Abstract
Little effort has been made to quantitate adverse outcomes of monoclonal gammopathy of undetermined significance (MGUS) of the immunoglobulin M (IgM) class, which progresses to lymphoma or Waldenström macroglobulinemia, whereas IgA and IgG MGUS progress to multiple myeloma, primary amyloidosis (AL), or a related plasma cell disorder. From 1960 to 1994, IgM MGUS was diagnosed in 213 patients in southeastern Minnesota. The end point was progression to lymphoma or a related disorder, as assessed with the Kaplan-Meier method. The 213 patients were followed up for 1567 person-years (median, 6.3 years per patient). Lymphoma developed in 17 patients (relative risk [RR], 14.8), Waldenström macroglobulinemia in 6 (RR, 262), primary amyloidosis in 3 (RR, 16.3), and chronic lymphocytic leukemia in 3 (RR, 5.7). The relative risk of progression was 16-fold higher in the patients with IgM MGUS than in the white population of the Iowa Surveillance, Epidemiology, and End Results Program. Cumulative incidence of progression was 10% at 5 years, 18% at 10 years, and 24% at 15 years. On multivariate analysis, the serum monoclonal protein and serum albumin concentrations at diagnosis were the only risk factors for progression to lymphoma or a related disorder. Risk for progression to lymphoma or a related disorder at 10 years after the diagnosis of MGUS was 14% with an initial monoclonal protein concentration of 0.5 g/dL or less, 26% with 1.5 g/dL, 34% for 2.0 g/dL, and 41% for more than 2.5 g/dL. (Blood. 2003;102:3759-3764)
Introduction
Monoclonal gammopathy of undetermined significance (MGUS) is found in more than 1% of persons 50 years or older and in approximately 3% of those older than 70 years.1-5 MGUS is defined by the presence of a serum monoclonal protein (M protein) concentration less than 3 g/dL; no M protein or only small amounts of monoclonal light chains in the urine; absence of lytic bone lesions, anemia, hypercalcemia, and renal insufficiency related to the M protein; and less than 10% plasma cells in the bone marrow (if tested).6,7 This condition is clinically significant because of the high likelihood that in some patients MGUS will progress to multiple myeloma or some other plasma cell malignancy.8 Among patients with MGUS, 15% to 20% have the IgM subclass. Little effort has been made to determine the nature and frequency of adverse outcomes of MGUS of the IgM class, which has different clinical features and progresses to lymphoma or Waldenström macroglobulinemia rather than to multiple myeloma.9-13
Among 242 patients with MGUS of the IgM type recognized at the Mayo Clinic from 1956 to 1978, a group of malignant lymphoid disorders developed in 40 (17%) patients an average of 4 years after recognition of the M protein.9 These disorders included Waldenström macroglobulinemia in 22 patients, lymphoproliferative disorder in 9, lymphoma in 6, primary amyloidosis (AL) in 2, and chronic lymphocytic leukemia in 1. In addition, 9 patients with MGUS had an increased concentration of serum M protein of more than 0.5 g/dL, and 10 others had an increase of more than 1 g/dL. Of these 19 patients with an increase in M protein concentration, none contracted symptomatic Waldenström macroglobulinemia or a related disorder.9 In a larger series of MGUS, the IgM type has not been evaluated as a separate entity.8,14,15
Therefore, our knowledge is limited by the small numbers of patients and the modest follow-up in most series. Furthermore, most of the reports emanate from tertiary medical centers, where the results may be distorted by selective referral of patients at greater risk for adverse outcomes. Consequently, we evaluated the prognosis and predictors of outcome in a cohort of patients with IgM MGUS from southeastern Minnesota. We previously reported on 1384 patients in southeastern Minnesota with IgG, IgA, and IgM MGUS,8 but the current analysis is restricted to the 214 patients with MGUS of the IgM class. Because IgM monoclonal gammopathies have different features and different outcomes, we believe it is important to evaluate a large cohort of patients with IgM MGUS for whom there is long-term follow-up to quantify their prognoses more accurately.
Patients, materials, and methods
From January 1, 1960, through December 31, 1994, MGUS of the IgM class was diagnosed in 213 Mayo Clinic patients who resided in the 11 counties (including Olmsted County) of southeastern Minnesota. All patients had an IgM M protein concentration less than 3 g/dL at diagnosis. The M proteins were identified by cellulose acetate or agarose gel electrophoresis.16 If there was an abnormal band or an equivocal pattern, immunoelectrophoresis or immunofixation was performed. Patients with smoldering macroglobulinemia, lymphoma, or related disorders at the time of recognition of the IgM M protein were excluded. The medical records linkage system of the Rochester Epidemiology Project17 makes possible the complete ascertainment of clinically diagnosed cases among Olmsted County residents. The 1980 population of Olmsted County was 92 006, and 312 559 people resided in the remaining 10 counties of southeastern Minnesota in that year. We obtained a waiver of consent from the Institutional Review Board for these studies.
The primary end point of the study was progression to lymphoma or related disorders. Patients with an M protein were advised to undergo serum protein electrophoresis annually and were contacted if they did not. In addition, follow-up included the review of each person's inpatient and outpatient medical record at the Mayo Clinic and the review of death certificates for all who died. The end points with respect to progression to lymphoma or a related malignancy were calculated in terms of cumulative probability and cumulative incidence of progression. Cumulative probability was calculated using a Kaplan-Meier estimate,18 in which the data on patients who died were censored; curves were compared using the log-rank test.19 The cumulative incidence curve, however, explicitly accounted for other causes of death and was computed according to the method of Gooley et al.20 The effects of potential risk factors on progression rates were examined in a Cox proportional hazards model.21
The risk for progression to each disease also was assessed relative to the risk in the general population by applying age- and sex-specific incidence rates for these conditions in the white cohort from the Iowa Surveillance, Epidemiology, and End Results Program22 to the age-, sex-, and calendar-year-specific person-years of follow-up in our study cohort. Confidence intervals for the relative risks are based on a Poisson approach.23
All statistical tests were 2-sided. Analyses were performed with SAS software version 6.12 (SAS Institute, Cary, NC) and S-Plus version 3.4 (Insightful, Seattle, WA).
Results
Baseline characteristics
Of the 213 patients with IgM MGUS, 123 (58%) were men and 90 (42%) were women. Median age at diagnosis was 74 years (range, 24-94 years). Only 3 (1%) patients were younger than 40 years, and 64% were older than 70 years. Thirty-five percent of patients had a history of cancer in first-degree relatives, and 24% had a personal history of malignancy. Skin cancer accounted for 27%, genitourinary cancer 18%, and breast cancer 16%.
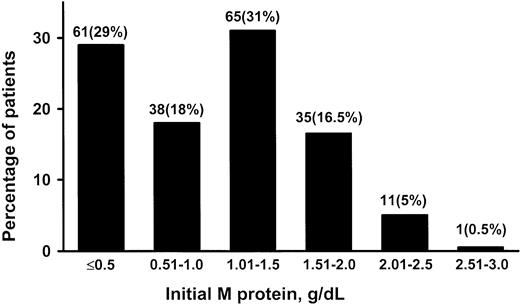
The value for the serum M protein at diagnosis ranged from unmeasurable (visible as a small band on electrophoresis but not quantifiable by densitometry) to 2.6 g/dL (median, 1.2 g/dL) (Figure 1). Only 53% of the patients had an M-protein value greater than 1.0 g/dL at diagnosis. IgM gammopathy was associated with a small non-IgM gammopathy (biclonal) in 3 (1%) patients. In 1 patient, the non-IgM component disappeared during follow-up. The light chain was κ in 70% and λ in 30%. The mobility of the M spike was γ in 85.5%, β-γ in 10%, β in 4%, and α2-globulin in 0.5%. The quantitative IgM value ranged from 40 to 4800 mg/dL (median, 675 mg/dL). Fifteen percent of patients had a normal nephelometric IgM value of 300 mg/dL or less. The concentration of uninvolved (normal, polyclonal, or background) immunoglobulins was reduced in 35% of 129 patients whose immunoglobulin concentration was determined quantitatively. Electrophoresis, immunoelectrophoresis, and immunofixation were performed on urine samples from 64 patients with IgM MGUS (19% had a monoclonal κ light chain, 8% had a λ light chain, and 73% were negative for a monoclonal light chain). Only 3 patients had more than 100 mg of light chain per 24 hours.
Initial monoclonal (M) protein values in 213 patients. Patients were residents of southeastern Minnesota in whom monoclonal gammopathy of undetermined significance (MGUS) of IgM class was diagnosed from 1960 through 1994.
Initial monoclonal (M) protein values in 213 patients. Patients were residents of southeastern Minnesota in whom monoclonal gammopathy of undetermined significance (MGUS) of IgM class was diagnosed from 1960 through 1994.
The initial hemoglobin level ranged from 5.9 to 18.9 g/dL (median, 14.0 g/dL) (Table 1). Only 17% of patients had a hemoglobin level lower than 12.0 g/dL, and in 4% it was lower than 10 g/dL. However, the anemia was caused by conditions other than monoclonal gammopathy (eg, myelodysplasia, renal insufficiency, iron deficiency). Only 1% had platelet counts lower than 100 × 109/L, and 3% had platelet levels higher than 500 × 109/L. The sedimentation rate was less than 30 mm in 1 hour in 51% of the patients and more than 100 mm in 1 hour in only 7% of the patients. The serum albumin level ranged from 1.9 to 4.5 g/dL (median, 3.4 g/dL), but only 14% of patients had albumin levels lower than 3 g/dL. The serum creatinine value was 2.0 mg/dL or more in 7% of patients, but this was not related to the monoclonal gammopathy in any instance. Of 126 patients in whom cryoglobulins were sought, 8 (6%) had positive results. Serum viscosity, determined in only 9 patients, ranged from 1.5 to 3.2 cP (median, 1.9 cP).
Laboratory values in 213 patients with lgM monoclonal gammopathy of undetermined significance
. | Median . | Range . | Other . |
|---|---|---|---|
| Hemoglobin, g/dL | 14 | 5.9-18.9 | < 12 in 17% |
| Platelets, × 109/L | 275 | 78-783 | < 100 in 1% |
| Sedimentation rate, in 1 h | 29 | 0-135 | ≥ 30 in 49% |
| Creatinine, mg/dL | 1.1 | 0.5-5.4 | ≥ 2 in 7% |
| Serum monoclonal protein, g/dL | 1.2 | 0.3-2.6 | ≥ 2 in 7% |
| Urine monoclonal protein, g/24 h | 0.05 | 0.01-0.54 | > 100 in 19% |
| Serum albumin, g/dL | 3.4 | 1.9-4.5 | < 3 in 14% |
. | Median . | Range . | Other . |
|---|---|---|---|
| Hemoglobin, g/dL | 14 | 5.9-18.9 | < 12 in 17% |
| Platelets, × 109/L | 275 | 78-783 | < 100 in 1% |
| Sedimentation rate, in 1 h | 29 | 0-135 | ≥ 30 in 49% |
| Creatinine, mg/dL | 1.1 | 0.5-5.4 | ≥ 2 in 7% |
| Serum monoclonal protein, g/dL | 1.2 | 0.3-2.6 | ≥ 2 in 7% |
| Urine monoclonal protein, g/24 h | 0.05 | 0.01-0.54 | > 100 in 19% |
| Serum albumin, g/dL | 3.4 | 1.9-4.5 | < 3 in 14% |
MGUS was diagnosed between 1960 and 1994 in patients in southeastern Minnesota.
Bone marrow examination was performed in 27 patients: 24 had normal or nondiagnostic results, 2 had inadequate specimens, and 1 had disseminated vasculitis. All had less than 10% plasma cells in the bone marrow aspirate. Patients who were asymptomatic and who did not have hepatosplenomegaly, lymphadenopathy, constitutional symptoms, or anemia were not subjected to bone marrow examination, in accordance with our clinical practice.
Livers were palpable in 10% of patients and ranged in size from 1 to 11 cm (median, 2.5 cm) below the right costal margin. Spleens were palpable in only 5 patients—1 cm below the left costal margin in 4 patients and 6 cm below the costal margin in 1 patient.
Outcomes
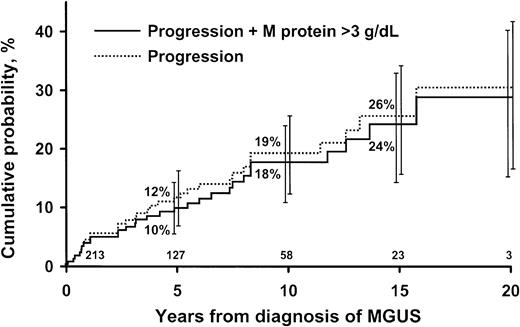
The 213 patients were monitored for 1567 person-years (median, 6.3 years; range, 0-20.6 years), during which 152 (71%) died. During follow-up, non-Hodgkin lymphoma, Waldenström macroglobulinemia, primary amyloidosis, and chronic lymphocytic leukemia developed in 29 (14%) patients (Table 2). The rate of progression did not increase immediately after recognition of the IgM MGUS, but it gradually increased throughout the period of observation. This strongly suggested that patients with early or smoldering macroglobulinemia were not included in our IgM MGUS cohort. Non-Hodgkin lymphoma was classified as lymphoplasmacytic (6 patients), diffuse large B-cell (5 patients), mucosa-associated lymphoid tissue (MALT) (2 patients), small lymphocytic (1 patient), follicular (1 patient), large cell (presumably B-cell) (1 patient), and B-cell unclassified (1 patient). Cumulative probability of progression to one of these disorders was 10% at 5 years, 18% at 10 years, and 24% at 15 years (Figure 2). The overall average risk for progression was approximately 1.5% per year. Patients were at risk for progression even after having stable MGUS for 20 years or more. Of the 17 patients in whom lymphoma developed, progression occurred after 5 years of observation in 3 patients. Of the 6 patients in whom Waldenström macroglobulinemia developed, progression occurred more than 5 years after recognition of the M protein in 5 patients. In 2 additional patients, the M-protein concentration was greater than 3 g/dL, but no therapy was required. Another patient had IgM λ monoclonal gammopathy (982 mg/dL), but biclonal gammopathy (IgM 386 mg/dL + IgA λ 2840 mg/dL) developed 5 years later. The bone marrow contained more than 15% plasma cells, and the patient was thought to have smoldering multiple myeloma that did not require immediate therapy.
Observed and expected progression and standardized incidence rates among 213 patients with IgM monoclonal gammopathy of undetermined significance
Disease progression . | Observed . | Expected* . | SIR . | 95% CI . |
|---|---|---|---|---|
| Non-Hodgkin lymphoma | 17 | 1.1 | 14.8 | 8.6-23.7 |
| Amyloidosis | 3 | 0.18 | 16.3 | 3.4-47.5 |
| Macroglobulinemia | 6 | 0.02 | 262 | 96.0-569.5 |
| Chronic lymphocytic leukemia | 3 | 0.53 | 5.7 | 1.2-16.5 |
| Total group | 29 | 1.83 | 15.9 | 11.0-22.8 |
Disease progression . | Observed . | Expected* . | SIR . | 95% CI . |
|---|---|---|---|---|
| Non-Hodgkin lymphoma | 17 | 1.1 | 14.8 | 8.6-23.7 |
| Amyloidosis | 3 | 0.18 | 16.3 | 3.4-47.5 |
| Macroglobulinemia | 6 | 0.02 | 262 | 96.0-569.5 |
| Chronic lymphocytic leukemia | 3 | 0.53 | 5.7 | 1.2-16.5 |
| Total group | 29 | 1.83 | 15.9 | 11.0-22.8 |
MGUS was diagnosed between 1960 and 1994 in patients in southeastern Minnesota.
SIR indicates standardized incidence rate; 95% CI, 95% confidence interval.
Iowa SEER Registry.
Probability of progression in 213 patients. Patients were residents of southeastern Minnesota in whom monoclonal gammopathy of undetermined significance (MGUS) of IgM class was diagnosed from 1960 through 1994. Curve shows probability of progression of MGUS to lymphoma, Waldenström macroglobulinemia, primary amyloidosis, or chronic lymphocytic leukemia. Bars show 95% confidence intervals. Numbers at bottom of the horizontal axis are numbers of patients at risk at each interval.
Probability of progression in 213 patients. Patients were residents of southeastern Minnesota in whom monoclonal gammopathy of undetermined significance (MGUS) of IgM class was diagnosed from 1960 through 1994. Curve shows probability of progression of MGUS to lymphoma, Waldenström macroglobulinemia, primary amyloidosis, or chronic lymphocytic leukemia. Bars show 95% confidence intervals. Numbers at bottom of the horizontal axis are numbers of patients at risk at each interval.
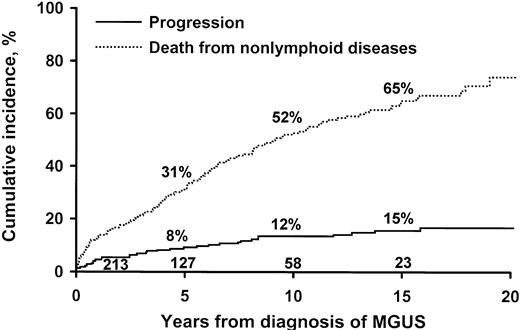
Rates of death due to other diseases, which included cardiovascular and cerebrovascular diseases and cancers that were not of lymphoid or plasmacytic origin, were 31% at 5 years, 52% at 10 years, and 65% at 15 years. In comparison, in a competitive model, the rates of progression due to lymphoplasma cell cancers were 8% at 5 years, 12% at 10 years, and 15% at 15 years (Figure 3). Patients with IgM MGUS had a shorter median survival time than expected for Minnesota residents of matched age and sex—7.0 versus 10.8 years (P < .001) (Figure 4).
Competitive model in 213 patients. Patients were residents of southeastern Minnesota in whom monoclonal gammopathy of undetermined significance (MGUS) of IgM class was diagnosed from 1960 through 1994. Upper curve shows probability of dying of nonlymphoid diseases. Lower curve shows probability of progression to lymphoma or a related disorder.
Competitive model in 213 patients. Patients were residents of southeastern Minnesota in whom monoclonal gammopathy of undetermined significance (MGUS) of IgM class was diagnosed from 1960 through 1994. Upper curve shows probability of dying of nonlymphoid diseases. Lower curve shows probability of progression to lymphoma or a related disorder.
Survival curves. Lower curve shows median survival of 213 patients with monoclonal gammopathy of undetermined significance (MGUS) of IgM class. Upper curve shows expected survival of Minnesota residents of matched age and sex.
Survival curves. Lower curve shows median survival of 213 patients with monoclonal gammopathy of undetermined significance (MGUS) of IgM class. Upper curve shows expected survival of Minnesota residents of matched age and sex.
The number of patients with progression to lymphoid neoplasm or a related disorder (29 patients) was 15.9 times that expected on the basis of incidence rates for those conditions in the general population (Table 2). The risk for disease was increased by a factor of 14.8 for non-Hodgkin lymphoma, 262 for macroglobulinemia, 16.3 for primary amyloidosis, and 5.7 for chronic lymphocytic leukemia.
The M protein disappeared in 9 patients during follow-up. All of these patients had low initial concentrations of M protein; concentration exceeded 1.0 g/dL (1.2 g/dL) at diagnosis in only 1 patient.
Risk factors for progression
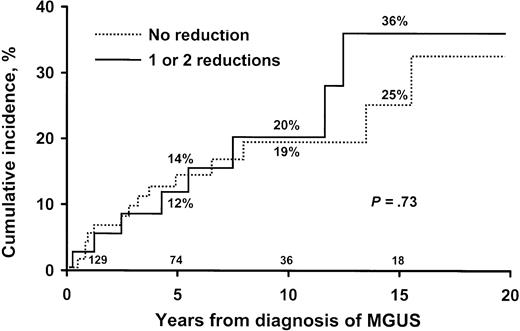
Baseline factors evaluated in a univariate model included age; sex; presence of hepatosplenomegaly; values for hemoglobin, serum calcium, alkaline phosphatase, creatinine, and albumin; concentration of serum M protein; presence, type, and amount of monoclonal urinary light chain; reduction in uninvolved immunoglobulins; platelet value; prothrombin time; and sedimentation rate (Table 3). Hemoglobin and serum albumin levels and the sedimentation rate were significant, whereas the serum M-protein concentration was of borderline significance (P = .06). Factors, including splenomegaly, platelet value, prothrombin time, sedimentation rate, reduction of uninvolved immunoglobulins, and size of urine M protein, in which more than 10% of the values were missing, were excluded from multivariate analysis. Only the concentration of the serum M protein (P = .03) at diagnosis and the serum albumin value (P = .01) were independent predictors of progression with multivariate analysis. Reductions in one or more uninvolved immunoglobulins and the presence of a monoclonal light chain in the urine were not risk factors for progression (Figure 5).
Risk factors for progression in 213 patients with IgM monoclonal gammopathy of undetermined significance
Factor . | Univariate analysis . |
|---|---|
| Age | 0.43 |
| Sex | 0.96 |
| Hepatomegaly | 0.56 |
| Hemoglobin value | 0.002 |
| Creatinine value | 0.70 |
| Serum albumin value | 0.03 |
| Serum monoclonal protein concentration | 0.06 |
| Serum monoclonal M-κ or M-λ | 0.49 |
| Urinary monoclonal protein | |
| Presence | 0.28 |
| Type κ or λ | 0.43 |
| Reduction of uninvolved immunoglobulins | 0.73 |
| Serum alkaline phosphatase value (log) | 0.55 |
| Platelet value | 0.90 |
| Sedimentation rate | 0.004 |
Factor . | Univariate analysis . |
|---|---|
| Age | 0.43 |
| Sex | 0.96 |
| Hepatomegaly | 0.56 |
| Hemoglobin value | 0.002 |
| Creatinine value | 0.70 |
| Serum albumin value | 0.03 |
| Serum monoclonal protein concentration | 0.06 |
| Serum monoclonal M-κ or M-λ | 0.49 |
| Urinary monoclonal protein | |
| Presence | 0.28 |
| Type κ or λ | 0.43 |
| Reduction of uninvolved immunoglobulins | 0.73 |
| Serum alkaline phosphatase value (log) | 0.55 |
| Platelet value | 0.90 |
| Sedimentation rate | 0.004 |
MGUS was diagnosed between 1960 and 1994 in patients in southeastern Minnesota.
Risk of progression in 213 patients. Patients were residents of southeastern Minnesota in whom monoclonal gammopathy of undetermined significance (MGUS) of IgM class was diagnosed from 1960 through 1994. Risk is shown with and without reduction of uninvolved immunoglobulins. Numbers at bottom of horizontal axis are numbers of patients at risk at each interval.
Risk of progression in 213 patients. Patients were residents of southeastern Minnesota in whom monoclonal gammopathy of undetermined significance (MGUS) of IgM class was diagnosed from 1960 through 1994. Risk is shown with and without reduction of uninvolved immunoglobulins. Numbers at bottom of horizontal axis are numbers of patients at risk at each interval.
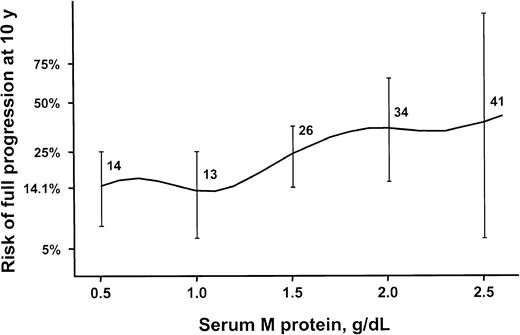
The relative risk for progression was directly related to the concentration of the M protein in the serum at the time of diagnosis of MGUS. The risk for progression to lymphoma or a related malignancy 10 years after recognition of MGUS was 14% for an initial M-protein value of 0.5 g/dL or less, 26% for 1.5 g/dL, 34% for 2.0 g/dL, and 41% for 2.5 g/dL (Figure 6). The risk for progression to lymphoma or a related disorder at 10 years with an initial M-protein value of 1.5 g/dL was 1.8 times the risk for progression with an initial value of 0.5 g/dL or less; for an initial value of 2.0 g/dL, it was 2.8 times; and for an initial value of 2.5 g/dL, it was 3.1 times.
Relative risk of disease progression in 213 patients. Patients were residents of southeastern Minnesota in whom monoclonal gammopathy of undetermined significance (MGUS) of IgM class was diagnosed from 1960 through 1994. Risk is by monoclonal protein value at diagnosis. Error bars show 95% CI.
Relative risk of disease progression in 213 patients. Patients were residents of southeastern Minnesota in whom monoclonal gammopathy of undetermined significance (MGUS) of IgM class was diagnosed from 1960 through 1994. Risk is by monoclonal protein value at diagnosis. Error bars show 95% CI.
Discussion
Patients with MGUS are at increased risk for progression to multiple myeloma, lymphoma, Waldenström macroglobulinemia, primary amyloidosis (AL), or a related plasma cell disorder. In patients with IgG or IgA MGUS, the risk for development of multiple myeloma and related disorders is well characterized and recently was the subject of 2 large studies.8,15 Although patients with IgM MGUS were included in these 2 studies, there was no specific analysis of progression in patients with IgM MGUS. A detailed and specific study of IgM MGUS is needed because the rate and nature of progression differ from those of IgG or IgA MGUS. IgG and IgA MGUS arise from mature, somatically mutated, postswitch plasma cells, and approximately 50% have evidence of translocations in the immunoglobulin heavy-chain region, 14q32.24 In contrast, IgM MGUS arises from somatically mutated postgerminal center B lymphocytes that have not undergone isotype class switching. Thus, translocations of 14q32 are not found in IgM MGUS (Fonseca R, personal communication, December 2002). As a result, the phenotype of progression in IgM MGUS is completely different from that in IgG or IgA MGUS. In essence, IgM MGUS can be considered a distinct biologic and clinical entity whose only relationship to IgA and IgG MGUS is the presence of secreted monotypic immunoglobulin.
Among older studies of MGUS, only a few series of modest size and with suboptimal durations of follow-up have focused on IgM monoclonal gammopathies.9-13 Patients with IgM MGUS constituted only a small proportion of patients in these studies. In 1 study,10 IgM monoclonal gammopathy was an incidental finding in 31% of 160 asymptomatic patients, but only 11 were classified as having benign disease (MGUS) after follow-up. In most of these patients, Waldenström macroglobulinemia or malignant lymphoma developed, and the authors emphasized the need for follow-up of more than 10 years. In another study,11 13 of 34 patients had benign IgM monoclonal gammopathy, but most patients were followed up for only 1 to 3 years. In another series, 3 of 28 patients with IgM gammopathy appeared to have benign disease, but 4 others had unrelated conditions.12 Waldenström13 reported that 44 of 67 patients with IgM gammopathy remained stable without treatment for a “long time.” In another report, 26 of 263 patients had MGUS of the IgM class. Macroglobulinemia (4 patients), lymphoma (3 patients), and chronic lymphocytic leukemia (1 patient) developed during a follow-up of 5 to 20 years. Macroglobulinemia was diagnosed at a median of 6 years after the recognition of MGUS, whereas MGUS had been present for 6, 13, and 15 years in 3 patients in whom malignant lymphoma developed.25 Duration and results of follow-up were not given in a series of 106 patients with IgM paraprotein, of whom 22 had IgM MGUS.26 In a report of 242 patients with MGUS of the IgM type diagnosed at Mayo Clinic from 1956 to 1978,9 malignant lymphoid disorders developed in 40 (17%) patients an average of 4 years after recognition of the M protein. Most patients with progression had Waldenström macroglobulinemia or lymphoma.
MGUS of the IgM type has been included in large series of MGUS, but there has been no effort for separate analysis of patients with MGUS of the IgM type. In the Gregersen et al14 series from Jutland, 246 of 1324 patients with MGUS had the IgM type, but their follow-up data were not separated from those with IgG or IgA proteins. Of our 1384 patients with MGUS, 214 had the IgM type, but detailed laboratory features and prognoses were evaluated for the entire group, though there was initial evidence that the rate of progression in IgM MGUS was higher than that in IgG or IgA MGUS.8 Cesana et al15 reported that 130 of 1104 patients with MGUS had the IgM type, but, again, features of IgM monoclonal gammopathy were not examined separately. Therefore, our knowledge is limited by the small number of patients and the modest follow-up in most series and by the fact that data on IgM MGUS were combined with data on IgG and IgA MGUS in the larger studies.
This article describes 213 patients with IgM MGUS who were observed for 1567 person-years. The relative risk for progression to lymphoma was 14.8-fold, and the risk for Waldenström macroglobulinemia was increased 262-fold in the IgM MGUS group. In contrast, the risk of progression to lymphoma was not increased in the cohort of 1170 non-IgM patients from southeastern Minnesota. Furthermore, multiple myeloma was increased 30-fold in the non-IgM patients. In the 1170 non-IgM patients, the median age was 72 years, the percentage of patients older than 70 years was 54%, and the percentage of patients younger than 40 years was 2%; the respective values in the 213 patients with IgM MGUS were 74 years, 64%, and 1%. Men constituted 54% of the non-IgM cohort and 58% of the IgM MGUS patients. The size of the M protein in both cohorts was the same (1.2 g/dL). Serum monoclonal κ light chain was found in 21% and λ light chain in 10% of the non-IgM cohort; in the IgM MGUS group, these percentages were 19% and 8%.
Importantly, the study is population-based because all patients came from a defined geographic area (southeastern Minnesota) in which referral occurs almost exclusively to the Mayo Clinic in Rochester. We defined MGUS of the IgM type by the presence of an IgM M protein with a concentration of 3 g/dL or less and only modest amounts of monoclonal light chain in the urine and the absence of lymphadenopathy, hepatosplenomegaly, lytic bone lesions, anemia, hypercalcemia, and renal insufficiency related to the M protein. If bone marrow was examined, the marrow had to contain less than 10% plasma cells. Ideally, with unlimited resources and the consent of the patient, bone marrow examination would be of interest in all patients with MGUS of IgM type and in those with IgG and IgA types. We do not believe that a patient's medical care is compromised by delaying a bone marrow examination until the M-protein value increases or until constitutional symptoms, hepatosplenomegaly, lymphadenopathy, or anemia develops. Agreement is universal that patients with IgM MGUS or smoldering (asymptomatic) macroglobulinemia should not be treated (Kyle RA, Therneau TM, Rajkumar SV, et al, read at the 2nd International Workshop on Waldenström's Macroglobulinemia, Athens, Greece, September 2002).
The study demonstrates that the risk for lymphoma, Waldenström macroglobulinemia, or a related condition occurred at a rate of 1.5% per year throughout follow-up. This rate of progression is higher than the 1% we reported earlier when all forms of MGUS were considered together, and it is an important finding.8 Overall, non-Hodgkin lymphoma developed in 17 patients, Waldenström macroglobulinemia in 6 patients, and primary amyloidosis and chronic lymphocytic leukemia in 3 patients each. The relative risk for development of one of these related malignant lymphoid diseases was 15.9-fold greater than expected on the basis of incidence rates in the Iowa Surveillance, Epidemiology, and End Results Program.22 The risk for development of non-Hodgkin lymphoma, 14.8, is an underestimate because only lymphomas associated with IgM protein were included as observed cases, whereas the incidence rates for all lymphomas were used to calculate the number of expected cases. The risk for progression was highest for Waldenström macroglobulinemia. In contrast, distinguishing the patient with stable monoclonal gammopathy from one in whom lymphoma, Waldenström macroglobulinemia, or a related disorder will eventually develop is difficult when MGUS is initially recognized. In fact, in the past, if an M protein in a patient with MGUS remained stable for 3 to 5 years, the process was believed to be benign. However, as with other forms of MGUS, this study clearly demonstrates that the risk for progression persists even after 20 years of follow-up. On multivariate analysis, only the initial concentration of the serum M protein and the serum albumin level at diagnosis were independent predictors of progression.
In a comparison of patients with various M-protein values in which 0.5 g/dL or less was used as a reference value, we found that the initial concentration of M protein was a statistically significant predictor of progression. Patients with an IgM protein value of 2.5 g/dL had a 3.1-fold greater risk for progression than patients with a value of 0.5 g/dL. These results are similar to those in our earlier study of 1384 patients with all forms of MGUS.8 The adverse prognostic effect of the concentration of the M protein is likely a reflection of the impact of a higher tumor burden on progression. For instance, with a higher tumor burden, more cells are at risk for critical genetic changes necessary for progression.
A low serum albumin level was also an important independent indicator of progression. The prognostic value of serum albumin levels has not been described previously, and the possible mechanism for this effect is unclear. Although there was a reduction of uninvolved immunoglobulins in more than one third of the patients, this was not indicative of progression, a finding similar to that in other forms of MGUS. The presence of a monoclonal light chain in the urine was also not a risk factor for the development of lymphoma or a related disorder. Future studies must use cytogenetic analysis, high-throughput gene expression arrays, and other molecular studies to identify other, more specific risk factors for progression. However, these studies are difficult to perform in this patient population because routine baseline and follow-up bone marrow biopsies generally are not indicated for clinical purposes.
Lymphoma or a related disorder probably would have developed in the 3 patients who had a significant increase in their gammopathy. However, it is important to keep in mind that patients with IgM MGUS are far more likely to die of an unrelated disease than to have progression to a malignant lymphoid disorder, as shown in this study. The actual risk for death from lymphoma and related disorders is overstated when one ignores the greater risk for death from other causes such as cardiovascular and cerebrovascular diseases or nonlymphoid malignancies in these older patients.
Prepublished online as Blood First Edition Paper, DOI 10.1182/blood-2003-03-0801.
Supported in part by research grant CA 62242 from the National Cancer Institute.
This manuscript was previously published as a short paper in Kyle RA, Therneau TM, Rajkumar SV, et al. Long-term follow-up of IgM monoclonal gammopathy of undetermined significance. Semin Oncol. 2003;30:169-171. By permission of W. B. Saunders Co.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal