Abstract
Eosinophilia is common in myeloproliferative disorders (MPDs) with abnormalities of chromosome band 5q31-33, including those that present with t(1;5)(q23;q33). With the development of rational drug therapy, characterization of the molecular targets for these translocations could guide treatment and affect patient survival. We cloned the t(1;5)(q23;q33) and showed that it fuses platelet-derived growth factor receptor beta (PDGFRB) to the coiled-coil domains of a novel partner protein, myomegalin. Using two-color interphase fluorescence in situ hybridization (FISH), we also demonstrated that the eosinophils are clonal in these disorders. Imatinib mesylate has recently been shown to be efficacious in MPDs with PDGFR activation. Therefore, following our molecular studies, we were able to redirect this patient's treatment. Although she had refractory and progressive disease, once imatinib was started, complete clinical and hematologic remission, as well as major cytogenetic response, was achieved. Given the therapeutic implications, our findings stress the need to aggressively investigate the molecular basis of these diseases, with emphasis on the PDGFR family. (Blood. 2003;102: 4187-4190)
Introduction
The cloning of genes affected by recurrent chromosomal translocations has furthered our understanding of the molecular basis of hematologic malignancies and allowed the development of molecular assays that have improved diagnosis and monitoring of therapy. It has also led to the development of rational targeted drug therapy, including the specific tyrosine kinase inhibitor, imatinib mesylate1 (Gleevec; Novartis, Basel, Switzerland).
Eosinophilia is common in several myeloproliferative disorders (MPDs), notably chronic myeloid leukemia (CML) and the 8p11 myeloproliferative syndrome.2,3 Less common causes of eosinophilia include MPDs associated with translocations that involve chromosome band 5q31-33.4 Cloning of these translocations4-9 showed that they consistently target the PDGFRB gene. In all cases, partner genes contribute oligomerization domains that constitutively activate the tyrosine kinase of platelet-derived growth factor receptor beta (PDGFRB). Recently, cryptic deletions of chromosome 4q12 resulting in activation of PDGFRA were found in cases of hypereosinophilic syndrome (HES),10 suggesting that activation of the PDGFR receptor tyrosine kinase family is a common theme in this phenotypically similar collection of disorders.
Study design
An 11-month-old girl presented with malaise, poor feeding, and hepatosplenomegaly. The initial blood count was hemoglobin (Hb) 60 g/L (6.0 g/dL), white blood cell (WBC) count 43.9 × 109/L with a neutrophilic left shift, marked eosinophilia, monocytosis, and thrombocytopenia. After a bone marrow examination, the diagnosis of an myelodysplastic syndrome (MDS)/MPD syndrome with eosinophilia was established. Karyotype revealed a t(1;5)(q23;q33) in 100% of the bone marrow metaphases. The patient was refractory to conventional chemotherapy, including etoposide, cytarabine, and interferon. At the start of imatinib, 1 year after the diagnosis, the patient had progressive disease, and a new cytogenetic study confirmed the presence of the t(1;5) in 100% of metaphases.
All of the laboratory investigations and treatment decisions were approved by the ethics committee of the patient's institution of origin (Hospital AC Camargo, Brazil). Written consent was obtained from the patient's guardians.
Molecular cloning
RT-PCR
Single step reverse transcriptase (RT)-PCR was used for detection of the PDE4DIP-PDGFRB and reciprocal PDGRFB-PDE4DIP fusions. To define the specific PDE4DIP (myomegalin) isoform fused to PDGFRB, a long-range nested RT-PCR was performed. All oligo sequences are available on request. All relevant PCR products were sequenced.
Western blot
Protein was isolated from the patient's peripheral blood cells at diagnosis and 2 months into imatinib treatment. Immunoblotting was performed as previously described,13 with total AKT and phosho-AKT (S473) antibodies used as directed by the manufacturer (Cell Signaling, Beverly, MA).
Two-color fluorescence in situ hybridization
An eosinophil-enriched cell population was isolated from the patient's peripheral blood as described previously.14 Bacterial artificial chromosome (BAC) clones mapping centromeric (CTC-307M15, CTB-46E9, and CTB-13H5) and telomeric (CTB-5M9, CTB-108B20, and CTB-171P15) to the PDGFRB locus were from ResGen (Carlsbad, CA). BAC DNAs were hybridized to the patient's slides as described previously.3 After counterstaining with DAPI (4′,6′diamedino-2-phenylindole), images were processed using an Olympus AX70 fluorescence microscope and Genus imaging software (Applied Imaging, Santa Clara, CA). To assess posttreatment samples for cytogenetic response, cells were spun onto slides, fixed, and hybridized to PDGFRB probes as described earlier. Cells were scored for the presence of PDGFRB rearrangements on the BioView Duet Imaging system (Rehovot, Israel).
Results and discussion
Molecular cloning of the t(1;5)(q23;q33)
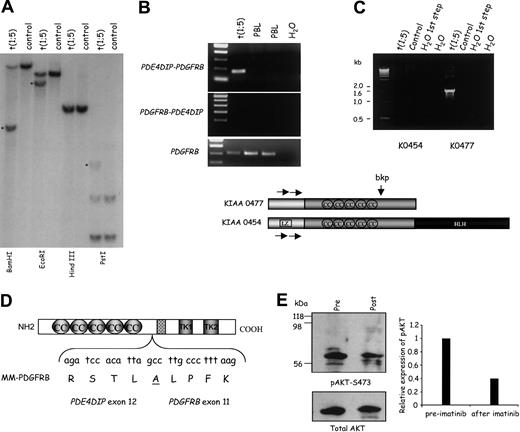
With Southern blot analysis, we detected rearrangement of the PDGRFB gene in the t(1;5) DNA (Figure 1A). Subsequently, sequencing of several 5′ RACE-PCR clones consistently showed the same novel DNA sequence fused in-frame to PDGFRB exon 11. Database search confirmed that these sequences mapped to chromosome 1q21-23 and corresponded to a novel gene, PDE4DIP (phosphodiesterase 4D interacting protein, myomegalin).15 Southern blot analysis with a PDE4DIP probe confirmed its disruption in this translocation (data not shown).
PDGFRBis fused toPDE4DIPin an MPD associated with eosinophilia and t(1;5)(q23;q33). (A) Southern blot analysis of the t(1;5) and control DNA with a HindIII-XhoI PDGFRB genomic probe spanning exon 11. Rearranged bands are indicated by a star. Cloning of the genomic breakpoints for this fusion confirmed that the wild-type PDGFRB and PDE4DIP-PDGFRB HindIII restriction fragments are of very similar size and, therefore, comigrated in the Southern blot. At DNA level, the fusion of these genes occurs at IVS 12-767 (PDE4DIP) and IVS 10+289 (PDGFRB). (B) Single-step RT-PCR confirms the expression of the PDE4DIP-PDGFRB fusion. The reciprocal fusion is not expressed. PDGFRB RT-PCR confirms the integrity of the cDNAs. (C) Top: Isoform-specific nested RT-PCR showing that the PDE4DIP isoform lacking the LZ domain (KIAA 0477) is fused to PDGFRB in the t(1;5). Below: Diagram of the primary protein structure of human myomegalin, putative oligomerization domains, the breakpoint in the t(1;5), and the location of the primers (horizontal arrows) used in the isoform-specific nested RT-PCR. (D) Diagrammatic representation of the myomegalin (MM)-PDGFRB protein fusion and contributing nucleotides, amino acids, and domains. (E) Left panel: Western blot analysis showing lower levels of phosphorylated AKT (top subpanel) in the patient's primary cells 2 months into imatinib treatment. Total AKT is shown in the lower subpanel. Right panel: Densitometry of relevant bands showed a 60% reduction in the expression of phospho-AKT after imatinib.
PDGFRBis fused toPDE4DIPin an MPD associated with eosinophilia and t(1;5)(q23;q33). (A) Southern blot analysis of the t(1;5) and control DNA with a HindIII-XhoI PDGFRB genomic probe spanning exon 11. Rearranged bands are indicated by a star. Cloning of the genomic breakpoints for this fusion confirmed that the wild-type PDGFRB and PDE4DIP-PDGFRB HindIII restriction fragments are of very similar size and, therefore, comigrated in the Southern blot. At DNA level, the fusion of these genes occurs at IVS 12-767 (PDE4DIP) and IVS 10+289 (PDGFRB). (B) Single-step RT-PCR confirms the expression of the PDE4DIP-PDGFRB fusion. The reciprocal fusion is not expressed. PDGFRB RT-PCR confirms the integrity of the cDNAs. (C) Top: Isoform-specific nested RT-PCR showing that the PDE4DIP isoform lacking the LZ domain (KIAA 0477) is fused to PDGFRB in the t(1;5). Below: Diagram of the primary protein structure of human myomegalin, putative oligomerization domains, the breakpoint in the t(1;5), and the location of the primers (horizontal arrows) used in the isoform-specific nested RT-PCR. (D) Diagrammatic representation of the myomegalin (MM)-PDGFRB protein fusion and contributing nucleotides, amino acids, and domains. (E) Left panel: Western blot analysis showing lower levels of phosphorylated AKT (top subpanel) in the patient's primary cells 2 months into imatinib treatment. Total AKT is shown in the lower subpanel. Right panel: Densitometry of relevant bands showed a 60% reduction in the expression of phospho-AKT after imatinib.
The chimeric PDE4DIP-PDGFRB mRNA was readily detectable in the patient with the t(1;5)(q23;q33), but not in 2 healthy control subjects (Figure 1B). The reciprocal PDGFRB-PDE4DIP fusion is not expressed (Figure 1B). Myomegalin is the protein encoded by PDE4DIP, and it was characterized because of its binding to the phosphodiesterase PDE4D.14 Of relevance for this report, myomegalin encodes several putative oligomerization domains capable of activating PDGFRB. They include a leucine zipper (LZ) domain and several coiled-coil structures (Figure 1C). In humans, there are at least 2 major isoforms of myomegalin (KIAA0454 and KIAA0477),15 encoding unique N and C termini (Figure 1C). This feature is of significance because one of these isoforms encodes an N-terminal LZ domain. Surprisingly, we found that in our case PDGFRB is fused to the PDE4DIP isoform lacking the LZ domain (KIAA0477) (Figure 1C), indirectly implicating the coiled-coil domains in the constitutive activation of PDGFRB.
In this fusion protein the first 905 amino acids of myomegalin are joined in-frame to the transmembrane and tyrosine kinase domains of PDGFRB (Figure 1D). It is highly likely that deregulation of PDGFRB activity is the major pathogenic defect in this MPD.
Clonality of the eosinophils in MPD with t(1;5)(q23;q33)
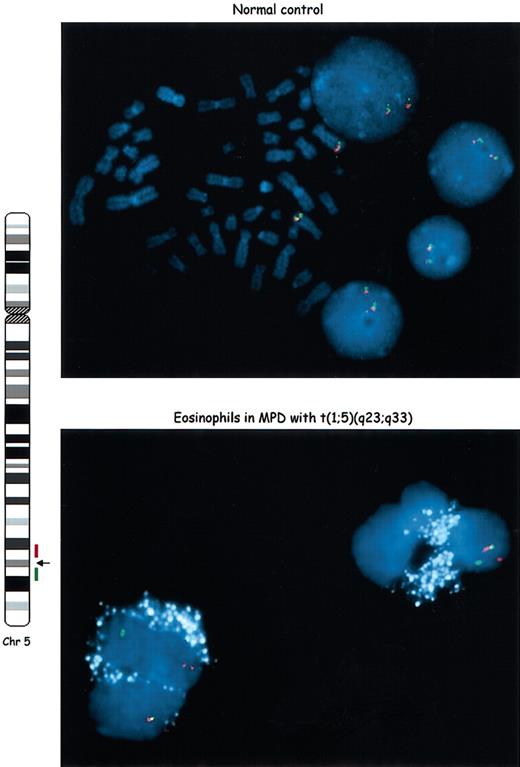
Eosinophilia is a common feature of several hematologic malignancies. It is generally assumed that, when a clonal cytogenetic abnormality has been demonstrated in a MDS/MPD with eosinophilia, these cells are part of the neoplastic clone. However, this is not always true,16-18 and only rarely has the clonality of the eosinophils been clearly established.19 As seen in Figure 2, eosinophils are readily identified on the basis of the autofluorescence of eosinophilic granules, a feature that is unique among cells of the granulocytic series.19-21 These granules were pseudo-colorized white to allow for clear visualization of the fluorescence in situ hybridization (FISH) signals. Using a two-color interphase FISH assay with probes flanking the PDGFRB locus, we observed that this patient's eosinophils have rearrangements of PDGFRB and are, therefore, components of the malignant clone (Figure 2).
The eosinophils have a rearrangedPDGFRBlocus and are part of the neoplastic clone. We used a two-color interphase FISH assay in an eosinophil-enriched preparation to define the clonality of these cells. Differentially labeled BAC clones located centromeric (red) or telomeric (green) to the PDGFRB breakpoint region in the t(1;5) were used as probes. Schematic representation of chromosome 5, location of breakpoint and probes is shown on the left side. In normal metaphases and interphases (top panel) the red and green signals are juxtaposed (yellow) in both chromosomes 5. In the patient's peripheral blood eosinophils (bottom panel), one pair of signals is juxtaposed (normal chromosome 5), whereas the other signals are split, indicating rearrangement of the PDGFRB locus. The eosinophilic granules were pseudo-colorized in white because of their auto-fluorescence. Original magnification, × 1000.
The eosinophils have a rearrangedPDGFRBlocus and are part of the neoplastic clone. We used a two-color interphase FISH assay in an eosinophil-enriched preparation to define the clonality of these cells. Differentially labeled BAC clones located centromeric (red) or telomeric (green) to the PDGFRB breakpoint region in the t(1;5) were used as probes. Schematic representation of chromosome 5, location of breakpoint and probes is shown on the left side. In normal metaphases and interphases (top panel) the red and green signals are juxtaposed (yellow) in both chromosomes 5. In the patient's peripheral blood eosinophils (bottom panel), one pair of signals is juxtaposed (normal chromosome 5), whereas the other signals are split, indicating rearrangement of the PDGFRB locus. The eosinophilic granules were pseudo-colorized in white because of their auto-fluorescence. Original magnification, × 1000.
Response to imatinib in MPD with t(1;5)(q23;q33)
After documenting the PDGFRB disruption in this patient, she received a trial of imatinib therapy. All clinical and hematologic abnormalities rapidly resolved. The bone marrow values were normal at 5 months. FISH of peripheral blood cells after 7 months of therapy showed the t(1;5) in only 7.1% of the cells (split apart PDGFRB signals in 8 of 106 cells, versus 0 of 210 cells from a healthy control). We also evaluated the effects of imatinib on known PDGFRB targets in vivo, comparing AKT phosphorylation in the patient's primary cells before and after treatment. We demonstrated that after treatment, there was a marked decrease in the phosphorylation of AKT (Figure 1E). Because AKT is one of the well-defined targets of PDGFRB mitogenic and transforming effects in hematopoietic cells, via activation of the phosphatidylinositol 3 kinase (PI3K) pathway,4 these studies are consistent with imatinib specifically targeting the deregulated PDGFRB in this patient's MPD.
In summary, we have shown that the t(1;5)(q23;q33) targets PDGFRB and that complete remission can be achieved with imatinib in a heavily pretreated patient with progressive disease. Of interest, although only a few cases of MPD with t(1;5)(q23;q33) have been described,11,12 this disease appears to be more common in infants. It also lacks the extreme male bias found in other cases of MPD associated with PDGRB activation.4 Our results confirm and extend the clinical relevance of imatinib treatment in cases of MPD with eosinophilia.22,23
Prepublished online as Blood First Edition Paper, August 7, 2003; DOI 10.1182/blood-2003-04-1150.
Supported in part by the Leukemia and Lymphoma Society of America
(R.C.T.A.). R.C.T.A. is a V Foundation Scholar.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Patricia Dahia, Charles Stiles, and James Griffin (Dana-Farber Cancer Institute); Beatriz Beitler and Pedro Dorlhiac (Hospital das Clinicas, University of São Paulo); Nick Cross (Wessex Regional Genetics Laboratory, United Kingdom); and David Parkinson (Novartis, United States). Imatinib was provided on a compassionate basis by Novartis, Brazil.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal