, a prerequisite of the cytoplasmic apoptosome complex.35 We were able to confirm these results, as Δψm was reduced in all CCCP-treated (Figure 4B) and a subpopulation of aged platelets (not shown). Furthermore, our data strongly suggest that the reduction of the mitochondrial transmembrane potential plays a key role in the clearance of damaged platelets, as both CCCP-treated and in vitro-aged platelets have a markedly decreased recovery in vivo (Figure 1A, Figure 3A). In addition, we found striking similarities between CCCP-treated and in vitro-aged platelets with regard to platelet morphology, membrane asymmetry, and surface expression of glycoproteins (Figure 2, Figure 3), changes that may account for the clearance of these cells. Preservation of discoid shape has long been considered as a prerequisite for platelet survival. However, platelets from tubulin-β1-deficient mice are spherical due to their genetic defect but show a normal posttransfusion recovery and lifespan.36,37 Furthermore, platelets spherical due to activation have been shown to circulate in mice and baboons.38,39 The latter studies also demonstrate that surface expression of P-selectin, another characteristic of PSL, does not play a major role in the clearance of activated platelets in vivo. Measuring the level of P-selectin expression initially showed some promise for predicting recovery and survival of transfused platelets in humans. However, this could not be confirmed in various clinical studies.40 Our data confirm this lack of correlation for damaged mouse platelets, as we observed similar amounts of P-selectin on the surface of platelets that were incubated with CCCP in the absence or presence of the metalloproteinase inhibitor GM6001, the latter resulting in profoundly increased posttransfusion recovery of these platelets (Figure 4A).
PS exposure on dying cells is widely accepted as a signal for phagocytosis by scavenger receptor-bearing cells.41 Surface expression of PS is induced during PSL14,42 and could be observed on aging platelets in vivo.43 However, it is currently unclear whether PS exposure is the trigger for the clearance of damaged platelets upon transfusion. We found PS expression on less than 10% of all cells treated with CCCP for up to 90 minutes, conditions resulting in clearance of more than 90% of these cells upon transfusion into mice (Figure 1A, Figure 2B). Similar results were observed with aged platelets (Figure 3). These results strongly suggest that PS exposure is not involved in the clearance of damaged platelets in mice.
We now provide evidence that metalloproteinase activity plays a key role in the clearance of damaged platelets, as posttransfusion recovery of in vitro-aged or CCCP-treated platelets was markedly improved in the presence of the broad-range metalloproteinase inhibitor GM6001 (Figure 4). When studied in vitro, no difference between platelets damaged in the presence or absence of GM6001 with regard to Δψm, shape change, PS exposure, or surface expression of P-selectin was observed. In contrast, profound loss of surface GPIbα as seen on CCCP-treated and aged platelets was almost completely inhibited in the presence of GM6001 (Figure 5). Proteolytic cleavage of GPIbα in response to cellular activation has been shown for mouse and human platelets,21,44,45 the mechanism, at least in humans, involving a membrane-bound divalent cation-dependent proteinase, which is possibly a metalloproteinase.44 GM6001 also prevented CCCP-induced loss of GPIbα on human platelets (Figure 6), demonstrating that endogenous metalloproteinase(s) play a key role in the proteolytic cleavage of human as well as mouse GPIbα. Our results further indicate an increased relative susceptibility of mouse versus human platelets with regard to GPIbα proteolysis. Whereas mouse platelets shed more than 90% of their GPIbα within 90 minutes of treatment with CCCP (Figure 2D), approximately 75% of human GPIbα was shed after 8 hours of incubation in CCCP (Figure 6).
So far, 4 MMPs (MMP-1, MMP-2, MMP-3, and MMP-9) have been identified in human platelets. As in other cell types, MMPs are stored in resting platelets in an inactive form. Activation requires translocation to the cell surface and proteolytic cleavage of the proenzyme.17,20,46 Experiments directed at identifying the metalloproteinase(s) involved in the clearance of damaged platelets are in progress. Our data point to the possibility that cleavage of GPIbα may play a role in the clearance of damaged platelets. GPIbα has been shown to be a powerful modulator of platelet survival in vivo as antibody-induced clustering/dimerization of this receptor leads to rapid clearance of circulating platelets, whereas antibodies against other platelet-surface glycoproteins have no such effects.22,47 Furthermore, it was recently demonstrated that the N-terminal domain of GPIbα plays a role in the clearance of refrigerated platelets in mice,48 as chilling of platelets induces clustering of GPIb-V-IX on the platelet surface, eliciting recognition of platelets by hepatic macrophage receptor integrin αMβ2 (CR3, CD11b/CD18). However, our data strongly suggest a different mechanism for clearance of CCCP-treated as well as in vitro-aged platelets, as such platelets were also cleared in αMβ2-deficient mice (W.B., unpublished observation, February 2003). Furthermore, both CCCP treatment and storage induce almost complete removal of the glycocalicin portion of GPIbα from the platelet surface (Figure 2D-E, Figure 3B), demonstrating that clearance of these cells does not involve the N-terminal domain of the GPIbα subunit.
Irrespective of the underlying mechanism, our results demonstrate that inhibition of metalloproteinase activity during platelet injury markedly improves the recovery and hemostatic function of these cells (Figure 7). We found a close relationship between cleavage of GPIbα and the recovery of transfused platelets in mice, whereas no such relationship between recovery and shape change, membrane asymmetry, microvesiculation, or surface expression of P-selectin could be observed.
In summary, we propose mitochondrial damage as a model for PSL, allowing studies on the mechanism responsible for the reduced posttransfusion recovery of damaged platelets in vivo. Our study provides evidence that endogenous metalloproteinases play a key role in the clearance of damaged platelets, and demonstrate that inhibition of metalloproteinase activity not only prevents platelet clearance but also results in reduced proteolysis of GPIbα and therefore a markedly improved hemostatic function of these cells. We propose that GPIbα surface expression is a sensitive marker for the determination of metalloproteinase activity in platelet concentrates and therefore may serve as an indicator of their quality.
Prepublished online as Blood First Edition Paper, August 7, 2003; DOI 10.1182/blood-2003-04-1305.
Supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health grant P01 HL56949 (D.D.W.). W.B. was supported by the Deutsche Forschungsgemeinschaft.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Lesley Cowan for help with preparing the manuscript and Jennifer Jesneck for excellent technical assistance.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal