Abstract
The plasma membrane calcium pump (PMCA) is the only active Ca2+ transporter in human red blood cells (RBCs). Previous measurements of maximal Ca2+ extrusion rates (Vmax) reported only mean values in the RBC population. Despite early evidence for differences in Ca2+ extrusion capacity among RBCs, the precise Vmax distribution remained unknown. It was important to characterize this distribution to assess the range and modality (uni- or multimodal) of PMCA Vmax variation and the likelihood of RBCs with elevated [Ca2+]i in the circulation participating in physiologic and pathologic processes. We report here the application of a new method to investigate the detailed distribution of PMCA Vmax activity in RBCs. The migrating profile of osmotic lysis curves was used to identify and quantify the fraction of cells that extrude a uniform Ca2+ load at different rates. The results revealed that RBCs from single donors have large variations in PMCA activity that follow a unimodal, broad distribution pattern consistently skewed toward higher Vmax values, suggesting an excess of cells with Vmax higher than the mean value. The method applied may provide a way of evaluating whether the observed variation in PMCA Vmax is related to cell age. (Blood. 2003;102:4206-4213)
Introduction
The plasma membrane Ca2+ pump (PMCA), in its various isoforms, is expressed in all animal cells, where it plays a major role in the control of resting intracellular-free Ca2+ levels ([Ca2+]i).1-4 The PMCA was originally discovered and extensively studied in human red blood cells (RBCs) in which it is the only active Ca2+ extrusion transporter. The maximal Ca2+ transport capacity (Vmax) of the PMCA in human RBCs (approximately 10 mmol [340 g hemoglobin {Hb}]-1h-1) is high compared with the normal pump-leak turnover rate of Ca2+ (approximately 50 μmol [340 g Hb]-1h-1).3 Although early work indicated that Ca2+ pump activity may vary from cell to cell over a much broader range than any other known hematologic parameter,5 available measurements of PMCA Vmax in intact RBCs always reported mean values in the RBC population6 ; the actual distribution of PMCA Vmax activity among RBCs was unknown. The aim of the present work was to characterize the distribution of PMCA Vmax activity in normal human RBCs.
It is important to ascertain the extent of PMCA Vmax variation among RBCs because differences in pump-leak [Ca2+]i levels may determine the degree of vulnerability of individual cells to certain physiologic and pathologic stresses. Human RBCs express in their plasma membrane a Ca2+-sensitive K+ channel (the “Gardos” channel7-9 ). Hoffman et al10 recently showed that the isoform hSK4 represents the small conductance Gardos channel of human RBCs. In conditions with elevated [Ca2+]i, Gardos channel activation causes rapid net loss of KCl and water, leading to irreversible cell dehydration. Elevated [Ca2+]i has also been implicated in the activation of cell proteases and in the cross-linking of cytoskeletal proteins, with cumulative effects on cell aging and viability.11-15
The first indirect evidence of substantial cell-to-cell variations in PMCA Vmax arose when Tiffert et al16 examined the dehydration response of RBCs uniformly permeabilized to Ca2+ with the ionophore A23187. As Ca2+ influx into RBCs was progressively increased by increasing the ionophore concentration in a stepwise manner, RBCs suspended in plasmalike media dehydrated following an all-or-none rather than a graded pattern: Instead of all cells dehydrating at progressively faster rates—as expected with a uniform response—only a fraction of RBCs dehydrated and this fraction increased with increasing Ca2+ influx. Further experiments showed that Gardos channel activity among the RBCs was fairly uniform and could not have accounted for this all-or-none pattern.17 These results suggested that uniform Ca2+ permeabilization of RBCs did not generate uniformly elevated [Ca2+]i among the cells and that the differences arose from variations in Ca2+ pump activity.
Further evidence for marked variations in Ca2+ pump activity among RBCs from single donors was provided by Garciáa-Sancho and Lew18,19 who detected large differences in the total Ca2+ content of RBCs ([CaT]i) uniformly permeabilized to Ca2+ with A23187.20 As Ca2+ influx increased so did the fraction of high-Ca2+-containing cells, indicating a gradual variation of some property among the RBCs rather than a sharp distinction between subpopulations. This unexpected behavior suggested that the measured total Ca2+ content of Ca2+-permeabilized RBCs did not represent a uniform Ca2+ content but rather the mean of extremely heterogeneous distributions. To interpret this phenomenon, Garciáa-Sancho and Lew19 proposed a relatively wide distribution of PMCA Vmax activities among RBCs: the fraction of cells with Vmax below the set Ca2+ influx would gain Ca2+ continuously, while their pumps ran at the Ca2+-saturated Vmax rate consuming cell adenosine triphosphate (ATP).6,19 In those cells in which the Vmax of the pump exceeded the glycolytic capacity, ATP depletion would eventually inhibit the pump and [Ca2+]i would approach the Ca2+ equilibrium distribution determined by the ionophore with very high [CaT]i. On the other hand, in those RBCs with a pump Vmax higher than the set Ca2+ influx and with a glycolytic capacity to match ATP consumption by the pump, pump-leak balance would maintain [Ca2+]i at levels below pump saturation in the micromolar or submicromolar range. More recent work provided additional evidence for Vmax differences among RBCs.21,22 However, the actual distribution of PMCA Vmax among RBCs has never been directly investigated. We report here an investigation of the distribution of PMCA Vmax in normal RBCs using a new method developed ad hoc. The results exposed a broad unimodal variation of Ca2+ pump activity among RBCs from single donors, with a large coefficient of variation and a right-skewed Vmax distribution. The significance of such a distribution is discussed.
Materials and methods
Experimental design
Finding and characterizing RBCs that differ in the speed at which they can pump out an ionophore-induced uniform Ca2+ load20 requires a procedure to detect cells with different Ca2+ contents during the Ca2+-extrusion process. We designed a method based on the property of RBCs to become rapidly dehydrated when their [Ca2+]i is elevated. The RBC components that participate in Ca2+-induced dehydration are illustrated in Figure 1 (modified from Tiffert et al22 with permission from Elsevier). The PMCA Vmax distribution method was based on the “Ca2+ load-extrusion” protocol designed by Dagher and Lew6 to measure the mean PMCA Vmax in RBCs. This is illustrated in the computer simulations of Figure 2A.
Diagram of the RBC transport systems that participate in Ca2+-induced dehydration. The top part of the figure shows the transporters of Ca2+, Cl-, and H+ responsible for the net gain of CaCl2 by RBCs when exposed to the ionophore A23187. With RBCs suspended in a Ca2+-containing plasmalike medium, addition of the ionophore A23187 triggers an electroneutral entry of Ca2+ in exchange for protons with a Ca2+:2H+ stoichiometry. With CO2 concentrations at equilibrium across the RBC membrane, the anion exchanger (AE) and CO2 shunt operate jointly like an electroneutral Cl-:H+ cotransport,23 known as the Jacob-Stewart mechanism (JS).24-26 Together, the ionophore and JS mediate net CaCl2 transport since the proton fluxes cancel out. Ca2+ transport is rate limited by the ionophore concentration because the high constitutive expression of the AE ensures nonlimiting speed in the co- and counter-ion transfers. The cytoplasmic Ca2+-buffering behavior of RBCs (B, CaB) may be approximated by [Ca2+]i = α[CaT]i, where α is approximately 0.3 over a wide range of [Ca2+]i values and [CaT]i is the total calcium content of the cells.27,28 Thus, normal RBC [CaT]i levels are hardly detectable, among the lowest of any cell in nature.29-31 At sufficiently high ionophore concentrations (usually > 10 μM in 10% hematocrit RBC suspensions) and appropriate external Ca2+ concentrations ([Ca2+]o > 100 μM), the induced Ca2+ influx exceeds the Vmax of the PMCA in all the RBCs causing their [Ca2+]i levels to approach equilibrium with [Ca2+]o in a uniform manner.20 The ionophore-induced equilibrium sets [Ca2+]i /[Ca2+]o = ([H+]i/[H+]o).2,32 Addition of Co2+ in excess of [Ca2+]o instantly blocks Ca2+ transport by the ionophore allowing the PMCA to extrude the induced Ca2+ load.6,33 Co2+ is itself transported by the ionophore but at normal [Mg2+]i levels it has no effect on PMCA-mediated Ca2+ fluxes.34 The bottom part of the figure shows the transport systems that participate in the Ca2+-induced rapid dehydration response. Elevated [Ca2+]i triggers the dehydration process by activating the Gardos channels. Gardos channel activation hyperpolarizes the cell (E, membrane potential) driving Cl- out, resulting in the net loss of KCl and water. With full Gardos activation, dehydration is rate limited by the Cl- permeability.35 Replacement of approximately 10 mM Cl- by the more permeable anion SCN- maximizes the dehydration rate because rapid SCN-:Cl- exchange via AE, or SCN- diffusion across the membrane (dashed line), continually replenish the intracellular SCN- supply.18 Gardos-mediated dehydration has a very low temperature coefficient. With nonlimiting anion movement, maximal dehydration of RBCs may be attained within 10 to 30 minutes at 0°C to 4°C.18
Diagram of the RBC transport systems that participate in Ca2+-induced dehydration. The top part of the figure shows the transporters of Ca2+, Cl-, and H+ responsible for the net gain of CaCl2 by RBCs when exposed to the ionophore A23187. With RBCs suspended in a Ca2+-containing plasmalike medium, addition of the ionophore A23187 triggers an electroneutral entry of Ca2+ in exchange for protons with a Ca2+:2H+ stoichiometry. With CO2 concentrations at equilibrium across the RBC membrane, the anion exchanger (AE) and CO2 shunt operate jointly like an electroneutral Cl-:H+ cotransport,23 known as the Jacob-Stewart mechanism (JS).24-26 Together, the ionophore and JS mediate net CaCl2 transport since the proton fluxes cancel out. Ca2+ transport is rate limited by the ionophore concentration because the high constitutive expression of the AE ensures nonlimiting speed in the co- and counter-ion transfers. The cytoplasmic Ca2+-buffering behavior of RBCs (B, CaB) may be approximated by [Ca2+]i = α[CaT]i, where α is approximately 0.3 over a wide range of [Ca2+]i values and [CaT]i is the total calcium content of the cells.27,28 Thus, normal RBC [CaT]i levels are hardly detectable, among the lowest of any cell in nature.29-31 At sufficiently high ionophore concentrations (usually > 10 μM in 10% hematocrit RBC suspensions) and appropriate external Ca2+ concentrations ([Ca2+]o > 100 μM), the induced Ca2+ influx exceeds the Vmax of the PMCA in all the RBCs causing their [Ca2+]i levels to approach equilibrium with [Ca2+]o in a uniform manner.20 The ionophore-induced equilibrium sets [Ca2+]i /[Ca2+]o = ([H+]i/[H+]o).2,32 Addition of Co2+ in excess of [Ca2+]o instantly blocks Ca2+ transport by the ionophore allowing the PMCA to extrude the induced Ca2+ load.6,33 Co2+ is itself transported by the ionophore but at normal [Mg2+]i levels it has no effect on PMCA-mediated Ca2+ fluxes.34 The bottom part of the figure shows the transport systems that participate in the Ca2+-induced rapid dehydration response. Elevated [Ca2+]i triggers the dehydration process by activating the Gardos channels. Gardos channel activation hyperpolarizes the cell (E, membrane potential) driving Cl- out, resulting in the net loss of KCl and water. With full Gardos activation, dehydration is rate limited by the Cl- permeability.35 Replacement of approximately 10 mM Cl- by the more permeable anion SCN- maximizes the dehydration rate because rapid SCN-:Cl- exchange via AE, or SCN- diffusion across the membrane (dashed line), continually replenish the intracellular SCN- supply.18 Gardos-mediated dehydration has a very low temperature coefficient. With nonlimiting anion movement, maximal dehydration of RBCs may be attained within 10 to 30 minutes at 0°C to 4°C.18
Method used to investigate the PMCA Vmaxdistribution in RBCs. (Panel A) Computer simulation of the Ca2+ load-extrusion protocol in an RBC population whose symmetrical distribution of PMCA Vmax activity is shown in the inset. Substrate-fed RBCs are assumed to be suspended at an Hct of approximately 10% in an iso-osmotic buffer containing 80 mM K+ and 100 μM CaCl2 at 37°C with magnetic stirring. A high ionophore concentration is used to rapidly induce a large and uniform RBC Ca2+ load. After about 2 minutes (to prevent significant reduction of cell ATP levels) Co2+ is added in excess of Ca2+ in the medium to block ionophore-mediated Ca2+ transport and to expose the uphill extrusion of Ca2+ by the pump. [CaT]i was estimated in timed samples with the use of 45Ca by dividing the measured cell-contained 45Ca radioactivity by the specific activity of 45Ca (there are no endogenous Ca2+ pools that could dilute the specific activity set by the addition of tracer). Each of the Ca2+ extrusion curves was obtained by solving numerically the differential equation d[Ca2+]i /dt = (Vmax)([Ca2+]i2/(Kd2 + [Ca2+]i2)), using a different Vmax value for each curve and applying the conversion [CaT]i = [Ca2+]i /α (Figure 1 legend). The thick line was computed as the weighted mean of the Vmax distribution shown in the inset. Note that the Ca2+-extrusion curves with different Vmax remain linear until [CaT]i falls to about 2 to 4 μM, as expected from a Ca2+ desaturation kinetics with Kd values in the range of 0.2 to 0.5 μM.2,4,36 The mean-Vmax curve also renders a linear segment with a slope close to the true mean Vmax of the distribution (approximately 14 mmol (340 g Hb)-1h-1) but with an apparent Ca2+ desaturation pattern starting at [CaT]i approximately 100 μmol (340 g Hb)-1 a level much higher than that of the single-Vmax desaturation curves. This specious desaturation effect results from retention of Ca2+ within the low-Vmax cells. Points A to E mark sampling times for hemolysis curves expected to have the patterns illustrated in panel B. The horizontal dashed line is at the [CaT]i level above which Gardos channels are assumed to open maximally. (Panel B) Actual hemolysis curves from preliminary experiments illustrate the patterns expected with samples taken at the time points A (▴) to E (•) of the load-extrusion protocol simulated in panel A.
Method used to investigate the PMCA Vmaxdistribution in RBCs. (Panel A) Computer simulation of the Ca2+ load-extrusion protocol in an RBC population whose symmetrical distribution of PMCA Vmax activity is shown in the inset. Substrate-fed RBCs are assumed to be suspended at an Hct of approximately 10% in an iso-osmotic buffer containing 80 mM K+ and 100 μM CaCl2 at 37°C with magnetic stirring. A high ionophore concentration is used to rapidly induce a large and uniform RBC Ca2+ load. After about 2 minutes (to prevent significant reduction of cell ATP levels) Co2+ is added in excess of Ca2+ in the medium to block ionophore-mediated Ca2+ transport and to expose the uphill extrusion of Ca2+ by the pump. [CaT]i was estimated in timed samples with the use of 45Ca by dividing the measured cell-contained 45Ca radioactivity by the specific activity of 45Ca (there are no endogenous Ca2+ pools that could dilute the specific activity set by the addition of tracer). Each of the Ca2+ extrusion curves was obtained by solving numerically the differential equation d[Ca2+]i /dt = (Vmax)([Ca2+]i2/(Kd2 + [Ca2+]i2)), using a different Vmax value for each curve and applying the conversion [CaT]i = [Ca2+]i /α (Figure 1 legend). The thick line was computed as the weighted mean of the Vmax distribution shown in the inset. Note that the Ca2+-extrusion curves with different Vmax remain linear until [CaT]i falls to about 2 to 4 μM, as expected from a Ca2+ desaturation kinetics with Kd values in the range of 0.2 to 0.5 μM.2,4,36 The mean-Vmax curve also renders a linear segment with a slope close to the true mean Vmax of the distribution (approximately 14 mmol (340 g Hb)-1h-1) but with an apparent Ca2+ desaturation pattern starting at [CaT]i approximately 100 μmol (340 g Hb)-1 a level much higher than that of the single-Vmax desaturation curves. This specious desaturation effect results from retention of Ca2+ within the low-Vmax cells. Points A to E mark sampling times for hemolysis curves expected to have the patterns illustrated in panel B. The horizontal dashed line is at the [CaT]i level above which Gardos channels are assumed to open maximally. (Panel B) Actual hemolysis curves from preliminary experiments illustrate the patterns expected with samples taken at the time points A (▴) to E (•) of the load-extrusion protocol simulated in panel A.
To detect Vmax heterogeneity and characterize its distribution, the load-extrusion protocol in Figure 2A was used as follows: At the points A to E on the model-generated Ca2+ load-extrusion curve, RBCs are sampled and delivered into a low-K+ medium (LK medium; see “Solutions”) consisting of a large volume of ice-cold, iso-osmotic buffer containing EGTA (ethylene glycol-bis(2-aminoethylether)-N,N,N′,N′,-tetraacetic acid) and no added Ca2+, to prevent any Ca2+ gains; 1 mM vanadate to ensure complete pump inhibition; low-K+; and 10 mM SCN- to allow rapid dehydration of the cells with elevated Ca2+. This medium instantly arrests Ca2+ fluxes mediated by ionophore and pump so that each RBC retains the Ca2+ it contained at the time of sampling, which determines whether or not it will dehydrate.
When Gardos channels are fully activated at nonlimiting anion permeabilities, full dehydration proceeds without profile changes in the hemolysis curves indicating that the distribution of Gardos channel-mediated dehydration capacity is fairly uniform among RBCs.17 Thus, any alterations in the profile of hemolysis curves from cells actively extruding a Ca2+ load could be attributed to differences in Ca2+ pump extrusion capacity among the cells. Let us assume provisionally that maximal Gardos channel activation is attained when [CaT]i exceeds 20 μmol (340 g Hb)-1 as indicated by the dashed horizontal line in Figure 2A. Then all RBCs with [CaT]i more than 20 μmol (340 g Hb)-1 will dehydrate maximally, whereas those with lower [CaT]i will dehydrate partially or not at all. If we induce Ca2+ loads much higher than the Gardos channel deactivation threshold, the time required to pump out the subthreshold Ca2+ becomes negligible in relation to the time required to pump out the full Ca2+ load, justifying the assumption that nondehydrating cells have practically emptied their Ca2+ loads. After a brief incubation at 4°C in LK medium to allow the cells containing suprathreshold [CaT]i to dehydrate maximally, the tubes are spun, most of the supernatant is discarded, and the cell pellets are resuspended in the remaining medium for hemolysis curve sampling.
The predicted results were obtained in preliminary experiments whose real hemolysis curves, from samples taken at the times A to E in Figure 2A, are shown in Figure 2B. With samples A and E we see normal hemolysis curves since all the cells would have been essentially Ca2+ free (in sample A because they never experienced a Ca2+ load and in sample E because the Ca2+ load had been fully extruded, even from the slowest pumping cells). The identity of the 2 curves rules out any significant effects of the Ca2+ loads implemented here on the critical hemolytic volume of normal RBC populations, as might have been expected from Ca2+-induced membrane area losses.37 Sample B gives the hemolysis curve corresponding to full dehydration of all the cells: because RBCs dehydrated by net salt loss need to gain much more water than normal-volume cells to swell to their critical hemolytic volume, the curve is deeply left-shifted toward low relative tonicities. In samples C and D, variable fractions of high-Vmax cells did not dehydrate since they had pumped out nearly all their Ca2+ at the time of sampling, whereas the slower pumping cells retaining more than 20 μmol (340 g Hb)-1 of [CaT]i became fully dehydrated; the resulting lysis curves C and D are intermediate (between curves A and B) with an inflected quasi-plateau region, indicating the development of a marked bimodality in the distribution of hydration states in the RBC population. As progressively more cells pump out their Ca2+ loads, the mixed lysis curves with the plateau pattern would migrate upwards toward the fully recovered pattern of sample E. The fraction of cells that emptied their Ca2+ loads at each sampling time may be estimated from the Y-intercept values read near the midpoint of the quasi-plateau region of the hemolysis curves. An example is shown in Figure 2B by the dashed vertical line at a relative tonicity of 0.3. The important consideration in the choice of relative tonicity is that the analysis to be applied should be largely independent of the value chosen to represent the plateau. In the experiments to be reported below, the results agreed within a margin of ± 3% when the choice of relative tonicity for the plateau Y-intercepts was within the range 0.25 to 0.40. To estimate the percent cells with fully extruded Ca2+ loads at each sampling time (fX) we must first define the 100% value. We use the ordinate values (Y) at the intersection of curves A (or E) and B with the dashed vertical line to define YA and YB. The full lysis curve migration interval (100%) is YA - YB. The percent cells with emptied Ca2+ loads at the time of sample C (fC) may be estimated from the ordinate value of curve C at a relative tonicity of 0.3 (YC) as follows: fC = 100 (YC - YB)/(YA - YB). And for D, fD = 100 (YD - YB)/(YA - YB).
Rapid sampling for lysis curves during the Ca2+ extrusion phase of the load-extrusion protocol could thus provide a detailed characterization of fX as a function of the time interval between Co2+ addition and emptying of the Ca2+ load, ΔtX. This function is the integral of the distribution of PMCA Vmax values in the RBC population since for each fX the corresponding Vmax is computed from (Vmax)X = (Ca2+ load)/ΔtX, where ΔtX is the time interval between Co2+ addition and sampling for X.
Solutions
The 2 basic solutions used (to which specific additions were made, as noted) were (1) high-K+ (HK)-containing (80 mM KCl; 70 mM NaCl; 10 mM HEPES-Na [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid-Na], pH 7.5, at 37°C; and 0.15 mM MgCl2) and (2) low-K+ (LK) (140 mM NaCl; 10 mM NaSCN; 10 mM HEPES-Na, pH 7.5, at 37°C; and 0.15 mM MgCl2). Unless specified otherwise, the final concentrations of the most frequently added solutes was (in mM) EGTA, 0.1; CaCl2, 0.14; inosine, 5; sodium orthovanadate, 1; CoCl2, 0.4 in HK medium and 0.1 in LK medium; ionophore A23187 (from 2 mM stock in ethanol), 0.01. When radioactive 45Ca was used, the specific activity was set between 107 and 2 × 108 counts per minute (cpm)/μmol. All additions were done from stock solutions at least 100-fold more concentrated than in the final solutions or cell suspensions.
Preparation of cells
Venous blood was drawn from healthy volunteers into heparinized syringes after obtaining informed, written consent. Approval was obtained from the Institutional Review Boards of the Physiological Laboratory, University of Cambridge, United Kingdom; and from the Department of Medicine, Albert Einstein College of Medicine, Bronx, NY, for these studies. RBCs were washed twice by centrifugation and resuspension in large volumes of ice-cold HK solution with added EGTA and thrice more with solution HK alone. The buffy coat containing platelets and white cells was removed after each wash. After the last wash, the cells were suspended at 10% hematocrit (Hct) in medium HK supplemented with inosine and 45Ca(Ca2+) or Ca2+, as described in Figures 3 and 4, and incubated for about 10 minutes at 37°C for temperature equilibration before applying the load-extrusion protocol with the specific modalities described in the figure legends.
Generation of 45Ca content heterogeneity during the Ca2+ extrusion period of the load-extrusion protocol. One experiment with protocol 1 is shown (see “Experimental protocols”) representative of 3 with similar results. (A) Measurement of the PMCA Vmax by the load-extrusion extrusion protocol. RBCs were suspended at 10% Hct in medium HK supplemented with 5 mM inosine and 140 μM 45Ca(Ca2+). The ionophore A21387 was added at t = 0 (final concentration 10 μM) and CoCl2 was added at t = 2 minutes (final concentration 0.4 mM). (B) Comparison of the release of Hb and 45Ca induced by hypotonic lysis, as a function of relative tonicity. The measurements were performed on aliquots of the same samples used for the Vmax estimates in panel A, as per protocol 1. All samples were postincubated in LK medium. Solid and open symbols report percentages of 45Ca and Hb released at each relative tonicity, respectively. One hundred percent release corresponds to the values obtained at about 0 relative tonicity. (C) Direct comparison of lysis-induced release of Hb and 45Ca at each relative tonicity. The solid symbols and lines represent paired values from open and solid symbols of panel B. The open symbols and dashed lines are from duplicate samples from the same experiment treated as in panel A except for postincubation in HK medium to preclude volume changes.
Generation of 45Ca content heterogeneity during the Ca2+ extrusion period of the load-extrusion protocol. One experiment with protocol 1 is shown (see “Experimental protocols”) representative of 3 with similar results. (A) Measurement of the PMCA Vmax by the load-extrusion extrusion protocol. RBCs were suspended at 10% Hct in medium HK supplemented with 5 mM inosine and 140 μM 45Ca(Ca2+). The ionophore A21387 was added at t = 0 (final concentration 10 μM) and CoCl2 was added at t = 2 minutes (final concentration 0.4 mM). (B) Comparison of the release of Hb and 45Ca induced by hypotonic lysis, as a function of relative tonicity. The measurements were performed on aliquots of the same samples used for the Vmax estimates in panel A, as per protocol 1. All samples were postincubated in LK medium. Solid and open symbols report percentages of 45Ca and Hb released at each relative tonicity, respectively. One hundred percent release corresponds to the values obtained at about 0 relative tonicity. (C) Direct comparison of lysis-induced release of Hb and 45Ca at each relative tonicity. The solid symbols and lines represent paired values from open and solid symbols of panel B. The open symbols and dashed lines are from duplicate samples from the same experiment treated as in panel A except for postincubation in HK medium to preclude volume changes.
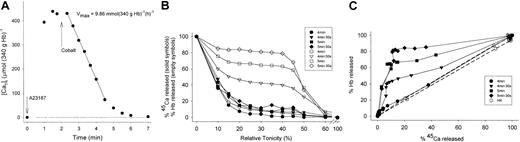
Distribution of PMCA Vmax among normal RBCs. One experiment with protocol 2 is shown (see “Experimental protocols”) representative of 5 with similar results. (A) Duplicate measurement of the PMCA Vmax by the load-extrusion protocol. The indicated mean Vmax values were obtained by linear regression through the first 6 time-points after cobalt addition. The mean rate of Ca2+ extrusion fell progressively when [CaT]i dropped below about 100 μmol (340 g Hb)-1. (B) RBCs from the same batch as those used for the Vmax measurements reported in panel A underwent the load-extrusion protocol without 45Ca tracer and were sampled for hemolysis curves at the indicated times after ionophore addition (the number of curves exceeded the repertoire of available symbols in the software used leading to cyclic repetition; however, the bottom-up sequence can be followed unambiguously). All samples were postincubated in LK medium. There was a monotonic upward progression of the curves with time from the fully dehydrated pattern (1-min point) to the fully recovered pattern (7-min point). (C) Percent cells with emptied Ca2+ loads as a function of time after cobalt addition (Δt). The percent of Ca2+-emptied RBCs was computed from the ordinate readings shown in panel B at a relative tonicity of 0.3, taking the distance between lowest and highest reading as 100% (see “Materials and methods”). The equation y = axn/(bn + xn) was found to give a good empirical fit of the experimental points in the 5 experiments of this series, slightly better than that obtained with other sigmoid saturation functions, as judged by eye and by least means squares analysis. The parameter values obtained by least mean squares fit to the equation in this experiment were as follows: a = 100, b = 2.2, and n = 5.5. (D) Derivative of the curve fit through the experimental points in panel C (continuous line) compared with a Gaussian curve fit to the rising branch of the derivative curve (dotted line). Both curves are plotted as a function of Vmax estimated from (Ca2+ load)/Δt. The Ca2+ load in the suspension used for hemolysis curve sampling (B) was 390 μmol (340 g Hb)-1. Compared with the Gaussian curve, the derivative curve reporting the actual Vmax distribution among RBCs shows a marked right-shifted skew, observed in all the experiments of this series (Table 3). The parameter values of the actual Vmax distribution are shown in the figure and were calculated as follows: mean = Σ(yixi)/Σyi;SD = ((Σ(yi(xi-mean))2)/Σyi)1/2; Skew = (Σ((yi(xi-mean))/SD)3)/Σyi.
Distribution of PMCA Vmax among normal RBCs. One experiment with protocol 2 is shown (see “Experimental protocols”) representative of 5 with similar results. (A) Duplicate measurement of the PMCA Vmax by the load-extrusion protocol. The indicated mean Vmax values were obtained by linear regression through the first 6 time-points after cobalt addition. The mean rate of Ca2+ extrusion fell progressively when [CaT]i dropped below about 100 μmol (340 g Hb)-1. (B) RBCs from the same batch as those used for the Vmax measurements reported in panel A underwent the load-extrusion protocol without 45Ca tracer and were sampled for hemolysis curves at the indicated times after ionophore addition (the number of curves exceeded the repertoire of available symbols in the software used leading to cyclic repetition; however, the bottom-up sequence can be followed unambiguously). All samples were postincubated in LK medium. There was a monotonic upward progression of the curves with time from the fully dehydrated pattern (1-min point) to the fully recovered pattern (7-min point). (C) Percent cells with emptied Ca2+ loads as a function of time after cobalt addition (Δt). The percent of Ca2+-emptied RBCs was computed from the ordinate readings shown in panel B at a relative tonicity of 0.3, taking the distance between lowest and highest reading as 100% (see “Materials and methods”). The equation y = axn/(bn + xn) was found to give a good empirical fit of the experimental points in the 5 experiments of this series, slightly better than that obtained with other sigmoid saturation functions, as judged by eye and by least means squares analysis. The parameter values obtained by least mean squares fit to the equation in this experiment were as follows: a = 100, b = 2.2, and n = 5.5. (D) Derivative of the curve fit through the experimental points in panel C (continuous line) compared with a Gaussian curve fit to the rising branch of the derivative curve (dotted line). Both curves are plotted as a function of Vmax estimated from (Ca2+ load)/Δt. The Ca2+ load in the suspension used for hemolysis curve sampling (B) was 390 μmol (340 g Hb)-1. Compared with the Gaussian curve, the derivative curve reporting the actual Vmax distribution among RBCs shows a marked right-shifted skew, observed in all the experiments of this series (Table 3). The parameter values of the actual Vmax distribution are shown in the figure and were calculated as follows: mean = Σ(yixi)/Σyi;SD = ((Σ(yi(xi-mean))2)/Σyi)1/2; Skew = (Σ((yi(xi-mean))/SD)3)/Σyi.
Experimental protocols
Two sets of experiments were performed with slightly different protocols. The first set of experiments was designed to test the assumptions implicit in the design of the Vmax distribution method by comparing the release of Hb and 45Ca induced by hypotonic lysis (Figure 3; Tables 1, 2). The second set of experiments aimed to characterize the distribution of PMCA Vmax values among RBCs from healthy subjects.
Simultaneous measurements of the fractions of Hb and Ca2+ lost from AA RBCs by lysis in hypotonic media at 4 sequential time points during the Ca2+ extrusion stage of the load-extrusion protocol
. | Percent 45Ca2+ lost . | . | . | . | Percent Hb lost . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Relative tonicity, % . | 4 min . | 4.5 min . | 5 min . | 5.5 min . | 4 min . | 4.5 min . | 5 min . | 5.5 min . | ||||||
| 0 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | ||||||
| 10 | 37.8 | 47.5 | 43.4 | 36.0 | 38.7 | 60.0 | 75.5 | 85.2 | ||||||
| 15 | 15.0 | 25.1 | 29.3 | 27.7 | 18.5 | 50.4 | 68.4 | 83.5 | ||||||
| 20 | 7.5 | 15.4 | 15.0 | 20.1 | 13.0 | 46.5 | 66.5 | 84.1 | ||||||
| 25 | 4.3 | 11.0 | 14.3 | 13.3 | 9.7 | 43.6 | 66.4 | 82.0 | ||||||
| 30 | 4.1 | 9.0 | 9.3 | 11.6 | 8.2 | 42.3 | 64.6 | 81.1 | ||||||
| 35 | 1.6 | 7.7 | 7.2 | 15.1 | 6.8 | 41.3 | 64.2 | 80.3 | ||||||
| 40 | 0.9 | 6.8 | 10.2 | 11.1 | 5.5 | 40.0 | 63.2 | 79.1 | ||||||
| 45 | 1.4 | 6.4 | 10.1 | 12.2 | 4.5 | 35.9 | 58.5 | 68.2 | ||||||
| 50 | 0.3 | 6.3 | 3.0 | 3.1 | 2.6 | 25.4 | 36.5 | 33.9 | ||||||
| 60 | 0.3 | 2.1 | 0.6 | 1.1 | 0.5 | 7.2 | 4.5 | 1.4 | ||||||
| 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||
| Plateau values mean ± SEM | — | 8.2 ± 0.7 | 10 ± 1 | 13 ± 1 | — | 41 ± 1 | 63 ± 1 | 81 ± 1 | ||||||
| Mean [CaT]i, μmol (340 g Hb)−1 | 157 | 73 | 39 | 17 | — | — | — | — | ||||||
. | Percent 45Ca2+ lost . | . | . | . | Percent Hb lost . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Relative tonicity, % . | 4 min . | 4.5 min . | 5 min . | 5.5 min . | 4 min . | 4.5 min . | 5 min . | 5.5 min . | ||||||
| 0 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | ||||||
| 10 | 37.8 | 47.5 | 43.4 | 36.0 | 38.7 | 60.0 | 75.5 | 85.2 | ||||||
| 15 | 15.0 | 25.1 | 29.3 | 27.7 | 18.5 | 50.4 | 68.4 | 83.5 | ||||||
| 20 | 7.5 | 15.4 | 15.0 | 20.1 | 13.0 | 46.5 | 66.5 | 84.1 | ||||||
| 25 | 4.3 | 11.0 | 14.3 | 13.3 | 9.7 | 43.6 | 66.4 | 82.0 | ||||||
| 30 | 4.1 | 9.0 | 9.3 | 11.6 | 8.2 | 42.3 | 64.6 | 81.1 | ||||||
| 35 | 1.6 | 7.7 | 7.2 | 15.1 | 6.8 | 41.3 | 64.2 | 80.3 | ||||||
| 40 | 0.9 | 6.8 | 10.2 | 11.1 | 5.5 | 40.0 | 63.2 | 79.1 | ||||||
| 45 | 1.4 | 6.4 | 10.1 | 12.2 | 4.5 | 35.9 | 58.5 | 68.2 | ||||||
| 50 | 0.3 | 6.3 | 3.0 | 3.1 | 2.6 | 25.4 | 36.5 | 33.9 | ||||||
| 60 | 0.3 | 2.1 | 0.6 | 1.1 | 0.5 | 7.2 | 4.5 | 1.4 | ||||||
| 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||
| Plateau values mean ± SEM | — | 8.2 ± 0.7 | 10 ± 1 | 13 ± 1 | — | 41 ± 1 | 63 ± 1 | 81 ± 1 | ||||||
| Mean [CaT]i, μmol (340 g Hb)−1 | 157 | 73 | 39 | 17 | — | — | — | — | ||||||
The results are representative of those obtained in 3 similar experiments. The quasi-plateau values were calculated from the measured Hb and 45Ca losses (numbers in bold) in the relative tonicity range from 25% to 45% and used as described in Table 2 to estimate the mean [CaT]i retained within the cells that failed to dehydrate during the postincubation period in LK medium. “Mean [CaT]i” reports the measured mean total RBC Ca2+ content at the indicated time points as shown in Figure 3A.
— indicates not applicable.
Estimate of the mean [CaT]i retained within the RBCs that failed to dehydrate during the postincubation period in LK medium
Time of sample, min . | Quasi-plateau percents . | . | Mean [CaT]i in the cell population, μmol (340 g Hb)−1 . | Mean [CaT]i in the dehydrated cells, μmol (340 g Hb)−1 . | Mean [CaT]i in the nondehydrated cells, μmol (340 g Hb)−1 . | |
|---|---|---|---|---|---|---|
| . | Hb lost . | 45Ca2+ lost . | . | . | . | |
| 4.5 | 41 | 8.2 | 73 | 114 | 15 | |
| 5.0 | 63 | 10 | 39 | 95 | 6.2 | |
| 5.5 | 81 | 13 | 17 | 78 | 2.7 | |
Time of sample, min . | Quasi-plateau percents . | . | Mean [CaT]i in the cell population, μmol (340 g Hb)−1 . | Mean [CaT]i in the dehydrated cells, μmol (340 g Hb)−1 . | Mean [CaT]i in the nondehydrated cells, μmol (340 g Hb)−1 . | |
|---|---|---|---|---|---|---|
| . | Hb lost . | 45Ca2+ lost . | . | . | . | |
| 4.5 | 41 | 8.2 | 73 | 114 | 15 | |
| 5.0 | 63 | 10 | 39 | 95 | 6.2 | |
| 5.5 | 81 | 13 | 17 | 78 | 2.7 | |
The estimates were applied to 3 samples taken at the indicated times after ionophore (A23187) addition during the Ca2+ extrusion stage of the load-extrusion protocol (Figure 3A) and are representative of the ranges found in 3 similar experiments. The results in the table may be read and interpreted as illustrated in the following example using the 5-minute data; at 5 minutes, in the plateau region of relative tonicities (25% to 45%, Table 1) the mean lysis-induced losses of Hb and 45Ca were 63% and 10%, respectively (Figure 3B). Since the mean [CaT]i in the cell population is partitioned between dehydrated and nondehydrated cells, mean − [CaT]i = fd mean − ([CaT]i)d + fnd mean − ([CaT]i)nd, where fd and fnd represent the fractions of dehydrated (d) and nondehydrated (nd) cells, respectively, and mean − ([CaT]i)(d or nd) is the mean Ca2+ content within each d or nd subpopulation. Continuing with the 5-minute example, 10% of 39 μmol (340 g Hb)−1 is within 63% nondehydrated cells and 90% of 39 μmol (340 g Hb)−1 is within 37% of dehydrated cells, rendering mean − ([CaT]i)d = 95 and mean − ([CaT]i)nd = 6.2 in units of μmol (340 g Hb)−1.
For the first set of experiments (Figure 3 protocol 1) washed RBCs were suspended at 10% Hct in medium HK, supplemented with inosine and high-specific activity 45Ca(Ca2+) (2 × 108 cpm/μmol) and incubated for about 10 minutes at 37°C for temperature equilibration. The ionophore A23187 was added at t = 0 followed 2 minutes later by CoCl2. Samples (0.5 mL) were drawn once before ionophore addition, twice or thrice after ionophore, and just before cobalt addition to estimate the size of the Ca2+ load. After cobalt, samples drawn every 30 seconds were delivered into centrifuge tubes containing 12 mL of ice-cold LK solution with added EGTA and vanadate to inhibit the Ca2+ pump (preliminary experiments had shown that exposure of the RBCs to 1 mM vanadate at 0°C to 4°C produced irreversible and near-complete Ca2+ pump inhibition). In this medium the Ca2+ content of the RBCs at the time of sampling was maintained, since low temperature, vanadate, and intracellular cobalt prevented Ca2+ loss through the ionophore or extrusion by the pump. After about 30 minutes of this “postincubation” in the ice bath to allow maximal dehydration by the Ca2+-containing cells, the RBCs were washed once by centrifugation and resuspension in 10 to 12 mL of LK+EGTA medium, and the cell pellet was finally resuspended in 1 mL LK+EGTA. The wash and dilution reduced external 45Ca to near background and rendered about 1 mL of a 5% Hct suspension for each sample, whose intracellular 45Ca distribution was maintained from the time of sampling. Of this, 0.1 mL was centrifuged for 15 seconds in 1.5-mL microfuge tubes, and after discarding the supernatant the cell pellet was resuspended in 0.6 mL of 6% trichloroacetic acid (TCA) and centrifuged again. From the clear TCA supernatant, 0.5 mL was taken for scintillation counting of 45Ca to estimate [CaT]i (Figure 3A). The remaining 0.9 mL of each sample was used for osmotic lysis curves (see next paragraph), after which the Hb and 45Ca2+ released by hypotonic lysis was determined for each of the 12 different microwells.
Osmotic lysis curves
A solution containing 155 mM NaCl and 2 mM HEPES-Na, pH 7.5, at room temperature, iso-osmotic with the HK and LK solutions, was diluted with a medium containing only 2 mM HEPES-Na to render 12 “lysis” solutions with the relative tonicities (RT) indicated in the figures. Two hundred fifty microliters of each of these lysis solutions were delivered into each of the 8 wells of columns 1 to 12 of 96 × round-bottom microwell plates. Between 0.5 and 0.7 mL of the 0.9-mL 5% Hct sample was placed in a grooved container and, using a multichannel pipette, 10 μL was delivered and mixed into each of the 12 wells of the assigned row so that each sample provided a complete lysis curve. The round-bottom plates were centrifuged at 1020g × 5 minutes, and 150 μL of the cell-free supernatant from each well was transferred to corresponding wells on 96 × flat-bottom microwell plates for measurement of Hb concentrations by light absorbance at 415 nm (Soret band) using a microplate reader (Molecular Devices, Sunnyvale, CA).5 The absorbance in the 2 mM HEPES-Na buffer (relative tonicity = “0”) was taken as 100% lysis, and the readings at the higher tonicities were compared to it. After the Hb measurement, 130 μL of 11% TCA were added to each well, the plate was centrifuged to sediment denatured protein, and 200 μL of the clear TCA supernatant from each well was transferred to scintillation vials to measure the 45Ca released by lysis. These measurements provided a direct comparison of the Hb and Ca2+ released from the RBCs at each relative tonicity and at each sampling time.
The second set of experiments (Figure 4 protocol 2) differed from the first in that the original cell suspension was divided in 2, one for measuring the mean Vmax of the PMCA by the Dagher-Lew method6 using low specific activity 45Ca(Ca2+) (Figure 4A), the other for measuring lysis curve migration with high time resolution in tracer-free conditions (Figure 4B). Except for the presence of tracer 45Ca in the aliquot for measurement of the mean Vmax, the load extrusion protocol was the same for both suspensions. Samples for lysis curves (0.5 mL) from the tracer-free suspension were drawn at 10-second intervals after cobalt addition and processed as described in the preceding paragraph, except for the TCA treatment.
Results
We first tested the following assumptions made for the experimental design shown in Figure 2: (1) starting from a high, saturating, and uniform Ca2+ load, cell-to-cell differences in Ca2+ pumping rates would generate a progressive heterogeneity of Ca2+ contents among the RBCs; (2) deactivation of the Gardos channels would occur when [CaT]i fell below approximately 20 μmol (340 g Hb)-1 (Figure 2A dashed line); and (3) the vertical migration of the quasi-plateau region of hemolysis curves (Figure 2B) reports the progressive fraction of RBCs that have fully extruded the imposed Ca2+ load.
A typical result is shown in Figure 3 and Tables 1 and 2. During the load-extrusion protocol parallel samples were taken at the indicated times to estimate the mean PMCA Vmax from the initial slope of the Ca2+ extrusion curve after cobalt addition (approximately 10 mmol [340 g Hb]-1h-1; Figure 3A). Apparent Ca2+ desaturation of the pump began after 4.5 minutes when the mean [CaT]i was below 73 μmol (340 g Hb)-1.
The losses of Hb and Ca2+ induced by hypotonic lysis (Table 1; Figure 3B) were compared for 4 selected samples taken between 4 and 5.5 minutes of ionophore addition (2 and 3.5 minutes after addition of cobalt). With the 4-minutes sample (as with earlier samples taken after cobalt; not shown) the Hb and Ca2+ release curves had very similar patterns (Figure 3B ○ and •, respectively). But the following 3 samples showed a progressive divergence between the Ca2+ release curves and the Hb release curves. The change in Hb release pattern shows that the proportion of dehydrated cells decreased progressively from more than 80% in the 4-minutes sample to less than 20% in the 5.5-minutes sample. In contrast, the 45Ca release pattern remained essentially unchanged; more than 80% of the Ca2+ was released at relative tonicities below 0.20. Because dehydrated cells lyse only at these low relative tonicities, the Ca2+ release pattern indicates that in all samples, more than 80% of the mean [CaT]i was always contained within cells that dehydrated during the postincubation period. Thus, the increasing fraction of RBCs that failed to dehydrate during the postincubation period in LK medium always contained less than 20% of the RBCs' mean total Ca2+ content.
These findings indicate that during PMCA-mediated Ca2+ extrusion of a uniform Ca2+ load, a large and progressive heterogeneity of Ca2+ contents is generated among the RBCs, which must be attributed to cell-to-cell differences in PMCA Vmax.
In Figure 3C, the fractions of Hb and Ca2+ released at each relative tonicity are compared directly. The progressive departures from single linearity expose developing heterogeneities of Ca2+ content. At 4.5 minutes and later, with the emergence of low-[CaT]i cells, the curves show 2 distinct slopes reflecting the progressive dissociation between lysis-induced Hb and Ca2+ release. It can be seen that the increase in fractional Hb loss with time (steep slope component) is contributed by RBCs that contain less than 20% of the mean [CaT]i in the cell population.
Two additional samples in Figure 3C (dashed lines) were from cells postincubated in HK media so that they retained their original volume distribution from before the experiment. In these samples, as with all HK postincubated RBCs, regardless of the sampling time during the load-extrusion protocol, the points fell within 5% of the unit-slope line, indicating that when the original volume distribution of RBCs was retained during the load-extrusion protocol and subsequent sample processing, cells with different PMCA Vmax and [CaT]i remained evenly distributed in the cell population and cannot be distinguished by any of the properties that determine the normal lysis curve distribution such as cell volume, area, Hb, and total osmolyte content.5,38 A slope of 1 was also obtained from samples drawn just before cobalt addition. Although not unexpected, since the 45Ca distribution was uniform at that stage, the result is important because it confirms an important assumption that hypotonic swelling of cells to prelytic states, as must take place within all the microwells with hypotonic media, does not increase their Ca2+ permeability.18
The [CaT]i level at which Gardos channels deactivate was estimated from the analysis in Table 2 (from selected data in Table 1). Note that for each of the time points of partial Ca2+ extrusion in Table 2 when the mean RBC [CaT]i was 73, 39, and 17 μmol (340 g Hb)-1 and that of the dehydrated cells was 114, 95, and 78, respectively, the [CaT]i within the cells that did not dehydrate was 15, 6.2, and 2.7 μmol (340 g Hb)-1. Thus, Gardos channel deactivation always occurred when [CaT]i fell below this chosen boundary of 20 μmol (340 g Hb)-1. The results also document the large differences of Ca2+ contents generated shortly after the initiation of net Ca2+ extrusion that result from cell-to-cell variations in PMCA Vmax.
The detailed distribution of PMCA Vmax was investigated in RBCs from 4 donors in 5 experiments that rendered very similar patterns (compiled in Table 3). Figure 4 shows a typical experiment in which the mean PMCA Vmax, measured by linear regression through the first 6 time points after Co2+ addition, was 12.8 mmol (340 g Hb)-1h-1 (Figure 4A). After the mean RBC [CaT]i fell below approximately 100 μmol (340 g Hb)-1, the Ca2+ extrusion rate dropped progressively. During Ca2+ extrusion, frequent sampling for hemolysis curves gave the patterns shown in Figure 4B. Beginning about one minute after Co2+ addition, there was a progressive increase in the fraction of RBCs that failed to dehydrate during the postincubation period in low-K+ media, indicating that they had pumped out their initial Ca2+ loads to levels below those that activate the Gardos channels. The increase in the fraction of such cells was assessed by the upward progression of the quasi-plateau portions of the lysis curves (see “Materials and methods”). The result is shown in Figure 4C where the percent calcium-emptied cells, estimated from the readings at a relative tonicity of 0.3, was plotted as a function of time after Co2+ addition. The experimental points were well-fitted by a sigmoid, saturation-like curve (Figure 4C legend). About 90% of the RBCs emptied their Ca2+ loads within 3 minutes of Co2+ addition, while approximately 10% took longer to complete Ca2+ extrusion.
Statistical parameters of the PMCA Vmax distribution: compiled results from 5 experiments with RBCs from 4 donors
. | PMCA Vmax distribution parameters . | . | . | ||
|---|---|---|---|---|---|
| Experiment . | Mean Vmax* . | SD* . | Skew . | ||
| 1302 | 13.1 | 6.1 | 1.67 | ||
| 0503 | 11.5 | 5.2 | 1.40 | ||
| 1503 | 15.7 | 8.1 | 1.58 | ||
| 1306 | 11.4 | 6.0 | 1.52 | ||
| 0507 | 12.9 | 5.9 | 1.55 | ||
. | PMCA Vmax distribution parameters . | . | . | ||
|---|---|---|---|---|---|
| Experiment . | Mean Vmax* . | SD* . | Skew . | ||
| 1302 | 13.1 | 6.1 | 1.67 | ||
| 0503 | 11.5 | 5.2 | 1.40 | ||
| 1503 | 15.7 | 8.1 | 1.58 | ||
| 1306 | 11.4 | 6.0 | 1.52 | ||
| 0507 | 12.9 | 5.9 | 1.55 | ||
The results were obtained as described in detail in the legend of Figure 4 (experiment 0507).
SD indicates standard deviation.
Values given as mmol (340 g Hb)−1h−1.
Figure 4D (solid line) shows the derivative of the curve fit in Figure 4C estimated by dividing the Ca2+ load by the time interval between Co2+ addition and Ca2+ emptying, plotted as a function of Vmax. This represents the distribution of PMCA Vmax activities among the RBCs.
The results show a broad distribution of PMCA Vmax values with a right-skewed appearance. A symmetrical Gaussian distribution curve (Figure 4D dotted line) was superimposed on the rising branch of the Vmax distribution to highlight its skewed nature. Vmax values varied about 7-fold in this experiment, between 5 and 35 mmol (340 g Hb)-1h-1 with mean and standard deviation of 12.9 and 5.9 mmol (340 g Hb)-1h-1, respectively, and a positive skew of 1.55. The mean Vmax was higher than the mode, as expected from the right skew of the distribution. In all 5 experiments of this series the Vmax distribution was unimodal, broad, and positively skewed with an identical pattern and a range of Vmax variation similar to that shown for the experiment of Figure 4 (6- to 9-fold).
Discussion
The results presented here provide the first detailed description of the distribution of PMCA Vmax activity in normal human RBCs. This distribution characterizes the differences in Ca2+ extrusion capacity among RBCs but carries no information on whether these differences result from disparities in the number of pumps, in the fraction of active pumps, in the factors controlling the turnover rate of the pumps in each cell, in a differential response of the 2 pump isoforms, or in a combination of these.
Earlier findings had indicated the existence of differences in Ca2+ pumping rate among RBC subpopulations, but the actual pattern and extent of variation was unknown. The present results show that there is a large variation in PMCA Vmax among RBCs from single donors and that this variation follows a unimodal, broad distribution pattern with a consistent positive skewness toward higher Vmax values, suggesting an excess of cells with Vmax higher than the mean value. The apparent range of Vmax variation measured in the 5 experiments of this series was 6- to 9-fold. However, it should be noted that the method can detect the early upward deflections in hemolysis curve profiles with high sensitivity (Figure 4B-C), whereas the resolution in the late periods, when nearly all cells have emptied their Ca2+ loads, is less precise. Thus, the possibility cannot be ruled out that there are some RBCs with Vmax values much lower than those apparent from the indicated range.
It is important to rule out a potential artifactual origin of the skewed distribution. Differences in PMCA Vmax among RBCs may result in uneven ionophore-induced Ca2+ loads. The cells with the highest Vmax would be expected to sustain a pump-leak balance with [CaT]i below the measured mean load. The early emptying of their Ca2+ load could result not only from high Vmax values but also from lower Ca2+ loads. Using the mean Ca2+ load to calculate their Vmax could then overestimate their true Vmax values, thus creating a false right-shift in the Vmax distribution. However, Tiffert and Lew27(Fig8) showed that at the high ionophore and [Ca2+]o concentrations used in the present experiments, the difference between measured mean Ca2+ loads in the presence and absence of 1 mM vanadate, was negligible (this finding was repeatedly confirmed in other experiments, not shown). Vanadate at this concentration inhibited more than 99.5% of PMCA-mediated Ca2+ fluxes. Therefore, the differences in Ca2+ load among RBCs must have been minimal and cannot account for the large positive skew observed here.
The 3 main properties of the PMCA Vmax distribution documented here are unimodality, wide-spread, and right-shifted skew. Unimodality indicates that the variations in PMCA Vmax in normal human RBCs are smooth and continuous, thus ruling out distinct cell subpopulations with large differences in Vmax. The 2 isoforms of the PMCA known to be expressed in human RBCs39 may contribute to the observed variation in Ca2+ extrusion capacity among the cells. The unimodal pattern imposes constraints on this potential contribution but does not provide any information about possible functional differences between the 2 isoforms.
The question arises of how the large spread and positive skew of the PMCA Vmax distribution compares with those of other hematologic and transport parameters of RBCs. Thus far, only a few constitutive and functional RBC properties have been examined for population variations. RBC volume, osmotic fragility, Hb content, and Hb concentration all follow symmetric Gaussian distributions with coefficients of variation (in percent) of 13.7, 5.4, 12.5, and 7.0, respectively.5 RBC membrane transporters, whether highly expressed (anion exchanger 1.1 × 106 per cell40,41 ) or weakly expressed (Gardos channels, 100-200 per cell42-44 ), also appear to have parameter distributions with coefficients of variation of less than 7%,41 indicating relative uniformity. On the other hand, prostaglandin E2 was reported to induce Ca2+-dependent Gardos channel activation but only in about 15% of the RBCs, suggesting that receptor expression occurs in selected RBC subpopulations.45 Against this background, the PMCA Vmax distribution shows an unprecedented spread with a coefficient of variation of about 50% (range of 45% to 53% in the 5 experiments [Table 3], 46% in the experiment of Figure 4) and a positive skew, unique among the distributions investigated.
What might be the origin of this unusual distribution? Does it represent a static, constitutive feature of mature RBC populations generated during normal “production line” erythropoiesis or is it a dynamic, age-related property with an age-declining pattern? In other words, are individual RBCs “born” with the Vmax activity they will sustain throughout their approximate 120 days in the circulation, or does PMCA Vmax decline with age? At present, there is no conclusive evidence to distinguish between these 2 alternatives, and results of earlier experiments testing variations in Ca2+ pump Vmax in density-fractionated RBCs were conflicting.21,46,47
If PMCA Vmax does decline with RBC age the pattern would appear to differ from that of most other RBC transporters. In the transition from reticulocyte to mature RBC, the ion transport mediated by the sodium pump and the KCl cotransport (KCC) symport become substantially reduced within the first 2 to 3 days in the circulation.48-52 On the other hand, there is no evidence of early decline in PMCA activity from reticulocyte to mature RBC.53 In the context of the age-decline hypothesis, the broad PMCA Vmax variation pattern points toward a progressive fall in pump activity throughout the life of the mature cell. The possible mechanism of Vmax declines with cell age, such as progressive protease-induced falls in viable pump units per cell,54 metabolically-linked,15,47 progressive glycation,55 or other, remains speculative. The method applied here to study the distribution of PMCA Vmax, by enabling the segregation of RBCs with different Vmax values, may provide a way to investigate the possible age-Vmax relation more directly in the future.
A final consideration arising from the present results concerns the reduction in Ca2+ extrusion rate at relatively high mean [CaT]i levels seen during the Ca2+ extrusion phase of the load-extrusion protocol (Figures 3A,4A). This pattern has been interpreted in the past as reflecting desaturation of Ca2+ by the PMCA and was used to derive kinetic parameters of the pump.21,56 As explained above in the analysis of Figure 2A, the wide PMCA Vmax distribution documented here (Figure 4D) fully accounts for the observed apparent Ca2+ desaturation pattern that starts at high mean [CaT]i levels. Thus, apparent desaturation at high [CaT]i is an indicator of the presence of low-Vmax RBCs and carries no information on the kinetics of PMCA activation by [Ca2+]i.
Prepublished online as Blood First Edition Paper, August 14, 2003; DOI 10.1182/blood-2003-06-1787.
Supported by grants from the Wellcome Trust (United Kingdom) and National Institutes of Health (HL28018, HL58512 and RR12248).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are grateful to the Wellcome Trust, United Kingdom; and to the National Institutes of Health, United States, for funds.

![Figure 1. Diagram of the RBC transport systems that participate in Ca2+-induced dehydration. The top part of the figure shows the transporters of Ca2+, Cl-, and H+ responsible for the net gain of CaCl2 by RBCs when exposed to the ionophore A23187. With RBCs suspended in a Ca2+-containing plasmalike medium, addition of the ionophore A23187 triggers an electroneutral entry of Ca2+ in exchange for protons with a Ca2+:2H+ stoichiometry. With CO2 concentrations at equilibrium across the RBC membrane, the anion exchanger (AE) and CO2 shunt operate jointly like an electroneutral Cl-:H+ cotransport,23 known as the Jacob-Stewart mechanism (JS).24-26 Together, the ionophore and JS mediate net CaCl2 transport since the proton fluxes cancel out. Ca2+ transport is rate limited by the ionophore concentration because the high constitutive expression of the AE ensures nonlimiting speed in the co- and counter-ion transfers. The cytoplasmic Ca2+-buffering behavior of RBCs (B, CaB) may be approximated by [Ca2+]i = α[CaT]i, where α is approximately 0.3 over a wide range of [Ca2+]i values and [CaT]i is the total calcium content of the cells.27,28 Thus, normal RBC [CaT]i levels are hardly detectable, among the lowest of any cell in nature.29-31 At sufficiently high ionophore concentrations (usually > 10 μM in 10% hematocrit RBC suspensions) and appropriate external Ca2+ concentrations ([Ca2+]o > 100 μM), the induced Ca2+ influx exceeds the Vmax of the PMCA in all the RBCs causing their [Ca2+]i levels to approach equilibrium with [Ca2+]o in a uniform manner.20 The ionophore-induced equilibrium sets [Ca2+]i /[Ca2+]o = ([H+]i/[H+]o).2,32 Addition of Co2+ in excess of [Ca2+]o instantly blocks Ca2+ transport by the ionophore allowing the PMCA to extrude the induced Ca2+ load.6,33 Co2+ is itself transported by the ionophore but at normal [Mg2+]i levels it has no effect on PMCA-mediated Ca2+ fluxes.34 The bottom part of the figure shows the transport systems that participate in the Ca2+-induced rapid dehydration response. Elevated [Ca2+]i triggers the dehydration process by activating the Gardos channels. Gardos channel activation hyperpolarizes the cell (E, membrane potential) driving Cl- out, resulting in the net loss of KCl and water. With full Gardos activation, dehydration is rate limited by the Cl- permeability.35 Replacement of approximately 10 mM Cl- by the more permeable anion SCN- maximizes the dehydration rate because rapid SCN-:Cl- exchange via AE, or SCN- diffusion across the membrane (dashed line), continually replenish the intracellular SCN- supply.18 Gardos-mediated dehydration has a very low temperature coefficient. With nonlimiting anion movement, maximal dehydration of RBCs may be attained within 10 to 30 minutes at 0°C to 4°C.18](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/12/10.1182_blood-2003-06-1787/6/m_h82335314001.jpeg?Expires=1767704247&Signature=fk5S886GMKWwQ8GXhjuuXEBrNwrEzxD81oPpyRs7lvIPhkEHDVNY2k2nTSRYEr10nZuuFo0CAUwpVKmoaTBKI0c1oi9R6h9BT3lLvmrykwCP~2dLr5PhveBPCCs~ri0dQeeogW5OkpmzHP95i55vxfNFqnKZ2lYaFHrGGPKuHqIr2cxFSDU~qXRevgpbLwf6lw~Ox5UmBjb9VZIWITU0Kd6lrDeI3Nu~5htbgGplZhn1UlRCgdqt72Ad2tzXEiNd72TuLHkZO5DzZ7PdcIe4dsSniKwYkaejVOfG5oOMUDs6xAl~4yvAweLxBihb64E~WRxJ8Qkj4cL6OKtWgF3~RA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 2. Method used to investigate the PMCA Vmax distribution in RBCs. (Panel A) Computer simulation of the Ca2+ load-extrusion protocol in an RBC population whose symmetrical distribution of PMCA Vmax activity is shown in the inset. Substrate-fed RBCs are assumed to be suspended at an Hct of approximately 10% in an iso-osmotic buffer containing 80 mM K+ and 100 μM CaCl2 at 37°C with magnetic stirring. A high ionophore concentration is used to rapidly induce a large and uniform RBC Ca2+ load. After about 2 minutes (to prevent significant reduction of cell ATP levels) Co2+ is added in excess of Ca2+ in the medium to block ionophore-mediated Ca2+ transport and to expose the uphill extrusion of Ca2+ by the pump. [CaT]i was estimated in timed samples with the use of 45Ca by dividing the measured cell-contained 45Ca radioactivity by the specific activity of 45Ca (there are no endogenous Ca2+ pools that could dilute the specific activity set by the addition of tracer). Each of the Ca2+ extrusion curves was obtained by solving numerically the differential equation d[Ca2+]i /dt = (Vmax)([Ca2+]i2/(Kd2 + [Ca2+]i2)), using a different Vmax value for each curve and applying the conversion [CaT]i = [Ca2+]i /α (Figure 1 legend). The thick line was computed as the weighted mean of the Vmax distribution shown in the inset. Note that the Ca2+-extrusion curves with different Vmax remain linear until [CaT]i falls to about 2 to 4 μM, as expected from a Ca2+ desaturation kinetics with Kd values in the range of 0.2 to 0.5 μM.2,4,36 The mean-Vmax curve also renders a linear segment with a slope close to the true mean Vmax of the distribution (approximately 14 mmol (340 g Hb)-1h-1) but with an apparent Ca2+ desaturation pattern starting at [CaT]i approximately 100 μmol (340 g Hb)-1 a level much higher than that of the single-Vmax desaturation curves. This specious desaturation effect results from retention of Ca2+ within the low-Vmax cells. Points A to E mark sampling times for hemolysis curves expected to have the patterns illustrated in panel B. The horizontal dashed line is at the [CaT]i level above which Gardos channels are assumed to open maximally. (Panel B) Actual hemolysis curves from preliminary experiments illustrate the patterns expected with samples taken at the time points A (▴) to E (•) of the load-extrusion protocol simulated in panel A.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/12/10.1182_blood-2003-06-1787/6/m_h82335314002.jpeg?Expires=1767704247&Signature=IMj0jI-CfS95h0zIjY8ixX4NPqbugonpPM2AL32L-A~1GbUYrzv~cB-DxI99YCgCxfxR-~SS~QvtfkV2m4sfdhTeUkxXMzKBmPUM0utb9zod88vwsFt2niJRJynQgjGZY~5-xDwxIA0iVYPRWmgEHWNj8WkuE7JT07rYtP6P0zTbYlKWy-IDqS7mnHvnbjKdSH31gBrUmxLVmPCqCIu9I-rkoGRPKLe5cdePM6GgaW5f6Jnw47dL~M~Duvd30G-k8QuZeFonbNW-IMKh8KhZ~AUKOf5sNrZfS76EvtBqebSawk8DzSV99-XUCNPorTwk3KAMfDL9zig76cvdbNTR6w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. Distribution of PMCA Vmax among normal RBCs. One experiment with protocol 2 is shown (see “Experimental protocols”) representative of 5 with similar results. (A) Duplicate measurement of the PMCA Vmax by the load-extrusion protocol. The indicated mean Vmax values were obtained by linear regression through the first 6 time-points after cobalt addition. The mean rate of Ca2+ extrusion fell progressively when [CaT]i dropped below about 100 μmol (340 g Hb)-1. (B) RBCs from the same batch as those used for the Vmax measurements reported in panel A underwent the load-extrusion protocol without 45Ca tracer and were sampled for hemolysis curves at the indicated times after ionophore addition (the number of curves exceeded the repertoire of available symbols in the software used leading to cyclic repetition; however, the bottom-up sequence can be followed unambiguously). All samples were postincubated in LK medium. There was a monotonic upward progression of the curves with time from the fully dehydrated pattern (1-min point) to the fully recovered pattern (7-min point). (C) Percent cells with emptied Ca2+ loads as a function of time after cobalt addition (Δt). The percent of Ca2+-emptied RBCs was computed from the ordinate readings shown in panel B at a relative tonicity of 0.3, taking the distance between lowest and highest reading as 100% (see “Materials and methods”). The equation y = axn/(bn + xn) was found to give a good empirical fit of the experimental points in the 5 experiments of this series, slightly better than that obtained with other sigmoid saturation functions, as judged by eye and by least means squares analysis. The parameter values obtained by least mean squares fit to the equation in this experiment were as follows: a = 100, b = 2.2, and n = 5.5. (D) Derivative of the curve fit through the experimental points in panel C (continuous line) compared with a Gaussian curve fit to the rising branch of the derivative curve (dotted line). Both curves are plotted as a function of Vmax estimated from (Ca2+ load)/Δt. The Ca2+ load in the suspension used for hemolysis curve sampling (B) was 390 μmol (340 g Hb)-1. Compared with the Gaussian curve, the derivative curve reporting the actual Vmax distribution among RBCs shows a marked right-shifted skew, observed in all the experiments of this series (Table 3). The parameter values of the actual Vmax distribution are shown in the figure and were calculated as follows: mean = Σ(yixi)/Σyi;SD = ((Σ(yi(xi-mean))2)/Σyi)1/2; Skew = (Σ((yi(xi-mean))/SD)3)/Σyi.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/12/10.1182_blood-2003-06-1787/6/m_h82335314004.jpeg?Expires=1767704247&Signature=nV1Eu3TVEILIrfOgj5MEQPsOUEMEIPotT~Ectkj7cUoLHLBXAXsHchY3k0vTdGjdMU2jr1aMkko1yaAHjmGGx5NhKuSzrUVa0Nk1nGpbrU2ZbgVOPmbFfogMRbeQ3uoculYnoFK95IP3cPkoChH7RYx2Do5bjtFvhBqoLE7gWsD5Lp4S7o78wxYyJABkFTSTF3Nkd~b360IAWDTMCBmU2WyT3QrCJfaI3HTbAJf5O4VwV4nrbeKxbkzdfBilMn9l1oI2EIik08~s8hixDgY6LXVSorc5GEe327jQ3WSnJSWjcdvRdcC-7umEgm00uRnkP15KDfOIpx0xtmAt8uNzqA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal