Abstract
The major target of the pathogenic red blood cell (RBC) autoantibodies in New Zealand black (NZB) mice is the anion channel protein band 3, and CD4+ T cells from NZB mice respond to band 3. Here, we demonstrate that a band 3 peptide 861-875, which is the predominant sequence recognized by NZB T cells in vitro, bears a dominant helper epitope able to modulate the autoimmune hemolyic anemia in vivo. The development of RBC-bound autoantibodies and anemia was accelerated in NZB mice injected with peptide 861-874, which is relatively insoluble, and inhalation of the peptide primed T cells for both peptide 861-874 and band 3 responses. By contrast, inhalation of a soluble analog (Glu861, Lys875) of peptide 861-874 deviated the autoimmune response toward a T helper-2 (Th2) profile, with marked increases in the ratio of interleukin-4 to interferon-γ produced by splenic T cells responding in vitro to either peptide 861-874 or band 3. Moreover, in mice that had received such treatment, the proportion of RBC-bound immunoglobulin G (IgG) molecules that were of the Th2-associated IgG1 isotype was also increased, and anemia was less severe. It is concluded that NZB autoimmune hemolytic anemia is helper dependent and that nasal administration of different peptides containing the dominant T-cell epitope can have potentially detrimental or beneficial effects on the disease. (Blood. 2003; 102:3800-3806)
Introduction
Autoimmune hemolytic anemia (AIHA) is a classic example of an antibody-mediated autoimmune disease.1 It is well documented2 how autoantibody-coated red blood cells (RBCs) can be destroyed by splenic macrophages but, by contrast, the mechanisms that initiate production of the autoantibodies remain unclear. Efforts to identify possible mechanisms often focus on the New Zealand black (NZB) strain of mouse, which spontaneously develops AIHA.3 RBC-bound immunoglobulin G (IgG) autoantibodies can be detected by direct antiglobulin test (DAT) from 3 months of age, and the mice develop signs of anemia from about 6 months of age onward.4 It has been debated whether the production of this antibody is T-cell dependent. Early work showed that neonatal thymectomy failed to prevent the generation of RBC autoantibodies5 and that NZB bone marrow cells could transfer RBC autoantibody production to an irradiated H-2-matched healthy strain, even if the recipients were chronically T-cell depleted.4 More recently, it was demonstrated that oral administration of lipopolysaccharide activated B-1 cells and led to the production of autoantibody in trangenic mice expressing a pathogenic IgM monoclonal antibody derived from NZB mice.6,7 However, severe combined immunodeficient (SCID) mice repopulated with NZB pre-B cells fail to develop RBC autoantibodies,8 and production of autoantibody is retarded in NZB mice treated with anti-CD4 antibodies9 and in CD4 gene-deleted NZB mice.10 Thus, it would appear that CD4 T cells are required for optimal production of RBC autoantibodies in NZB mice, and the question arises as to their specificity. We have demonstrated that the autoantibodies eluted from the surface of DAT-positive RBCs,11 and many pathogenic monoclonal RBC autoantibodies from NZB mice,12 are specific for band 3, the RBC anion channel protein. This finding led us to determine if NZB mice harbor band 3-reactive T cells. The results revealed that splenic T cells from NZB mice, but not H-2-matched healthy strains, proliferate in vitro in response to band 3,13 and epitope mapping studies identified a number of peptides from band 3 that are recognized by the proliferating NZB splenocytes.14 Of these, peptide 861-874 invariably induces the strongest proliferation and is the only sequence to stimulate responses by T cells from NZB mice prior to the onset of disease. These in vitro properties suggest that peptide 861-874 contains a dominant autoreactive CD4+ T-cell epitope, but it remains to be determined whether the T cells that recognize this sequence provide help in vivo for the production of RBC autoantibodies in NZB mice.
It is now known that exposing rodents to peptides containing immunodominant T-cell epitopes can modulate the activity of autoaggressive T cells in helper-dependent autoimmune diseases. In particular, mucosal administration of such peptides has been recognized as a means to induce antigen-specific immune tolerance, an effect attributed to changes in the balance of T-cell regulation. For example, inhalation of the immunodominant myelin basic protein (MBP) peptide, Ac1-11, or analogs of it, prevents experimental allergic encephalomyelitis (EAE)15 in mice, while inhalation of a mixture of glutamic acid decarboxylase (GAD) peptides16 or insulin peptide B 9-26 17 by young nonobese diabetic (NOD) mice delays the onset and decreases the incidence of diabetes. Similar effects have also been demonstrated in autoantibody-mediated disease, because inhalation of the dominant peptide 150-169 prevents the development of experimental autoimmune myasthenia gravis (EAMG) in C57BL/6 mice immunized with Torpedo-fish acetylcholine receptor.18 By analogy, it would be predicted that exposing NZB mice to a band 3 peptide bearing a dominant helper T-cell epitope should modulate the response of the corresponding T cells and the production of RBC autoantibodies if band 3-reactive T cells do provide help for the production of RBC autoantibodies. Thus, the aim of the current work was to ascertain whether administration of the band 3 peptide 861-874 to NZB mice by different routes would modulate the response of the corresponding T cells and RBC autoantibody production. In particular, it was determined whether intranasal delivery of this peptide, or a more soluble analog containing the same helper epitope, would ameliorate NZB AIHA. The results have important implications for the design of future peptide immunotherapy in human AIHA and other autoimmune diseases.
Materials and methods
Mice
NZB (H-2d) mice were maintained under specific pathogen-free conditions in isolators in the animal facilities at the University of Bristol and University of Aberdeen.
Direct antiglobulin test (DAT)
Mice were bled and their blood was collected into saline containing 0.38% wt/vol trisodium citrate. The RBCs were washed 3 times in warm (37°C) phosphate-buffered saline (PBS), pH 7.4. A 2% to 5% (vol/vol) suspension of the RBCs was added to the wells of round-bottomed microtiter plates (Nunc, Roskilde, Denmark), and the agglutination titer was scored after incubation for 1 hour at 37°C with doubling dilutions of rabbit antiserum to mouse IgG Fc (Nordic, Maidenhead, United Kingdom).
Antigens and mitogen
Murine RBC antigens were prepared, and their purity assessed as described previously.13 Briefly, murine band 3 was purified by anion exchange chromatography from CBA RBC membranes that had been stripped of peripheral proteins and detergent solubilized in 1% C12E8 (Sigma, Poole, United Kingdom). Residual detergent was removed by cold acetone precipitation of the protein. The protein was concentrated (Amicon Centriprep concentrator; Stonehouse, United Kingdom) and dialyzed extensively against PBS, pH 7.4, before addition to cultures at a final concentration of 10 μg/mL.
Peptides
Peptide CLAVLWVVKSTPAS corresponding to 861-874 from the sequence of murine band 3 19 was synthesized by a solid-phase synthetic method using fluorenylmethoxycarbonyl-polyamide chemistry20 (Department of Biochemistry, University of Bristol, United Kingdom). The soluble analog of peptide 861-875 (Glu861, Lys875) and single alanine-substituted analogs of the sequence were synthesized similarly. The purity of the peptides was checked by mass spectroscopy. Peptides were used to stimulate cultures at 20 μg/mL, which has been found to be the optimal concentration in previous epitope mapping studies.21,22
Administration of peptides
Groups of 6-week-old NZB mice were given either 100 μg peptide 861-874 in PBS, or saline alone, by intraperitoneal injection. Groups of NZB mice of similar age were lightly ether-anesthetized and given 25 μL of peptide 861-874 or 861-875 (Glu861, Lys865) in PBS (4 mg/mL) intranasally on 3 occasions (300 μg in total) at 2-day intervals. Age- and sex-matched mice receiving saline or PBS acted as the control group.
Stimulation of T cells
T cells were isolated under aseptic conditions from the spleens of mice and stimulated in vitro with antigens, peptides, or mitogens. T cells were obtained from single-cell suspensions of macerated spleen by passage through a mouse immunoglobulin/rabbit antimouse immunoglobulin glass bead affinity column.23 This method typically yields T-cell preparations of more than 90% purity. In some experiments lymph nodes were removed and cell suspensions prepared. Unselected spleen cells, irradiated with 15 Gy (1500 rad) (cesium source; Gravatom Industries, Fareham, United Kingdom) to prevent their division, were used as the source of syngeneic antigen-presenting cells (APCs). T cells and APCs were cultured together in 2-mL wells at 1.25 × 106 mL-1 and 0.6 × 106 mL-1, respectively, in the presence of antigens, peptides, or mitogen, using the α modification of Eagle medium (Gibco, Paisley, United Kingdom) supplemented with fresh 0.5% heat-inactivated (56°C for 30 minutes) CBA mouse serum, 4 mM l-glutamine (Sigma), 20 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) (pH 7.2) (Sigma), 100 U/mL benzyl penicillin (Sigma), 100 μg/mL streptomycin sulfate (Sigma), and 5 × 10-5 M 2-mercaptoethanol (Sigma). The cultures were incubated in a humidified atmosphere of 5% CO2 and 95% air at 37°C. Proliferation was estimated from the incorporation of tritiated [3H]thymidine (Amersham, Bucks, United Kingdom) in duplicate 100-μL samples withdrawn from the wells between days 2 and 7 of culture whenever possible, using a 1450 Microbeta Liquid Scintillation Counter (LKB Wallac, Turku, Finland).
T-cell cytokine secretion assay
The method is described in detail elsewhere.24 Briefly, 100-μL samples of T-cell cultures were withdrawn from the 2 mL wells on the day of culture indicated and incubated for a further 24 hours in the wells of microtiter plates, which had been coated with monoclonal anticytokines. After washing, bound cytokines were detected by adding biotinylated anticytokine monoclonals directed to nonoverlapping epitopes on the cytokine. The levels of cytokines were estimated by regression analysis from a standard curve constructed using recombinant cytokine. For interferon-γ (IFN-γ) the capture antibody was clone R4-6A5 and the detecting antibody biotin-labeled XMG1.2; for interleukin-4 (IL-4) the capture antibody was clone 11B11 and the detecting antibody biotin-labeled BVD6-24G2; and for IL-10 the capture antibody was clone JES5-2A5 and the detecting antibody biotin-labeled SXC-1 (all from PharMingen, San Diego, CA).
Measurement of IgG subclasses bound to RBCs
The number of IgG molecules of each subclass bound to the surface of RBCs was determined using a quantitative and sensitive cellular enzyme-linked immunosorbent assay (ELISA) based on a direct enzyme-linked antiglobulin test (DELAT) developed in our laboratories25 and described in detail elsewhere.26 Briefly, RBCs were washed 6 times in PBS and fixed in glutaraldehyde. Fifty microliter volumes of a 2% suspension of fixed RBCs were added to duplicate wells of round-bottomed microtiter plates (Nunc) and incubated with subclass-specific sheep antimouse IgG antibody (The Binding Site, Birmingham, United Kingdom) for 1 hour at 37°C. After washing 3 times, the RBCs were incubated with alkaline phosphatase-conjugated donkey antisheep antibody (Sigma), washed a further 3 times, and allowed to react with phosphatase substrate solution (p-nitrophenyl phosphate; Sigma) for 1 hour at 37°C. The supernatant was transferred into flat-bottomed microtiter plates (Dynatech, Sussex, United Kingdom) and the optical density (OD) measured at 405 nm (Titertek Multiscan II; Labsystems, Hants, United Kingdom). The number of molecules of each murine IgG subclass bound to the RBCs was calculated by interpolation from a standard curve generated by tanning normal RBCs with a known concentration of each purified IgG subclass26 (Sigma). The lowest levels of detection in this assay were 102.68 molecules of IgG1 per RBC, 102.67 molecules of IgG2a, 102.78 molecules of IgG2b, and 102.81 molecules of IgG3.
Statistical analysis
Results were analyzed by Mann-Whitney U test or Student t test as appropriate, with the level of significance taken as a P level less than .05.
Results
Previous in vitro studies indicate that the band 3 sequence 861-874 may contain a dominant autoreactive CD4+ T-cell epitope.14,22 A series of experiments was designed to confirm that the corresponding T cells do provide help for NZB anti-RBC autoantibodies in vivo and to test whether NZB disease could be ameliorated by mucosal delivery of the peptide.
Effect of immunization with band 3 peptide 861-874 on NZB disease
If band 3 peptide 861-874 does contain a dominant autoreactive T-cell epitope, immunization of NZB mice with the sequence should exacerbate the autoimmune response. Accordingly, groups of 6-week-old NZB mice were given peptide 861-874 or saline by intraperitoneal injection and their autoantibody responses and development of anemia measured. The RBCs of mice that had received peptide 861-874 were coated with higher levels of IgG at 5 months of age than those from the control animals, with significant increases in both RBC-bound IgG1 and IgG2a (Figure 1A). Furthermore, the onset of anemia was accelerated in the peptide-immunized group, which demonstrated a significantly lower hematocrit at 5 months of age than the control mice (Figure 1B).
Effect of immunization with band 3 peptide 861-874 on NZB disease. Comparison of number of IgG molecules of each isotype present on RBCs (A) and the hematocrit (B) of individual NZB mice that were injected with saline (n = 11) or peptide 861-874 (n = 9) (*P < .01, **P < .001, Mann-Whitney U test).
Effect of immunization with band 3 peptide 861-874 on NZB disease. Comparison of number of IgG molecules of each isotype present on RBCs (A) and the hematocrit (B) of individual NZB mice that were injected with saline (n = 11) or peptide 861-874 (n = 9) (*P < .01, **P < .001, Mann-Whitney U test).
Effect of mucosal administration of peptide 861-874 on NZB T-cell responses
Administration of dominant peptides intranasally can ameliorate aggressive autoimmune responses by inducing tolerance of the corresponding T cells. Thus, the possibility that intranasal exposure of NZB mice to peptide 861-874 may modulate the response of the corresponding T cells, and RBC autoantibody production was examined. As a first step, groups of NZB mice aged 6 weeks were given peptide 861-874 or saline intranasally. Fourteen, 21, and 28 days after the last treatment, the ability of their splenic T cells to proliferate in response to peptide 861-874 and band 3 was compared. Figure 2 summarizes the results. At all 3 time points, significantly higher proliferative responses to peptide 861-874 were given by T cells from mice that had inhaled the peptide as compared with T cells from mice that had inhaled saline (Figure 2A). By comparison, the response of T cells from the peptide-treated group to band 3 increased steadily but was significantly higher than that of the saline-treated group on days 21 and 28 (Figure 2B). Thus, intranasal delivery of peptide 861-874 failed to induce tolerance but accelerated the priming of band 3-reactive T cells.
Effect of inhaling peptide 861-874 on T cells' proliferative response to peptide 861-874 and band 3. Splenic T cells were obtained from NZB mice that had inhaled saline or peptide 861-874 on days 14, 21, and 28 after treatment and were stimulated with peptide 861-874 (A) or band 3 (B). Each point represents the stimulation index of the peak responses (*P < .01, **P < .001, Mann-Whitney U test).
Effect of inhaling peptide 861-874 on T cells' proliferative response to peptide 861-874 and band 3. Splenic T cells were obtained from NZB mice that had inhaled saline or peptide 861-874 on days 14, 21, and 28 after treatment and were stimulated with peptide 861-874 (A) or band 3 (B). Each point represents the stimulation index of the peak responses (*P < .01, **P < .001, Mann-Whitney U test).
Design of a soluble analog of band 3 peptide 861-874
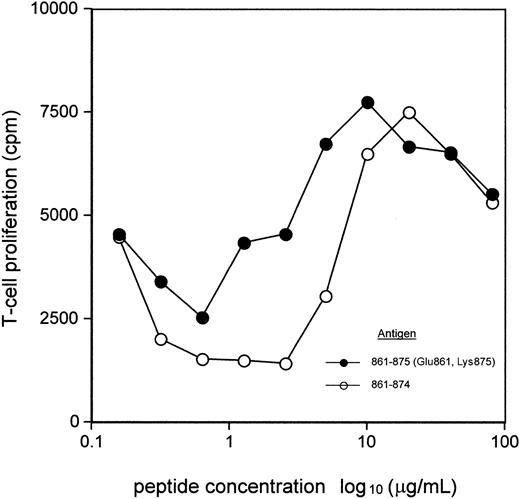
Peptide 861-874 is relatively insoluble in saline, a property that is often associated with immunogenicity rather than tolerogenicity. This generalization led us to consider whether intranasal administration of a soluble form of peptide 861-874 would have a different effect on T-cell responsiveness as compared with the native peptide. To examine this idea, a soluble form of peptide 861-874, namely peptide 861-875 (Glu861, Lys875), was synthesized, with substitutions made outside the core epitope, which has been characterized in previous studies.22 It was then asked if T cells from NZB mice responded to this peptide in vitro. As can be seen from Figure 3, peptide 861-875 (Glu861, Lys875) elicits a similar or higher response than peptide 861-874 from NZB T cells over the range of concentrations tested. The argument that NZB T cells may respond to different epitopes within each of the 2 peptides was addressed by taking advantage of the mapping of residues within peptide 861-874 that are necessary for NZB T-cell recognition or binding to the I-Ed restriction element.22 Analogs of peptide 861-875 (Glu861, Lys875) with single alanine substitutions at Val868, the critical T-cell-receptor contact residue within peptide 861-874, or at major histocompatibility complex (MHC) anchors Val864 and Lys865 22 each failed to stimulate proliferative responses by NZB T cells. Thus, in a representative experiment (n = 3) the response of NZB T cells to peptide 861-875 (Glu861, Lys875) was 11 463 ± 976 cpm, while peptides 861-875 (Glu861, Ala868, Lys875), 861-875 (Glu861, Ala864, Lys875), and 861-875 (Glu861, Ala865, Lys875) stimulated 3022 ± 196 cpm, 1444 ± 406 cpm, and 5140 ± 406 cpm, respectively, with background proliferation in unstimulated wells of 3553 ± 383 cpm.
Comparison between NZB T-cell proliferative response to peptide 861-874 and 861-875 (Glu861, Lys875). A splenic T-cell pool from DAT-positive NZB mice was cultured with varying concentrations of peptide 861-874 or 861-875 (Glu861, Lys875). The data are the mean values of the peak responses on day 6. In the absence of antigen, the proliferative response was less than 1200 cpm.
Comparison between NZB T-cell proliferative response to peptide 861-874 and 861-875 (Glu861, Lys875). A splenic T-cell pool from DAT-positive NZB mice was cultured with varying concentrations of peptide 861-874 or 861-875 (Glu861, Lys875). The data are the mean values of the peak responses on day 6. In the absence of antigen, the proliferative response was less than 1200 cpm.
Effect of mucosal administration of soluble peptide 861-875 (Glu861, Lys875) on NZB T-cell responses
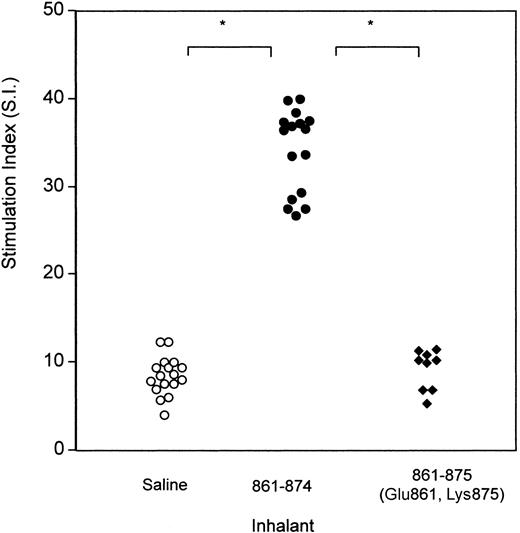
Having designed a soluble analog of band 3 peptide 861-874, experiments were designed to determine whether it differed from the wild-type sequence in the ability to prime T cells in vivo after mucosal administration. Accordingly, groups of NZB mice were given either saline, or peptide 861-874, or peptide 861-875 (Glu861, Lys875) intranasally on 3 occasions at 2-day intervals. Fourteen days after the last treatment, the ability of their splenic T cells to proliferate in response to peptide 861-874 was measured (Figure 4). As before, a significantly higher response to peptide 861-874 was obtained from mice that inhaled peptide 861-874 as compared with that generated in saline-treated mice. By contrast, the proliferative response of T cells from mice that inhaled peptide 861-875 (Glu861, Lys875) was similar to the T-cell response of control mice. In other words, the priming effect seen in mice treated with peptide 861-874 was absent in mice treated with the soluble peptide.
Comparison of proliferation in response to peptide 861-874 by T cells from NZB mice that had inhaled saline, peptide 861-874 (CLAVLWVVKSTPAS), or peptide 861-875 (Glu861, Lys875). Splenic T cells were obtained from the 2 groups of animals on day 14 after treatment and stimulated with peptide 861-874. Each point represents the stimulation index of peak responses by T cells from 1 mouse (*P < .001, Mann-Whitney U test).
Comparison of proliferation in response to peptide 861-874 by T cells from NZB mice that had inhaled saline, peptide 861-874 (CLAVLWVVKSTPAS), or peptide 861-875 (Glu861, Lys875). Splenic T cells were obtained from the 2 groups of animals on day 14 after treatment and stimulated with peptide 861-874. Each point represents the stimulation index of peak responses by T cells from 1 mouse (*P < .001, Mann-Whitney U test).
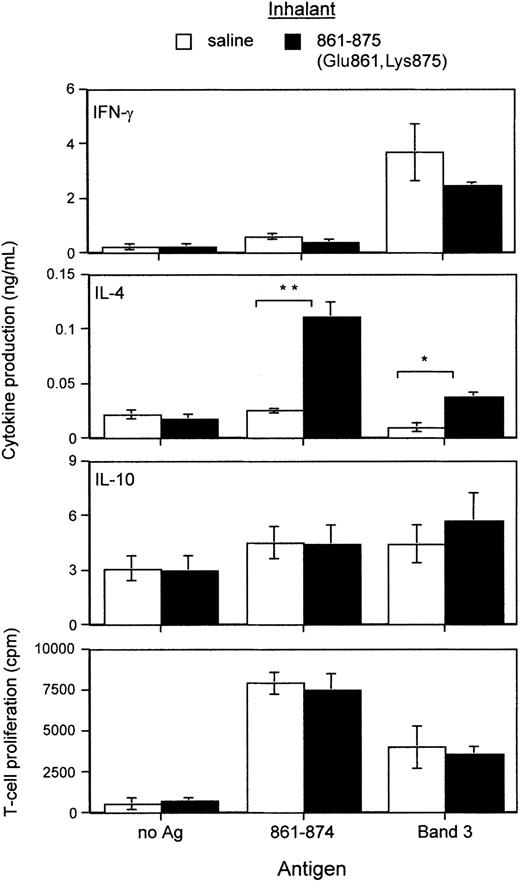
Splenic T cells derived from mice that had inhaled peptide 861-875 (Glu861, Lys875) were stimulated with peptide 861-874 or band 3 and their cytokine and proliferative responses compared with those given by T cells from saline-treated mice. It can be seen from Figure 5 that, in the absence of antigen, T cells from both saline- and peptide-treated mice produced similar levels of IFN-γ, IL-4, and IL-10. By contrast, in the presence of peptide 861-874, T cells from peptide-treated mice produced significantly more of the T helper-2 (Th2) cytokine IL-4 than those from the saline-treated group (Figure 5, second panel from top). A slight but insignificant decrease in IFN-γ secretion was also observed, while the level of IL-10 remained unchanged. Similarly, a significant increase in IL-4 was produced by T cells from peptide-treated mice cultured with band 3 as compared with T cells from saline-treated mice. There was also some decrease in IFN-γ and increase in IL-10 production, although the differences were not significant. The respective proliferative responses to peptide 861-874 and band 3 given by T cells from the 2 groups of mice were comparable (Figure 5, bottom panel).
Comparison of cytokine production driven by peptide 861-874 or band 3 between splenic T cells derived from NZB mice 3 weeks after they had inhaled saline or peptide 861-875 (Glu861, Lys875). The levels of the Th cytokines IFN-γ, IL-4, and IL-10 produced were measured by ELISA. No antigen was added to the negative control wells. Each bar represents the mean values (n = 4 or more) of peak responses (*P < .05, **P < .01, Student t test).
Comparison of cytokine production driven by peptide 861-874 or band 3 between splenic T cells derived from NZB mice 3 weeks after they had inhaled saline or peptide 861-875 (Glu861, Lys875). The levels of the Th cytokines IFN-γ, IL-4, and IL-10 produced were measured by ELISA. No antigen was added to the negative control wells. Each bar represents the mean values (n = 4 or more) of peak responses (*P < .05, **P < .01, Student t test).
Effect of mucosal administration of band 3 peptide 861-874, or the soluble analog 861-875 (Glu861, Lys875), on NZB autoantibody responses
An experiment was set up to determine if peptide inhalation affected the level of each IgG isotype of the RBC-bound autoantibodies, which may be dependent on the balance of helper cytokines. Groups of NZB mice that had inhaled saline, peptide 861-874, or peptide 861-875 (Glu861, Lys875) were bled at 5 months of age, and the number of antibody molecules of each isotype present on their RBCs was measured by DELAT (Figure 6). The number of IgG1 antibodies bound to RBCs from peptide 861-875- (Glu861, Lys875) treated mice was significantly higher than that bound to RBCs from either saline- or peptide 861-874-treated mice, while the number of bound IgG2a antibodies was less than that bound to RBCs from peptide 861-874-treated mice. The number of IgG3 antibodies bound to RBCs from both peptide 861-875- (Glu861, Lys875) and 861-874-treated mice was significantly higher than that bound to RBCs from saline-treated mice. In another experiment to determine whether or not the effect of inhaling peptide 861-875 (Glu861, Lys875) is specific, NZB mice were given mycobacterial-specific heat shock protein peptide 261-271 24 (n = 6) or saline (n = 5) by inhalation using the same protocol as for the previous experiment. The number of antibody molecules of each isotype present on their RBCs was measured by DELAT at 5 months of age, but no difference between the 2 groups was observed (results not shown).
Comparison of number of IgG molecules of each isotype present on RBCs from individual NZB mice that had inhaled saline (n = 16), peptide 861-874 (n = 16), or peptide 861-875 (Glu861, Lys875) (n = 8) (*P <.01, **P <.001, Mann-WhitneyUtest).
Comparison of number of IgG molecules of each isotype present on RBCs from individual NZB mice that had inhaled saline (n = 16), peptide 861-874 (n = 16), or peptide 861-875 (Glu861, Lys875) (n = 8) (*P <.01, **P <.001, Mann-WhitneyUtest).
Effect of mucosal administration of band 3 peptide analog 861-875 (Glu861, Lys875) on NZB anemia
The shift in RBC-bound autoantibody subclass resulting from mucosal administration of band 3 peptide analog 861-875 (Glu861, Lys875) would be predicted to affect the rate of hemolysis. To test this possibility, groups of NZB mice were given peptide 861-875 (Glu861, Lys875) or PBS intranasally and the development of anemia compared. At 5 months of age, the mean hematocrit of the peptide-treated mice was significantly higher than in the control group (Figure 7), and tests on the levels of RBC-bound immunoglobulin again showed a higher ratio of IgG1/IgG2a in the group that had received peptide (0.91:1) as compared with the control group (0.57:1).
Effect of inhaling band 3 peptide analog 861-875 (Glu861, Lys875) on NZB disease. Comparison of the hematocrit of individual NZB mice that inhaled PBS (n = 6) or peptide 861-875 (Glu861, Lys875) (n = 6) (**P < .002, Mann-Whitney U test).
Effect of inhaling band 3 peptide analog 861-875 (Glu861, Lys875) on NZB disease. Comparison of the hematocrit of individual NZB mice that inhaled PBS (n = 6) or peptide 861-875 (Glu861, Lys875) (n = 6) (**P < .002, Mann-Whitney U test).
Discussion
The results demonstrate that band 3 peptide 861-874 contains a dominant autoreactive helper T-cell epitope with the ability in vivo to modulate the course of AIHA in NZB mice. These findings support the view that NZB disease is helper dependent and illustrate that, while characterization of RBC autoantigen recognition by T cells can be exploited therapeutically, mucosal delivery of different peptides containing the dominant epitope can have detrimental or beneficial effects.
A number of earlier lines of evidence suggested that the dominant autoreactive T-cell epitope in NZB mice with AIHA is contained within band 3 peptide 861-874. Firstly, peptide 861-874 elicits a more vigorous response from NZB T cells than any other band 3 peptide. Next, T cells from 6-month-old NZB mice with high autoantibody titers often respond to peptides 110-125, 272-286, 301-307, 431-446, 441-456, 699-713, and 719-734 as well as peptide 861-874 but, by contrast, T cells from NZB mice aged less than 3 months also respond vigorously to peptide 861-874 but rarely to the other peptides. These results are reminiscent of the observation in murine EAE where immunization with peptide Ac1-11 from guinea pig MBP leads to both the induction of EAE and to epitope spreading, as judged by the recall proliferative responses by splenic T cells to 3 other endogenous peptides.27,28 Similarly, in NOD mice, the T-cell proliferative response to GAD spreads to numerous peptides as the disease develops.29 By analogy it would appear that in NZB mice the T-cell response is initially focused on peptide 861-874 and later spreads to other band 3 peptides. The evidence from in vitro studies that peptide 861-874 contains the dominant autoaggressive T-cell epitope is supported by the current work showing that NZB mice injected with peptide 861-874 had accelerated RBC autoantibody production and anemia.
It was asked whether administering peptide 861-874 to NZB mice by inhalation would modulate the response of the corresponding T cells in such a way as to ameliorate disease. To answer this question, NZB mice were given the native peptide intranasally. It was found that this procedure primed the T cells responsive to this peptide and to band 3. This result did not entirely surprise us because peptide 861-874 is relatively insoluble, and work in our laboratory on inhalation of another insoluble peptide30 suggested that it too primed the corresponding T cells.31 Consequently, the effects of a soluble analog of peptide 861-874, peptide 861-875 (Glu861, Lys875), were also assessed and different results were obtained. We first excluded the possibility that the difference is due to the reactivity of this analog, with 861-874-specific T cells being altered by the substitution of cysteine at position 861 with glutamic acid and the addition of lysine at position 875. First, NZB T cells proliferated in vitro, if anything, more vigorously to peptide 861-875 (Glu861, Lys875) than 861-874, presumably because of its better solubility. Secondly, the substitution and addition in peptide 861-875 (Glu861, Lys875) are made outside the minimal residual epitope, which we have demonstrated to lie between residues 862-870.22 Furthermore, NZB T cells recognize the same epitope from peptides 861-874 and 861-875 (Glu861, Lys875), because further substitution of the soluble analog at positions identified22 in the wild-type sequence as a T-cell receptor or MHC contact residues resulted in loss of stimulatory ability. Comparisons were then made between the ability of T cells from NZB mice that had inhaled saline, peptide 861-874, or peptide 861-875 (Glu861, Lys875) to proliferate in response to peptide 861-874. These experiments confirmed that exposing mice to peptide 861-874 primed the corresponding T cells. By contrast, the response of T cells from mice treated with peptide 861-875 (Glu861, Lys875) was the same as the control group. Thus, the priming effect seen in mice treated with 861-874 was absent in mice treated with the soluble peptide.
Strikingly, T cells from mice treated nasally with the soluble peptide 861-875 (Glu861, Lys875) produced an in vitro cytokine response to both peptide 861-874, and band 3 deviated toward Th2 cytokines. It would also be predicted from this result that if T cells reactive with peptide 861-874 help the autoantibody response, the isotype of RBC-bound autoantibodies should be altered in NZB mice that have inhaled peptide 861-875 (Glu861, Lys875). This deduction follows from the effect that different helper T-cell cytokines have on the isotype of antibody produced and, in particular, the association of murine Th2 cytokine-dominated responses with IgG1 antibodies.32 The prediction was borne out by the results. The number of IgG1 antibodies bound to RBCs from peptide 861-875- (Glu861, Lys875) treated mice was significantly higher, and the number of IgG2a antibodies lower, than those bound to RBCs from either saline- or peptide 861-874-treated mice. The effect is specific because the isotype of antibodies bound to RBCs from mice treated with an irrelevant peptide was not altered.
Further experiments were designed to test the effect of inhaling the soluble band 3 peptide analog on NZB anemia, because the changes in autoantibody subclass that resulted from this treatment would be expected to reduce hemolysis. This view is based on the capacity of a band 3-reactive12 monoclonal erythrocyte autoantibody derived from NZB mice to induce anemia in BALB/c recipients: The IgG2a autoantibody and the IgG2b switch variant were considerably more pathogenic than the IgG1 switch variant.33 The results showed that the hematocrit values of NZB mice that had received the analog peptide 86-875 (Glu861, Lys875) intranasally were significantly higher than those of control mice and confirmed that the change toward a Th2 cytokine-dominated autoantibody subclass is associated with amelioration of disease. Other examples of successful peptide therapy, based on nasal administration of peptides corresponding to dominant T-cell epitopes in models of autoimmunity such as EAE, are associated with a deviation from a Th1 to a regulatory IL-10 CD4+ T-cell response.34,35 In such models, protection is typically more complete than that seen in NZB disease. This difference may reflect the fact that naive T cells were manipulated in EAE, whereas in NZB disease T cells primed to peptide 861-874 were already generated by the time therapy was initiated. Another possibility is that there may be a generalized deficit in the ability to generate autoantigen-specific regulatory T cells in the autoimmune-prone NZB strain, which displays a number of other autoimmune abnormalities, such as elevated levels of immunoglobulin, anti-DNA antibodies, antithymocyte antibodies, and circulating immune complexes, in addition to AIHA.3-5 A switch to a suppressive subset would be expected to be more effective therapeutically than the deviation toward a Th2 response seen in NZB mice that had received peptide 861-875 (Glu861, Lys875). In this respect it is noteworthy that the dominant human RBC autoantigens, the Rh proteins, carry epitopes that stimulate IL-10 production by T cells from AIHA patients.36 Peptides that include these epitopes suppress the proliferative response by patients' T cells to the purified RhD protein.36 Preliminary work from our laboratories has identified band 3 peptides that stimulate IL-10 production by NZB T cells, but it remains to be determined whether such cells can be manipulated to ameliorate disease.
Current medical treatments for autoimmune diseases are unsatisfactory, because therapy is largely restricted to the use of immunosuppressive or antiinflammatory agents. This work supports the hypothesis that the production of RBC autoantibodies in NZB disease is truly T-cell dependent and that T cells reactive with a number of band peptides, particularly the sequence 861-874, are targets for therapy. As mentioned, peptide therapy of a variety of experimental and spontaneous rodent autoimmune diseases is a reality. However, the pathologies of the diseases that have been successfully controlled, including EAE, collagen-induced arthritis, NOD mouse diabetes, and EAMG, are primarily T-cell mediated or experimentally induced, and the effect of inhaling peptides bearing dominant helper T-cell epitopes in spontaneous autoantibody-mediated diseases remains to be determined. Our results demonstrate that the autoimmune response in NZB AIHA can also be modulated by nasal administration of a peptide containing the dominant T-cell epitope but reveal that this treatment can have potentially detrimental or beneficial effects, depending on the solubility of the sequence. This finding is clearly relevant to the development of safe immunotherapeutic regimens based on peptide inhalation for human autoimmune diseases.
Prepublished online as Blood First Edition Paper, June 26, 2003; DOI 10.1182/blood-2002-07-2125.
Supported by grants from the Wellcome Trust.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal