Abstract
We have recently identified 2 patients with a rare autosomal recessive form of hyper IgM disease, known as HIGM3, caused by mutations in the CD40 gene. These patients had opportunistic infections observed on X-linked hyper IgM syndrome (HIGM), suggesting that the CD40-CD40 ligand interaction is important for promoting T-cell-mediated immunity. To evaluate whether innate immunity signals may substitute CD154 for inducing the maturation of dendritic cells (DCs), we analyzed monocyte-derived DCs in these patients. Monocyte-derived DCs of HIGM3 subjects on ex vivo stimulation with tumor necrosis factor-α (TNF-α) or lipopolysaccharide (LPS) combined with interferon-γ (IFN-γ) normally express all the markers of mature DCs, such as CD83 and DC-LAMP. However, cell surface levels of HLA-DR in mature DCs are reduced, as is costimulatory activity of these cells for allogeneic naive T cells. In addition, CD40-deficient DCs secrete lower amounts of interleukin-12 (IL-12) but larger quantities of IL-10 than control subjects. Finally, analysis of circulating plasmacytoid DCs demonstrates a normal percentage of this subset in CD40-deficient cells, but IFN-α secretion in response to herpes simplex virus 1 (HSV-1) infection is severely reduced in patients. These observations suggest that the severe impairment of DC maturation may contribute to the defect of T-cell-mediated immunity observed in HIGM3 patients. (Blood. 2003;102:
Introduction
CD40-CD154 interaction is an essential step for triggering the adaptive immune response, as demonstrated by the immunologic defects observed in patients with mutations of the CD40-ligand gene (CD40L, CD154). In these patients a primary immunodeficiency known as type 1 hyper IgM syndrome (HIGM1) develops that is characterized by an X-linked pattern of inheritance and by a combination of recurrent bacterial and opportunistic infections.1-3 We have recently reported that patients with homozygous mutations of CD40 have a disease termed HIGM3. Its clinical phenotype overlaps with that of HIGM1 and is characterized by the defective production of secondary antibodies and severe opportunistic infections.4,5 In contrast, patients with the autosomal recessive form of hyper IgM have a disease termed HIGM2, which is caused by mutations of the B-cell-restricted molecule activation-induced cytidine deaminase (AID). These patients are not at risk for infections elicited by opportunistic pathogens.6 The increased occurrence of infections in patients with HIGM1 and HIGM3, particularly Cryptosporidium enteritis and Pneumocystis carinii pneumonia, suggests that CD40 and CD154 have nonredundant roles in controlling T-cell-mediated immunity,1 which is most likely dependent on the expression of CD40 by dendritic cells (DCs).
DCs are at the intercross between innate and adaptive immunity and have a prominent role in the initiation of the primary immune response because they are specialized in the uptake, processing, and presentation of antigens to T lymphocytes.7,8 Two distinct subsets of DCs have been identified in peripheral blood—myeloid DCs and plasmacytoid DCs. Myeloid DCs express CD11c. Plasmacytoid DCs are identified by the cell surface expression of CD123 and the secretion of interferon-α (IFN-α) during influenza virus or herpes simplex virus (HSV) infection. Myeloid and plasmacytoid DCs express CD40 and can be induced to maturation by CD154 stimulation.9,10
To stimulate naive T cells, DCs must undergo a maturation process that is characterized by several factors: the up-regulation of major histocompatibility complex (MHC) class II and costimulatory molecules CD80 and CD86, the expression of maturation markers CD83 and DC-LAMP, and the secretion of interleukin-12 (IL-12).11 DC maturation can be induced by various stimuli, some of which are provided by innate immunity while others, such as CD154, are dependent on the activation of adaptive immunity. Lack of cognate T-cell signals results in impaired production of IL-12, which is required to induce a protective response against opportunistic pathogens such as Cryptosporidium and Pneumocystis.12,13 Indeed, HIGM1 patients display fewer circulating memory T cells and poor IL-12 secretion by peripheral blood mononuclear cells (PBMCs), suggesting defective cooperation of T lymphocytes with antigen-presenting cells (APCs).12-14 Inflammatory and microbial signals may provide an alternative pathway for DC maturation15 that may be sufficient to substitute for the lack of T-cell-derived signals. Tumor necrosis factor-α (TNF-α), a proinflammatory cytokine homologous to CD154, and the gram-negative derivative lipopolysaccharide (LPS), have been shown to induce DC maturation by enhancing the expression of costimulatory molecules and MHC class II and the secretion of IL-12.7 However, the hypothesis that innate immunity signals, along with activated CD4+ cells, may completely substitute for the cognate interaction of DCs is challenged by the observation that patients who lack CD154 (HIGM1) or CD40 (HIGM3) acquire opportunistic infections suggestive of a profound defect in T-cell immunity.4,5,16
To evaluate the maturation and costimulatory function of DCs in the absence of CD40, we generated DCs from blood-derived monocytes in 2 subjects who lacked CD40 expression. We show here that monocytes obtained from HIGM3 patients can be differentiated in vitro into immature DCs with stellate morphology, normal macropinocytosis, and normal levels of costimulatory molecules such as CD80 and CD86. However, mature DCs of CD40-deficient cells that were obtained after culture with TNF-α or with LPS combined with IFN-γ displayed a consistent defect in the ability to induce the proliferation of allogeneic T cells and the secretion of IFN-γ. The defective costimulatory activity of DCs derived from patients with CD40 deficiency was associated with lower cell surface levels of MHC class II antigen and with a decreased release of IL-12. Compared with the DCs of healthy subjects, this suggests that the lack of CD40 triggering results in the generation of cells with impaired costimulatory activity for T cells.
Patients, materials, and methods
Patients
The patients studied were a 9-year-old Italian girl born of consanguineous parents (patient 1) and a 2-year-old Turkish girl (patient 2). Approval for these studies was obtained from the Spedali Civili of Brescia institutional review board. When blood samples were obtained, the patients displayed no evidence of severe infections except respiratory infections. Generating DCs from 2 age-matched controls who were admitted to the hospital for respiratory infections showed no differences in DC maturation markers or mixed-lymphocyte reaction (MLR). None of the experiments could be repeated with patient 2 because she underwent bone marrow transplantation. The clinical and immunologic descriptions of these patients have been previously reported.4,5 For each experiment, a healthy subject or an age-matched child, or both, were included for comparison. The control group was used with informed consent and consisted of healthy adult donors or age-matched children who were admitted to the hospital for minor head trauma.
Reagents
Human recombinant granulocyte macrophage-colony-stimulating factor (GM-CSF), IL-4, TNF-α, and IFN-γ were obtained from PeproTech (Rocky Hill, NJ) or from SanverTech (Bocheout, Belgium). CD154 trimer was obtained from R&D Systems (Minneapolis, MN). All reagents and media, which were tested by the endotoxin kit (Sigma, St Louis, MO), contained endotoxin at concentrations below detection levels (12 pg/mL). LPS (Escherichia coli 055:B5) was purchased from Sigma.
Flow cytometric analysis
Immunofluorescent staining was performed after washing the cells twice with phosphate-buffered saline (PBS) plus 0.5% fetal calf serum (FCS). Cells were incubated for 20 minutes at 4°C with one of the following murine monoclonal antibodies (mAbs): phycoerythrin (PE)-conjugated anti-CD40, anti-CD86, and anti-HLA-DR, all from Becton Dickinson (Mountain View, CA); anti-CD83 from Coulter-Immunotech (Marseilles, France); and fluorescein isothiocyanate (FITC)-conjugated anti-CD14 from DAKO (Copenhagen, Denmark). Anti-CD40 and anti-CD80 both from Becton Dickinson, and anti-CD1a from Coulter-Immunotech. Isotype-matched mAbs from Becton Dickinson were used for each experiment. Cells were analyzed by flow cytometry using the FACSCalibur and CELLQuest software from Becton Dickinson. For intracellular staining of T cells, FITC-conjugated anti-IFN-γ from R&D Systems was used. Intracellular staining was performed using a Cytofix/Cytoperm Plus Kit from BD PharMingen (San Diego, CA) according to the manufacturer's instructions.
Levels of circulating dendritic cells were determined using whole blood as previously described.17 Two hundred microliters blood was incubated at 4°C with a mixture of antilineage FITC-conjugated mAbs, including CD3, CD19, CD56, and CD14, all from Becton Dickinson. HLA-DR-PerCP (Becton Dickinson) was added to the mix with one of the following: CD123-biotin (BD PharMingen) + streptavidin-PE (Jackson Immunoresearch, West Grove, PA), or CD11c-PE. Isotype-matched mAbs from Becton Dickinson were used for each experiment. Myeloid DCs were identified as Lin-HLA-DR+CD11c+, and plasmacytoid DCs were identified as Lin-HLA-DR+CD123+ cells. After staining, red blood cells were lysed using lysing buffer from Becton Dickinson, washed twice with PBS plus 0.5% FCS, and analyzed in FACSCalibur with CellQuest software from Becton Dickinson. The fluorescence of at least 500 000 events was accumulated for analysis.
Cell culture
Generation and functional analysis of DCs was carried out independently in 2 different laboratories. Peripheral blood mononuclear cells (PBMCs) were purified by Ficoll-Hypaque separation density-gradient centrifugation (Amersham Pharmacia Biotech, Uppsala, Sweden). Monocytes were purified by Percoll separation medium from Pharmacia Biotech or by adherence as previously described.18 Monocytes were more than 97% pure as determined by direct immunofluorescence assay using the mAb CD14 from DAKO. Cells were cultured in RPMI 1640 (HyQ; Hy Clone Europe Ltd, Cramlington, United Kingdom), containing 100 U/mL penicillin, 100 μg/mL streptomycin, 2 mM l-glutamine (Life Technologies, Paisley, United Kingdom), 20 mM HEPES (N-2-hydroxyethylenepiperazine-N′-2-ethanesulfonic acid) (Imperial, United Kingdom), and 10% heat-inactivated fetal calf serum (FCS; Boehringer Mannheim, Germany). Dendritic cells were generated in vitro from blood-derived monocytes as previously described.19 Briefly, to obtain immature DCs, monocytes were cultured for 6 days at 1 × 106 cells/mL in 24-well tissue culture plates from Nunc, (Roskilde, Denmark). The monocytes were cultured in RPMI 1640 with 10% FCS supplemented with GM-CSF (250 ng/mL) and IL-4 (250 ng/mL). When indicated, DCs were cultured for 2 more days with one of the following: TNF-α, CD154 trimer, LPS, LPS+IFN-γ, or medium only as a control. For cytofluorometric and immunocytochemical stainings, 24-well macrotiter plates were coated with TNF-α or CD154 trimer at the concentration of 1 μg/mL for 1 hour at 37°C. Before DCs were added, the plates were washed with PBS. For IL-12 and IL-10 production assays, the wells were coated with CD154 trimer before DCs were added, and LPS (1 μg/mL) and IFN-γ (500 ng/mL) were added to the cultured cells. For allogeneic mixed-lymphocyte experiments and IL-10 neutralization assays, one of the following was added to the culture wells: TNF-α (50 ng/mL), LPS (250 ng/mL), or a combination of LPS (250 ng/mL) and IFN-γ (500 ng/mL). After 7 days of culture, DCs generated in vitro were CD1a+CD14-.
T-cell proliferation and MLR
T cells were isolated from PBMCs by incubation with anti-CD19-, -CD56-, and -CD14-coated magnetic beads followed by immunomagnetic depletion (Dynabeads). To evaluate T-cell proliferation, T lymphocytes from patients and healthy donors were stimulated for 96 hours in 96-well plates. The plates had been previously coated with anti-CD3 UCTH1 mAb (1 μg/mL) (BD PharMingen) or with medium only. For MLR experiments, immature DCs and LPS + IFN-γ mature DCs from controls and patients were added at graded doses to 1.5 × 105 freshly isolated CD3+ allogeneic T cells from healthy donors. T cells and dendritic cells were cocultured for 5 days in RPMI plus 10% human AB serum in 96-well, round-bottomed plates. Each group of experiments was performed in triplicate. T-cell proliferation was evaluated using [3H]-thymidine incorporation with 16-hour pulses (5 Ci/μmol [3.7 × 1010 Bq]; Amersham, United Kingdom). Naive T cells were isolated from the peripheral blood leukocytes of healthy donors using a human CD4+/CD45RA+ column kit with negative selection (R&D Systems). Approximately1.5 × 105 naive T cells were cocultured for 5 days in 96-well, flat-bottomed plates from Costar (Cambridge, MA) with 5 × 103 cells of TNF-α, LPS, or LPS + IFN-γ mature DCs, which were generated from the monocytes of controls and of patient 1 in the presence of 1 ng/mL Staphylococcus aureus enterotoxin B (SEB) from Toxin Technology (Sarasota, FL). Proliferation of naive T cells was measured using a Cell Proliferation Kit II (XTT; Roche). Tetrazolium salt XTT was added for the last 16 hours of culture, and formation of the formazan dye was measured using an enzyme-linked immunosorbent assay (ELISA) reader at 450 nm. Cord-blood-derived T cells (1 × 106 cells per well) instead of purified naive T cells were used to analyze the intracellular expression of IFN-γ. Allogeneic cord-blood-derived T cells (1.5 × 105 cells per well) were incubated with mature DCs (5 × 103 cells per well) for MLR experiments with anti-IL-10 neutralizing antibody or with control IgG at 10 μg/mL.
Macropinocytosis
Endocytosis was measured as the cellular uptake of FITC-Dextran (Sigma) as described previously.19 Approximately 2 × 105 immature DCs were incubated in media containing 1 mg/mL FITC-Dextran at 37°C. After 60 minutes, cells were stained at 4°C with PE-conjugated anti-HLA-DR mAb from Becton Dickinson. Uptake of FITC-Dextran was determined by flow cytometry using a FACScalibur, also from Becton Dickinson.
IFN-α secretion
PBMCs (2 × 105 cells/well) were cultured with a decreasing dilution titer of irradiated herpes simplex virus type-1, strain MacIntyre B at 106 psu/mL (American Type Culture Collection, Manassas, VA).17 After 24 hours, supernatants were collected and IFN-α secretion was assessed by ELISA following the manufacturer's instructions (Bender MedSystems Diagnostics, Vienna, Austria)
Immunocytochemistry
Monocyte-derived dendritic cells used for cytospin preparations were immature or matured on culture with CD154 trimer or TNF-α. Slides were air-dried and used for immunocytochemical staining. DC-LAMP expression was analyzed using anti-DC-LAMP mAb clone 104.G4 (1:200) from Immunotech (Marseilles, France). For antigen retrieval the slides were immersed in citrate buffer and microwaved for 5 minutes at 750 W, followed by the streptavidin-biotin complex immunoperoxidase technique, with diaminobenzidine as chromogen. Cells were photographed using an optic microscope. At least 5 fields of 200 × magnification for each sample were randomly selected and analyzed. Values are expressed as mean ± SD.
Cytokine determination
Cytokine production by dendritic cells or T cells was detected using sandwich ELISA systems. IL-12 (p70), IL-10, and IFN-γ production were measured using ELISA kits provided by Endogen (Woburn, MA) with a detection limit of 2 pg/mL. When indicated, cells were cultured with 10 μg/mL neutralizing antibody anti-IL-10, clone B-S10 from Diaclone Sas, (Besancon, France).
Statistical analysis
All experiments were performed at least twice, as indicated in the figure legends, and included one or more control subjects. Comparison between HIGM3 patients and control subjects was performed by Student t test for paired or unpaired data, as appropriate. For comparison among multiple groups, analysis of variance (ANOVA) followed by Bonferroni correction were used. Results of statistical analysis of the single experiment shown in figures are reported in the legend as P = .05 significance level to identify which of the means were significantly different from the others.
Results and discussion
Normal T-cell proliferation in patients with CD40 deficiency
Both patients with HIGM3 had a history of opportunistic infections. Susceptibility to these pathogens has been attributed to a defective T-cell priming by specialized APCs.12,14 In previous studies, we had reported that PBMCs of HIGM3 patients display normal blood distribution and functional response to mitogens.4,5 We evaluated cellular proliferation of purified T cells to expand in more detail this concept and to rule out a hypothetical intrinsic defect of T lymphocytes from HIGM3 patients. Before separating T cells, flow cytometric analysis demonstrated that lymphocyte subsets are normally distributed in the blood of HIGM3 subjects. Purified T cells isolated from 2 patients with CD40 deficiency displayed a normal proliferative response to immobilized anti-CD3 (Table 1). Because these data argue against an intrinsic defect of T cells and suggest that DCs may be primarily involved, we analyzed the in vitro differentiation of monocytes into DCs in patients with CD40 deficiency.
Characteristics of HIGM3 patients and controls
. | Control group, n = 9 . | Patient 1 . | Patient 2 . |
|---|---|---|---|
| Age, y | 4 (1-10) | 9 | 2 |
| CD3, % | 76.3 (62-81) | 68 | 60 |
| CD4+, % | 67.9 (33-47) | 55 | 24 |
| CD8+, % | 21.6 (20-30) | 13 | 34 |
| Medium, cpm × 10−3* | 0.3 (0.1-0.6) | 0.2 | 0.3 |
| Anti-CD3, cpm × 10−3* | 65 (40-120) | 94 | 39 |
. | Control group, n = 9 . | Patient 1 . | Patient 2 . |
|---|---|---|---|
| Age, y | 4 (1-10) | 9 | 2 |
| CD3, % | 76.3 (62-81) | 68 | 60 |
| CD4+, % | 67.9 (33-47) | 55 | 24 |
| CD8+, % | 21.6 (20-30) | 13 | 34 |
| Medium, cpm × 10−3* | 0.3 (0.1-0.6) | 0.2 | 0.3 |
| Anti-CD3, cpm × 10−3* | 65 (40-120) | 94 | 39 |
Control group values are averages of leukocyte subsets of the age-matched subjects. Ranges for control group values are presented in parentheses.
Lymphocyte proliferation in response to immobilized anti-CD3 or medium alone. T cells from HIGM3 patients and healthy subjects were purified as described in “Patients, materials, and methods” and were cultured for 96 hours in the presence or absence of immobilized anti-CD3 (1 μg/mL). Proliferation was assessed as [3H]-thymidine uptake during the last 16 hours. Results shown are representative of 3 experiments.
Normal in vitro differentiation of monocytes to immature DCs
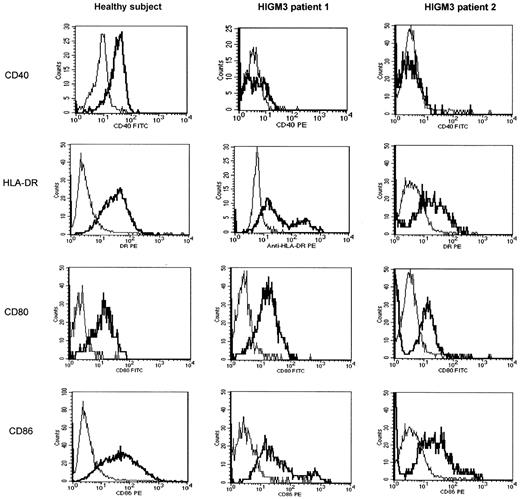
We evaluated the role of CD40 in the differentiation of monocytes to DCs by culturing purified monocytes of HIGM3 patients in the presence of GM-CSF and IL-4 for 6 days. Cultured cells displayed the typical morphology of myeloid DCs with ample cytoplasm and membrane ruffling and the expression of CD80 and CD86 at comparable levels for patients and healthy subjects. HLA-DR expression was slightly increased on the cell surface of DCs of patient 1 (Figure 1). As expected, we could not detect CD40 expression on the cell surface of patient DCs; however, CD40 was normally expressed in cells of the control subject (Figure 1). Because endocytosis of macromolecules is a prototypical activity of immature DCs, we investigated whether immature DCs from HIGM3 patients retain the capacity to uptake antigens. Macropinocytosis was evaluated by flow cytometry after incubating DCs at 37°C with FITC-Dextran dye for 1 hour. Immature DCs from HIGM3 patients showed normal macropinocytosis, suggesting that the absence of CD40 does not affect the antigen uptake ability of DCs (data not shown).
Fluorescence-activated cell sorter (FACS) analysis of expression of CD40, HLA-DR, CD80, and CD86 molecules by immature DCs from a healthy subject and from HIGM3 patients 1 and 2. Monocyte-derived immature DCs were cultured as described in “Patients, materials, and methods,” incubated with the appropriate antibody, washed twice, and analyzed using a flow cytometer. Expression of CD80 and CD86 was analyzed by CD80-FITC/CD86-PE double staining. Expression of CD40 was analyzed by CD40-FITC single staining (except for HIGM3 patient 1, in whom we used PE-conjugated anti-CD40 mAb) and HLA-DR expression by HLA-DR-PE staining. CD40, HLA-DR, CD80, and CD86 antibody stainings are presented (thick line) in comparison with mouse IgG antibody (thin line). The x-axis represents the intensity of green (or red) fluorescence expressed in a log scale as mean channel, and the y-axis represents the number of cells per channel. Data shown are from 1 of 3 representative, independent experiments.
Fluorescence-activated cell sorter (FACS) analysis of expression of CD40, HLA-DR, CD80, and CD86 molecules by immature DCs from a healthy subject and from HIGM3 patients 1 and 2. Monocyte-derived immature DCs were cultured as described in “Patients, materials, and methods,” incubated with the appropriate antibody, washed twice, and analyzed using a flow cytometer. Expression of CD80 and CD86 was analyzed by CD80-FITC/CD86-PE double staining. Expression of CD40 was analyzed by CD40-FITC single staining (except for HIGM3 patient 1, in whom we used PE-conjugated anti-CD40 mAb) and HLA-DR expression by HLA-DR-PE staining. CD40, HLA-DR, CD80, and CD86 antibody stainings are presented (thick line) in comparison with mouse IgG antibody (thin line). The x-axis represents the intensity of green (or red) fluorescence expressed in a log scale as mean channel, and the y-axis represents the number of cells per channel. Data shown are from 1 of 3 representative, independent experiments.
TNF-α, but not CD154 trimer, induces DC maturation in patients with CD40 deficiency
DC maturation achieved on CD40 triggering is characterized by the up-regulation of HLA-DR and the de novo expression of CD83 or DC-LAMP.20 To better define the role of CD40 in DC maturation, we asked whether other cytokines such as TNF-α may efficiently induce terminal maturation of DCs from HIGM3 patients.20 Phenotypical maturation of DCs was studied by evaluating the expression of HLA-DR and CD83 by flow cytometry after 48 hours of culture with coated TNF-α or CD154 trimer (Figure 2A). After stimulation with CD154 trimer, DCs from HIGM3 patient 1 failed to express CD83 and consistently displayed a fraction of cells with dim HLA-DR expression; in contrast, TNF-α stimulation induced CD83 expression in HIGM3 patient 1 (Figure 2A). Moreover, analysis of CD80 and CD86 expression after maturation of DCs with TNF-α or LPS combined with IFN-γ demonstrated normal up-regulation of cell surface expression of these molecules (data not shown).
Expression of CD14, HLA-DR, CD83, and DC-LAMP by DCs on maturation with CD154 trimer or TNF-α of HIGM3 patients. (A) FACS analysis of CD14, HLA-DR, and CD83 expression by DCs on maturation with CD154 trimer or TNF-α from a healthy subject and from HIGM3 patients. Monocyte-derived DCs were cultured and matured with coated CD154 trimer or TNF-α, as described in “Patients, materials, and methods,” and were incubated with the appropriate antibody, washed twice, and analyzed using a flow cytometer. Expression of CD14 and HLA-DR was analyzed by CD14-FITC/HLA-DR-PE double staining. Extent of CD83 expression was analyzed by CD83-PE single staining. HLA-DR, CD14, and CD83 antibody stainings are presented (thick line) in comparison with mouse IgG antibody (thin line). The x-axis represents the intensity of green or red fluorescence expressed in a log scale as mean channel, and the y-axis represents the number of cells per channel. Data shown are representative of 3 independent experiments. (B) HIGM3 patient DCs fail to express DC-LAMP on CD154 stimulation. Monocyte-derived immature and CD154 trimer or TNF-α matured DCs were cultured as described in “Patients, materials, and methods.” After cytospin preparation and immunocytochemical staining with DC-LAMP monoclonal antibody, cells were photographed using an optic microscope. Original magnification, × 200. Data are from 1 of 2 representative experiments.
Expression of CD14, HLA-DR, CD83, and DC-LAMP by DCs on maturation with CD154 trimer or TNF-α of HIGM3 patients. (A) FACS analysis of CD14, HLA-DR, and CD83 expression by DCs on maturation with CD154 trimer or TNF-α from a healthy subject and from HIGM3 patients. Monocyte-derived DCs were cultured and matured with coated CD154 trimer or TNF-α, as described in “Patients, materials, and methods,” and were incubated with the appropriate antibody, washed twice, and analyzed using a flow cytometer. Expression of CD14 and HLA-DR was analyzed by CD14-FITC/HLA-DR-PE double staining. Extent of CD83 expression was analyzed by CD83-PE single staining. HLA-DR, CD14, and CD83 antibody stainings are presented (thick line) in comparison with mouse IgG antibody (thin line). The x-axis represents the intensity of green or red fluorescence expressed in a log scale as mean channel, and the y-axis represents the number of cells per channel. Data shown are representative of 3 independent experiments. (B) HIGM3 patient DCs fail to express DC-LAMP on CD154 stimulation. Monocyte-derived immature and CD154 trimer or TNF-α matured DCs were cultured as described in “Patients, materials, and methods.” After cytospin preparation and immunocytochemical staining with DC-LAMP monoclonal antibody, cells were photographed using an optic microscope. Original magnification, × 200. Data are from 1 of 2 representative experiments.
Cell surface levels of MHC class II DR antigen were up-regulated after TNF-α stimulation but were consistently reduced compared with mature DCs of healthy subjects. Once again, this suggests a defect of DC maturation in cells derived from HIGM3 patients, even in response to stimuli different from CD154 (Figure 2A).
DC-LAMP (CD208) is an intracellular maturation marker of DCs that can be evaluated by immunohistochemistry.20,21 The mature phenotype of monocyte-derived DCs was evaluated on a cytospin preparation from patient 1 and an age-matched control. After stimulation with coated CD154 and TNF-α, most DCs (93.4% ± 2.8% and 86.6% ± 3.8%, respectively) from the controls displayed a typical lysosomal positivity for DC-LAMP. In contrast, DC-LAMP + DCs from the patient were severely reduced (26.6% ± 4.6%; P < .001) after TNF-α stimulation or scant (4% ± 3.5%; P < .001) after CD154 stimulation (Figure 2B).
Mature DCs from HIGM3 patients are defective for IL-12 production
Because DCs are the main producers of cytokines such as IL-12 and IL-10 that regulate T-cell activation, we chose to test the production of these cytokines by DCs cultured for 48 hours with CD154 trimer, LPS + IFN-γ, or medium alone. Normal DCs derived from healthy donors displayed a considerable increase in IL-12 production, from 91 ± 15 pg/mL to 745 ± 94 pg/mL after stimulation with LPS and IFN-γ and up to 206 ± 42 pg/mL after stimulation with CD154 trimer. In contrast, DCs from HIGM3 patients failed to respond to CD154 trimer with IL-12 secretion (64 ± 15 pg/mL) and secreted significantly lower amounts of this cytokine even after LPS and IFN-γ stimulation (407 ± 57 pg/mL; P < .05; Figure 3A). This indicates that DCs from HIGM3 patients can secrete IL-12 by alternative pathways other than CD40-CD154 trimer interactions and at a lower extent than in healthy subjects.
Production of IL-12 and IL-10 by mature DCs in patients with CD40 deficiency. (A-B) Comparison of IL-12 and IL-10 production by mature DCs in patients with CD40 deficiency. Monocyte-derived immature DCs (2 × 105 cells per well) were cultured for an additional 48 hours with either CD154 trimer (light gray bars), LPS + IFN-γ (dark gray bars), or medium alone (black bars), as described in “Patients, materials, and methods.” IL-12 (A) and IL-10 (B) concentrations in supernatants obtained after 48 hours of culture were evaluated by ELISA. Data are expressed in pg/mL (mean ± SD of triplicates). *Significant difference in patients compared with a healthy subject as assessed by statistical analysis (P < .05). (C) Production of IL-12 in the presence or absence of IL-10-neutralizing antibody by mature DCs from a healthy subject and from HIGM3 patient 1. Monocyte-derived DCs (2 × 105 cells per well) from a healthy subject and from patient 1 were matured with soluble TNF-α or LPS + IFN-γ 48-hour stimulation (see “Patients, materials, and methods”) in the presence of anti-IL-10 (10 μg/mL) to neutralize IL-10 action on the cells (black bars). An IgG antibody was used in control experiments (light gray bars). In the supernatant of these cultures, the cytokine IL-12 was measured using ELISA, and data are expressed in pg/mL (mean ± SD of triplicates). These experiments were performed in 2 different laboratories. Data shown are from 1 of 3 representative, independent experiments.
Production of IL-12 and IL-10 by mature DCs in patients with CD40 deficiency. (A-B) Comparison of IL-12 and IL-10 production by mature DCs in patients with CD40 deficiency. Monocyte-derived immature DCs (2 × 105 cells per well) were cultured for an additional 48 hours with either CD154 trimer (light gray bars), LPS + IFN-γ (dark gray bars), or medium alone (black bars), as described in “Patients, materials, and methods.” IL-12 (A) and IL-10 (B) concentrations in supernatants obtained after 48 hours of culture were evaluated by ELISA. Data are expressed in pg/mL (mean ± SD of triplicates). *Significant difference in patients compared with a healthy subject as assessed by statistical analysis (P < .05). (C) Production of IL-12 in the presence or absence of IL-10-neutralizing antibody by mature DCs from a healthy subject and from HIGM3 patient 1. Monocyte-derived DCs (2 × 105 cells per well) from a healthy subject and from patient 1 were matured with soluble TNF-α or LPS + IFN-γ 48-hour stimulation (see “Patients, materials, and methods”) in the presence of anti-IL-10 (10 μg/mL) to neutralize IL-10 action on the cells (black bars). An IgG antibody was used in control experiments (light gray bars). In the supernatant of these cultures, the cytokine IL-12 was measured using ELISA, and data are expressed in pg/mL (mean ± SD of triplicates). These experiments were performed in 2 different laboratories. Data shown are from 1 of 3 representative, independent experiments.
Analysis of IL-10 secretion by immature or mature DCs demonstrated that levels of this cytokine were consistently higher in patient 1 than in the control group (28 ± 6 vs 5 ± 1 pg/mL for immature DCs; 26 ± 4 vs 13 ± 3 pg/mL for CD154 matured). In contrast, IL-10 production by DCs treated with LPS and IFN-γ was higher in both patients than in healthy subjects (97 ± 10 pg/mL and 69 ± 12 pg/mL in patients 1 and 2, respectively, vs 20 ± 2 pg/mL in the control subject; P < .05; Figure 3B).
Because IL-10 is a potent suppressant of IL-12 secretion by activated DCs, we investigated IL-12 release by DCs stimulated with TNF-α or LPS combined with IFN-γ with or without adding neutralizing anti-IL-10 antibody.22 Adding anti-IL-10 to DC cultures increased IL-12 secretion by mature DCs of healthy donors and patients, but the amount of IL-12 secretion was lower in the cells of patient 1 than in a control subject, suggesting that the larger production of IL-10 did not account for the impairment of IL-12 secretion (Figure 3C).
Monocyte-derived DCs of HIGM3 patients are defective in MLR activity
Activated T lymphocytes provide a stimulatory signal to DCs by engaging CD40 mediated by CD154. To further characterize the function of CD40-deficient DCs in the context of T-cell-mediated immunity, an allogeneic MLR assay was performed using LPS + IFN-γ matured DCs generated from peripheral blood monocytes of HIGM3 patients. Normal allogeneic T lymphocytes were used as responder cells at 3 different DC/T-cell ratios (DCs at 0.5%, 3%, and 6% of T cells). In all cases analyzed, normal mature DCs were capable of inducing significant T-cell proliferation at 3% and 6% DC/T-cell ratios. In contrast, mature DCs generated from HIGM3 patients displayed significantly reduced ability to induce T-cell proliferation (P < .05; Figure 4A). Differences between patients and controls were consistently detected in 3 distinct experiments and were most evident at the highest DC/T-cell ratio. Analysis of the costimulatory capacity of mature DCs of HIGM3 patients obtained after stimulation with TNF-α demonstrated that cells from both HIGM3 patients induced significant up-regulation of the proliferative T-cell response at DC/T-cell ratios of 3% and 6%, but at a lower extent than cells from the control subjects (data not shown).
Proliferation of alloreactive T cells induced by mature DCs in patients with CD40 deficiency. (A) Proliferation of alloreactive T cells induced by mature DCs: allogeneic MLR. T cells were purified by negative selection from healthy donor PBMCs and were cultured with LPS + IFN-γ-matured DCs from controls and HIGM3 patients at 3 different ratios. DCs were 0.5%, 3%, and 6% of T cells (see “Patients, materials, and methods”). After 5 days at 37°C, proliferation of alloreactive T cells was assessed by [3H]-thymidine uptake. Results are expressed as mean cpm ± SD of 1 experiment performed in triplicate. *Significant difference in the control subject in comparison with patients as assessed by statistical analysis (P < .05). ♦ indicates healthy control; □, HIGM3 patient 1; and ▴, HIGM3 patient 2. (B) Proliferation of alloreactive naive T cells induced by TNF-α, LPS, or LPS+IFN-γ-matured DCs: allogeneic MLR. Naive lymphocytes were purified by negative selection from healthy donor PBMCs and cultured (1.5 × 105 cells per well) with soluble TNF-α (light gray bars), LPS (black bars), or LPS + IFN-γ-matured (dark gray bars) DCs (5 × 103 cells/well) from control subjects (adult and child) and HIGM3 patient 1, in the presence of 1 ng/mL SEB (see “Patients, materials, and methods”). After 5 days at 37°C, we measured proliferation of alloreactive naive T cells. The formation of a formazan salt was measured using an ELISA reader, as described in “Patients, materials, and methods.” Results are expressed as extinction coefficient at 450 nm (mean ± SD of an experiment performed in triplicate). Adult and child data are from control subjects. These MLR experiments were performed in 2 different laboratories. Data are from 1 of 3 representative, independent experiments. *Significant difference in the patient compared with healthy subjects as assessed by statistical analysis (P < .05).
Proliferation of alloreactive T cells induced by mature DCs in patients with CD40 deficiency. (A) Proliferation of alloreactive T cells induced by mature DCs: allogeneic MLR. T cells were purified by negative selection from healthy donor PBMCs and were cultured with LPS + IFN-γ-matured DCs from controls and HIGM3 patients at 3 different ratios. DCs were 0.5%, 3%, and 6% of T cells (see “Patients, materials, and methods”). After 5 days at 37°C, proliferation of alloreactive T cells was assessed by [3H]-thymidine uptake. Results are expressed as mean cpm ± SD of 1 experiment performed in triplicate. *Significant difference in the control subject in comparison with patients as assessed by statistical analysis (P < .05). ♦ indicates healthy control; □, HIGM3 patient 1; and ▴, HIGM3 patient 2. (B) Proliferation of alloreactive naive T cells induced by TNF-α, LPS, or LPS+IFN-γ-matured DCs: allogeneic MLR. Naive lymphocytes were purified by negative selection from healthy donor PBMCs and cultured (1.5 × 105 cells per well) with soluble TNF-α (light gray bars), LPS (black bars), or LPS + IFN-γ-matured (dark gray bars) DCs (5 × 103 cells/well) from control subjects (adult and child) and HIGM3 patient 1, in the presence of 1 ng/mL SEB (see “Patients, materials, and methods”). After 5 days at 37°C, we measured proliferation of alloreactive naive T cells. The formation of a formazan salt was measured using an ELISA reader, as described in “Patients, materials, and methods.” Results are expressed as extinction coefficient at 450 nm (mean ± SD of an experiment performed in triplicate). Adult and child data are from control subjects. These MLR experiments were performed in 2 different laboratories. Data are from 1 of 3 representative, independent experiments. *Significant difference in the patient compared with healthy subjects as assessed by statistical analysis (P < .05).
On the maturation of naive T cells with soluble TNF-α, LPS, or LPS combined with IFN-γ, an MLR test was performed to assess the DC costimulatory activity of the cells. To perform the test, DCs were taken from patient 1, a healthy age-matched donor, and a healthy adult donor. By using naive T cells (CD4+/CD45RA+) as targets, we found that the costimulatory activity of CD40-deficient mature DCs displayed an evident defect compared with cells derived from both healthy donors, even when LPS and IFN-γ were used to induce maturation (P < .05; Figure 4B).
Monocyte-derived DCs of HIGM3 patients are defective in inducing IFN-γ secretion by allogeneic T cells
IL-12 production by DCs is critical for inducing IFN-γ production by T cells in vitro.23,24 Therefore we used flow cytometric analysis to investigate the intracellular expression of IFN-γ by naive T cells after they were cocultured with mature DCs from a healthy subject or from patient 1. Analysis of IFN-γ-inducing activity on DCs matured after culture with CD154 trimer demonstrated that DCs derived from an HIGM3 subject were impaired in inducing IFN-γ expression by allogeneic naive T cells (less than 1% of IFN-γ-expressing T cells) compared with DCs generated from healthy donors (up to 12% of IFN-γ-secreting T cells; P < .05). Moreover, when naive T cells were cultured with DCs from a healthy control that had been induced to mature in vitro with LPS and IFN-γ, the production of IFN-γ by lymphocytes was higher than after coculture with mature DCs from the CD40-deficient patient (30% vs 16%; Figure 5A).
IFN-γ expression by naive T cells after activation with mature DCs in an HIGM3 patient. (A) Intracellular expression of IFN-γ by cord blood-derived T cells after activation with mature DCs in a healthy subject and HIGM3 patient 1. Monocyte-derived and CD154 trimer (black bars) or LPS + IFN-γ-matured (gray bars) DCs (medium only [white bars] is the internal negative control) (1 × 105/well) were cultured with 1 × 106 cord blood-derived allogeneic T cells, as described in “Patients, materials, and methods.” Intracellular IFN-γ production was evaluated by intracellular staining after cellular permeabilization. The number of cells expressing IFN-γ is shown on the y-axis as the percentage of cells. Significant difference in the patient compared with control subject as assessed by statistical analysis (P < .05). (B) IFN-γ production by alloreactive naive T cells induced to proliferate by TNF-α, LPS, or LPS + IFN-γ-matured DCs. Naive T lymphocytes (1.5 × 105/well) were purified by negative selection from healthy donor PBMCs and cultured with soluble TNF-α (white bars), LPS (black bars), or LPS + IFN-γ-matured (gray bars) DCs (5 × 103 cells) from controls (adult and child) and HIGM3 patient 1 in the presence of 1 ng/mL SEB (see “Patients, materials, and methods”). The supernatant was collected after 5 days at 37°C, and the production of IFN-γ was measured using ELISA. Cells used for this experiment correspond to those of the MLR assay described in Figure 4B. Data are expressed in pg/mL (mean ± SD of an experiment performed in triplicate). Adult and child data are from control subjects. *Significant difference in the patient in comparison with control subjects as assessed by statistical analysis (P < .05). Results shown are representative of 2 independent experiments.
IFN-γ expression by naive T cells after activation with mature DCs in an HIGM3 patient. (A) Intracellular expression of IFN-γ by cord blood-derived T cells after activation with mature DCs in a healthy subject and HIGM3 patient 1. Monocyte-derived and CD154 trimer (black bars) or LPS + IFN-γ-matured (gray bars) DCs (medium only [white bars] is the internal negative control) (1 × 105/well) were cultured with 1 × 106 cord blood-derived allogeneic T cells, as described in “Patients, materials, and methods.” Intracellular IFN-γ production was evaluated by intracellular staining after cellular permeabilization. The number of cells expressing IFN-γ is shown on the y-axis as the percentage of cells. Significant difference in the patient compared with control subject as assessed by statistical analysis (P < .05). (B) IFN-γ production by alloreactive naive T cells induced to proliferate by TNF-α, LPS, or LPS + IFN-γ-matured DCs. Naive T lymphocytes (1.5 × 105/well) were purified by negative selection from healthy donor PBMCs and cultured with soluble TNF-α (white bars), LPS (black bars), or LPS + IFN-γ-matured (gray bars) DCs (5 × 103 cells) from controls (adult and child) and HIGM3 patient 1 in the presence of 1 ng/mL SEB (see “Patients, materials, and methods”). The supernatant was collected after 5 days at 37°C, and the production of IFN-γ was measured using ELISA. Cells used for this experiment correspond to those of the MLR assay described in Figure 4B. Data are expressed in pg/mL (mean ± SD of an experiment performed in triplicate). Adult and child data are from control subjects. *Significant difference in the patient in comparison with control subjects as assessed by statistical analysis (P < .05). Results shown are representative of 2 independent experiments.
Then we evaluated IFN-γ secretion by naive allogeneic T cells activated after coculture with mature DCs from HIGM3 patient 1 and from healthy control subjects. Determining IFN-γ secretion in the supernatants generated by allogeneic naive T cells after coculture with TNF-α, LPS, or LPS combined with IFN-γ-matured DCs confirmed that cells from HIGM3 patients have a generalized and significant defect in the ability to induce IFN-γ production after stimulation with innate immunity signals (P < .05; Figure 5B).
Up-regulation of defective MLR activity and T-cell-dependent IFN-γ secretion by myeloid DCs in HIGM3 patients by IL-10 neutralization
IL-10 is a potent suppressor of T-cell activation and an inhibitor of TH1 cytokine secretion by immature DCs and regulatory T cells used to limit or ultimately terminate the immune response. We examined the effect of anti-IL-10 blocking mAb to assess the role of endogenous IL-10 secretion in the inability of mature DCs of HIGM3 patients to support MLR and to induce IFN-γ secretion from naive T cells. Incubating mature DCs with cord-blood-derived allogeneic T cells in the presence of anti-IL-10 antibody induced a significant increase in the capacity of DCs derived from the HIGM3 patient to activate allogeneic T cells (Figure 6A). Conversely, DCs of control subjects did not display up-regulated T-cell costimulatory activity.
IFN-γ expression by cord-blood-derived T cells after activation with mature DCs with or without the addition of anti-IL-10 neutralizing antibody. (A) Proliferation of cord blood-derived T cells induced by mature DCs, with or without the addition of anti-IL-10 neutralizing antibody. Cord blood-derived allogeneic T cells (1.5 × 105cells) were cultured with LPS + IFN-γ-matured DCs (5 × 103) from controls and HIGM3 patient 1 (see “Patients, materials, and methods”) in the presence of anti-IL-10 (10 μg/mL) to neutralize IL-10 action on the cells or with a control IgG antibody. After 5 days at 37°C, proliferation of alloreactive T cells was assessed by uptake of [3H]-thymidine. Results are expressed as mean cpm ± SD of triplicates. *Significant increase of thymidine incorporation after incubation with anti-IL-10 mAb as assessed by statistical analysis (P < .05). (B) IFN-γ production by alloreactive naive T cells induced to proliferate by mature DCs, with or without the addition of anti-IL-10 neutralizing antibody. Cord-blood-derived allogeneic T cells (1.5 × 105 cells/well) were cultured with LPS + IFN-γ-matured DCs (5 × 103) from controls or HIGM3 patient 1 (see “Patients, materials, and methods”), in the presence of anti-IL-10 (10 μg/mL) to neutralize IL-10 action on the cells or with a control IgG antibody. The supernatant was collected after 5 days at 37°C, and the production of IFN-γ was measured using ELISA. Cells used for this experiment correspond to those of the MLR assay described in panel A. Data are expressed in pg/mL (mean ± SD of triplicates). Asterisks indicate a significant increase of IFN-γ secretion after incubation with anti-IL-10 mAb as assessed by statistical analysis (P < .05). Results shown are representative of 2 independent experiments.
IFN-γ expression by cord-blood-derived T cells after activation with mature DCs with or without the addition of anti-IL-10 neutralizing antibody. (A) Proliferation of cord blood-derived T cells induced by mature DCs, with or without the addition of anti-IL-10 neutralizing antibody. Cord blood-derived allogeneic T cells (1.5 × 105cells) were cultured with LPS + IFN-γ-matured DCs (5 × 103) from controls and HIGM3 patient 1 (see “Patients, materials, and methods”) in the presence of anti-IL-10 (10 μg/mL) to neutralize IL-10 action on the cells or with a control IgG antibody. After 5 days at 37°C, proliferation of alloreactive T cells was assessed by uptake of [3H]-thymidine. Results are expressed as mean cpm ± SD of triplicates. *Significant increase of thymidine incorporation after incubation with anti-IL-10 mAb as assessed by statistical analysis (P < .05). (B) IFN-γ production by alloreactive naive T cells induced to proliferate by mature DCs, with or without the addition of anti-IL-10 neutralizing antibody. Cord-blood-derived allogeneic T cells (1.5 × 105 cells/well) were cultured with LPS + IFN-γ-matured DCs (5 × 103) from controls or HIGM3 patient 1 (see “Patients, materials, and methods”), in the presence of anti-IL-10 (10 μg/mL) to neutralize IL-10 action on the cells or with a control IgG antibody. The supernatant was collected after 5 days at 37°C, and the production of IFN-γ was measured using ELISA. Cells used for this experiment correspond to those of the MLR assay described in panel A. Data are expressed in pg/mL (mean ± SD of triplicates). Asterisks indicate a significant increase of IFN-γ secretion after incubation with anti-IL-10 mAb as assessed by statistical analysis (P < .05). Results shown are representative of 2 independent experiments.
Because IL-10 production by mature DCs suppresses IFN-γ synthesis by activated T cells, we investigated whether IL-10 neutralization could restore the ability of mature DCs of HIGM3 patient 1 to sustain IFN-γ production by alloreactive naive T cells. As shown in Figure 6B, IL-10 blocking by specific anti-IL-10 mAb strongly increased the levels of IFN-γ secretion by alloreactive naive T cells cultured with mature DCs of healthy donors and of HIGM3 patient. However, the largest difference was observed for T cells cocultured with DCs of HIGM3 patient. These results suggest that the increased levels of IL-10 secretion by mature DCs generated in patients with CD40 deficiency may partially account for the impaired ability of these cells to induce a TH1 response.
Impairment of IFN-α secretion after HSV-1 exposure in HIGM3 patients
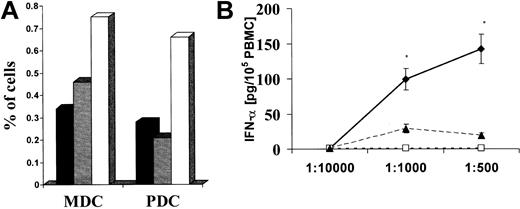
We investigated the number of peripheral blood DCs in HIGM3 patients compared with age-matched controls. In HIGM3 patients, the DC population represented less than 2% of the total cells in peripheral blood. Myeloid DCs and plasmacytoid DCs were analyzed for CD11c and CD123 expression, respectively. No evident imbalance between myeloid DC and plasmacytoid DC subsets was observed in HIGM3 patients, suggesting that the generation of DCs is not affected in the absence of CD40 expression (Figure 7A). Because plasmacytoid DCs correspond to the natural interferon-α-producing cells (IPCs), which are the major source of type 1 interferons in the antiviral immune response,10,19,25 we analyzed the levels of IFN-α secretion by total PBMCs in response to HSV-1 exposure in the patients with CD40 deficiency and in 2 age-matched control subjects. Both subjects displayed severe impairment in the functional response of IPCs to HSV-1 (P < .05; Figure 7), suggesting that the lack of CD40 could affect the activation of innate immunity after viral infection.
Analysis of circulating plasmacytoid DCs and IFN-α-secreting cells in HIGM3 patients. (A) Percentages of circulating myeloid and plasmacytoid DCs and IFN-α secretion after HSV-1 infection. Percentages of circulating DCs were calculated over total mononuclear leukocytes after exclusion of Lin+ cells stained, as described in “Patients, materials, and methods.” MDC indicates myeloid CD11c+ DCs; PDC, plasmacytoid CD123+ DCs; ▪, healthy control; ▦, patient 1; and □, patient 2. (B) Capacity of IFN-α secretion by total PBMCs (2 × 105 cells/well) after HSV-1 infection with a decreasing dilution titer of the virus isolate (1:10 000, 1:1000, 1:500) was assessed in patients with CD40 deficiency and in healthy controls. Culture supernatants were harvested after 24 hours, and IFN-α levels were determined using ELISA. Data are expressed in pg/10 × 105 PBMCs (mean ± SD of experiment performed in triplicate). Data from the healthy subject (♦) are representative of data from all the control group subjects. *Significant difference in the control subject in comparison with patients as assessed by statistical analysis (P < .05). ♦ indicates healthy subject; □, HIGM3 patient 1; and ▴, HIGM3 patient 2. Results are representative of 2 independent experiments.
Analysis of circulating plasmacytoid DCs and IFN-α-secreting cells in HIGM3 patients. (A) Percentages of circulating myeloid and plasmacytoid DCs and IFN-α secretion after HSV-1 infection. Percentages of circulating DCs were calculated over total mononuclear leukocytes after exclusion of Lin+ cells stained, as described in “Patients, materials, and methods.” MDC indicates myeloid CD11c+ DCs; PDC, plasmacytoid CD123+ DCs; ▪, healthy control; ▦, patient 1; and □, patient 2. (B) Capacity of IFN-α secretion by total PBMCs (2 × 105 cells/well) after HSV-1 infection with a decreasing dilution titer of the virus isolate (1:10 000, 1:1000, 1:500) was assessed in patients with CD40 deficiency and in healthy controls. Culture supernatants were harvested after 24 hours, and IFN-α levels were determined using ELISA. Data are expressed in pg/10 × 105 PBMCs (mean ± SD of experiment performed in triplicate). Data from the healthy subject (♦) are representative of data from all the control group subjects. *Significant difference in the control subject in comparison with patients as assessed by statistical analysis (P < .05). ♦ indicates healthy subject; □, HIGM3 patient 1; and ▴, HIGM3 patient 2. Results are representative of 2 independent experiments.
We report that the absence of CD40 does not completely impair terminal maturation of DCs as assessed by the expression of CD83 and DC-LAMP. Indeed, immature DCs of patients with CD40 deficiency can be induced to maturation by stimulation with TNF-α or with molecules of microbial origin, such as LPS combined with IFN-γ, that may help to overcome the CD40 defect.26-29 These maturation pathways may function independently of the CD40-CD154 pathway, as suggested by our observation that DCs from HIGM3 express CD83 and DC-LAMP on stimulation with TNF-α. However, mature DCs that lack CD40 display a profound defect in the ability to induce allogeneic stimulation of naive T cells, suggesting that CD40-CD154 interactions play a nonredundant role in the generation of fully functional mature DCs. An indirect proof to this hypothesis is offered by previous reports indicating that PBMCs from HIGM1 patients who lack CD154 have a defect in antigen-specific T-cell proliferation, whereas lectin responses and the maturation of monocyte-derived DCs are normal (12,13,30 ; and S.M., unpublished observation, July 2003).
Our observations provide an explanation for the peculiar susceptibility of HIGM3 patients to opportunistic pathogens and viruses. Infections by Pneumocystis or Cryptosporidium are characterized by the release of low levels of proinflammatory cytokines and the absence of LPS. This could result in inadequate priming for the maturation of DCs in patients such as those with HIGM1 and HIGM3, for whom CD40-CD154 interaction is abrogated. Moreover, an important contribution to the manifestations of the disease may also derive from the impairment of innate immune response against viruses. It is well known that all patients with hyper IgM, including those with CD40 deficiency, frequently acquire viral infections, such as from cytomegalovirus, that may cause interstitial pneumonia or liver disease.1,31 Indeed, we have shown that the activation of PBMCs by HSV-1 exposure was poorly effective in inducing IFN-α secretion, thus suggesting that plasmacytoid DC activation may also be affected by CD40 deficiency.
We found that immature DCs and LPS + IFN-γ-matured DCs of HIGM3 patients secrete larger amounts of IL-10 than the corresponding cells generated in healthy subjects. Endogenous secretion of IL-10 by immature DCs appears to be caused by a peculiar capacity of monocyte-derived DCs to accumulate at sites of chronic inflammation, and it prevents an exaggerated or potentially dangerous response against self-antigens that could lead to autoimmune reactions.32 In these conditions, IL-10 secretion serves as a mechanism to limit the maturation of monocyte-derived DCs and their capacity to initiate a TH1 response by reducing IL-12 secretion, T-lymphocyte activation, and IFN-γ secretion. Blocking IL-10 expression by mature DCs in HIGM3 patients leads to increases in MLR response and IFN-γ synthesis by alloreactive T cells, but not to concomitant increases in IL-12 secretion by anti-IL-10-treated DCs. It is likely that endogenous secretion of IL-10 by mature DCs may function in an autocrine fashion to suppress IL-12 secretion, thus preventing neutralization of the cytokine by the anti-IL-10 antibodies. Taken together, these results suggest that the inability of DCs in HIGM3 patients to sustain T-cell activation may, at least in part, rely on the large amounts of IL-10 secretion by these cells.
The fact that CD40-deficient DCs secrete large amounts of IL-10 suggests that CD40 is probably involved in controlling the release of IL-10 by DCs. Evidence shows that greater IL-10 secretion by DCs may impair the up-regulation of HLA-DR, thus affecting the DC costimulatory activity for allogeneic T cells.22,32,33 Indeed, low levels of HLA-DR expression were detected in mature DCs generated from the monocytes of HIGM3 patients. DCs generated from CD40 knock-out mice have a similar pattern of cytokine secretion, providing support to the model that CD40 activation of DCs constitutes an essential pathway for the induction of TH1-type immune responses.34 The fact that DCs from HIGM3 patients display impaired maturation and reduced costimulatory activity supports the notion that this disease is not an exclusive defect of humoral immunity but should be considered as a combined defect of B-cell and T-cell compartments.
Prepublished online as Blood First Edition Paper, July 31, 2003; DOI 10.1182/blood-2003-04-1244.
Supported by Ministero Istruzione Università, Ricerca (MIUR) Cofin 2002 grants (L.D.N., A.P.), MIUR-FIRB grant (L.D.N.), and European Union grant QLG1-GT-CT-2001-Q1536 (A.P.). Work at the AMC-UvA Laboratory was supported by grants from the Dutch Medical Research Council (P.K.D.), the Platform Alternative (nos. 97-32, 96-26), and the Neth-Leprosy Foundation (no. ILEP740204), and by the Dr. Hadwen Trust for Life Science Research (United Kingdom). S. Fontana and D.M. were supported by fellowships from Ministero Università e Ricerca Scientifica e Tecnologica (MURST) (Cofin 1999) and “Fondazione Camillo Golgi,” respectively.
Presented as an oral presentation at the Cytokines and Interferon Meeting: Joint Meeting of the International Cytokine Society, the International Society for Interferon and Cytokine Research, the European Cytokine Society, and the Society for Leukocyte Biology, Turin, Italy, October 6-10, 2002.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Joost J. Oppenheim for his comments, Antonietta Silini for reviewing the manuscript, and Silvana Festa for her technical expertise.



![Figure 4. Proliferation of alloreactive T cells induced by mature DCs in patients with CD40 deficiency. (A) Proliferation of alloreactive T cells induced by mature DCs: allogeneic MLR. T cells were purified by negative selection from healthy donor PBMCs and were cultured with LPS + IFN-γ-matured DCs from controls and HIGM3 patients at 3 different ratios. DCs were 0.5%, 3%, and 6% of T cells (see “Patients, materials, and methods”). After 5 days at 37°C, proliferation of alloreactive T cells was assessed by [3H]-thymidine uptake. Results are expressed as mean cpm ± SD of 1 experiment performed in triplicate. *Significant difference in the control subject in comparison with patients as assessed by statistical analysis (P < .05). ♦ indicates healthy control; □, HIGM3 patient 1; and ▴, HIGM3 patient 2. (B) Proliferation of alloreactive naive T cells induced by TNF-α, LPS, or LPS+IFN-γ-matured DCs: allogeneic MLR. Naive lymphocytes were purified by negative selection from healthy donor PBMCs and cultured (1.5 × 105 cells per well) with soluble TNF-α (light gray bars), LPS (black bars), or LPS + IFN-γ-matured (dark gray bars) DCs (5 × 103 cells/well) from control subjects (adult and child) and HIGM3 patient 1, in the presence of 1 ng/mL SEB (see “Patients, materials, and methods”). After 5 days at 37°C, we measured proliferation of alloreactive naive T cells. The formation of a formazan salt was measured using an ELISA reader, as described in “Patients, materials, and methods.” Results are expressed as extinction coefficient at 450 nm (mean ± SD of an experiment performed in triplicate). Adult and child data are from control subjects. These MLR experiments were performed in 2 different laboratories. Data are from 1 of 3 representative, independent experiments. *Significant difference in the patient compared with healthy subjects as assessed by statistical analysis (P < .05).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/12/10.1182_blood-2003-04-1244/6/m_h82335281004.jpeg?Expires=1765907489&Signature=Mc7Qw1pRpiifhMp~aN4gWXkU8wKY6tqdoM2hmW5ATO-P1e~3zvQbV5hAlO5sD21TWnAhnayxXAc438e2QWEBYcSHZMPtgUmtIFXytWi14f5l6htlpmTtXBhn6hX2UmlxxA0eekqDwp51O5pt8r4Tn8Eol7lHlKP~6fzzEvdgxF8hEGAWpyGocr94aQXprPJG9ZjIPZJ4MLUByuaGxV~N~rV8DQuPdj4bGX-OeDMLJWxzur2p3OnF2PdqmQXqS4SzuNgACPqEtp1De3M4ApC1C-Xc78zp96ToEyc3VnyWs74mVuMmh0mH9MPL7dSfSXLnz4lphtIuQGgHmA7v~ZvtYg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 5. IFN-γ expression by naive T cells after activation with mature DCs in an HIGM3 patient. (A) Intracellular expression of IFN-γ by cord blood-derived T cells after activation with mature DCs in a healthy subject and HIGM3 patient 1. Monocyte-derived and CD154 trimer (black bars) or LPS + IFN-γ-matured (gray bars) DCs (medium only [white bars] is the internal negative control) (1 × 105/well) were cultured with 1 × 106 cord blood-derived allogeneic T cells, as described in “Patients, materials, and methods.” Intracellular IFN-γ production was evaluated by intracellular staining after cellular permeabilization. The number of cells expressing IFN-γ is shown on the y-axis as the percentage of cells. Significant difference in the patient compared with control subject as assessed by statistical analysis (P < .05). (B) IFN-γ production by alloreactive naive T cells induced to proliferate by TNF-α, LPS, or LPS + IFN-γ-matured DCs. Naive T lymphocytes (1.5 × 105/well) were purified by negative selection from healthy donor PBMCs and cultured with soluble TNF-α (white bars), LPS (black bars), or LPS + IFN-γ-matured (gray bars) DCs (5 × 103 cells) from controls (adult and child) and HIGM3 patient 1 in the presence of 1 ng/mL SEB (see “Patients, materials, and methods”). The supernatant was collected after 5 days at 37°C, and the production of IFN-γ was measured using ELISA. Cells used for this experiment correspond to those of the MLR assay described in Figure 4B. Data are expressed in pg/mL (mean ± SD of an experiment performed in triplicate). Adult and child data are from control subjects. *Significant difference in the patient in comparison with control subjects as assessed by statistical analysis (P < .05). Results shown are representative of 2 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/12/10.1182_blood-2003-04-1244/6/m_h82335281005.jpeg?Expires=1765907489&Signature=gDPaImKE7otMs5yNtVoi-hGnTRS7gOxpZKKp-E2kXf9xs2TkXyannLuWg7Y3mF5ANonZR81Px6NbfLcOGZ~jy~YxfAvMTDRTThZTKbVWpyBUfHjbHpaSN8qfK8K6XhOg66-vgnJRcicDwJvEdfCs2lN0dAQaqXvl42pwnLbiiUekjWpWISVJqLxx-v5tpIw~V31nqTGcfVfShYCMVE5wmjW~kRNig~WsUIFppObCJz35LnUC4VymhmwClpdQowexeJArBblTh9DLILKVbCcB2mQ3t~62keOr0DlUXR~JOUsP08zQfq8EQI7p6A~988ZA2M6B0fPsb4VTjXCoIKV1nA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 6. IFN-γ expression by cord-blood-derived T cells after activation with mature DCs with or without the addition of anti-IL-10 neutralizing antibody. (A) Proliferation of cord blood-derived T cells induced by mature DCs, with or without the addition of anti-IL-10 neutralizing antibody. Cord blood-derived allogeneic T cells (1.5 × 105cells) were cultured with LPS + IFN-γ-matured DCs (5 × 103) from controls and HIGM3 patient 1 (see “Patients, materials, and methods”) in the presence of anti-IL-10 (10 μg/mL) to neutralize IL-10 action on the cells or with a control IgG antibody. After 5 days at 37°C, proliferation of alloreactive T cells was assessed by uptake of [3H]-thymidine. Results are expressed as mean cpm ± SD of triplicates. *Significant increase of thymidine incorporation after incubation with anti-IL-10 mAb as assessed by statistical analysis (P < .05). (B) IFN-γ production by alloreactive naive T cells induced to proliferate by mature DCs, with or without the addition of anti-IL-10 neutralizing antibody. Cord-blood-derived allogeneic T cells (1.5 × 105 cells/well) were cultured with LPS + IFN-γ-matured DCs (5 × 103) from controls or HIGM3 patient 1 (see “Patients, materials, and methods”), in the presence of anti-IL-10 (10 μg/mL) to neutralize IL-10 action on the cells or with a control IgG antibody. The supernatant was collected after 5 days at 37°C, and the production of IFN-γ was measured using ELISA. Cells used for this experiment correspond to those of the MLR assay described in panel A. Data are expressed in pg/mL (mean ± SD of triplicates). Asterisks indicate a significant increase of IFN-γ secretion after incubation with anti-IL-10 mAb as assessed by statistical analysis (P < .05). Results shown are representative of 2 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/12/10.1182_blood-2003-04-1244/6/m_h82335281006.jpeg?Expires=1765907489&Signature=D7YGG-43mRU9ttCY1X7~HHgQ7aWUjWyPenO0vLw0QZAGeZ631CY3T8IePEblgpyCuUneTIdAS4hEMR4JNyMliYolSrv9IhA6TrI~AjQTblJObULFS7Revg0VbUZviobyUHA3-ayrL68P1UR8kE0xNt2a0UIXqyHGbO7nJAdfmyHd81Q~PWvQvt6P9DV6U7dCvmXfgaSezmqUfPBTxQBjWr2RmSLkSoInEqj4MgBxPKFIam5obhlxAXQ91nky4UOW4llU9lI~x8gttqZ2W8thyLmNX9zIMdBdQCYwSNHDamkHlgLoy5kdAkunIloK9y0WYaXnS5BGAavZoyvG950SGg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal