Abstract
Endothelial intercellular adhesion molecule 1 (ICAM-1) and ICAM-2 are both involved in lymphocyte extravasation during immunosurveillance and inflammation. To define their exact role during T-cell extravasation, we used mouse T cells and ICAM-1-/-ICAM-2-/- brain endothelioma cells. ICAM-1-/-ICAM-2-/- brain endothelioma cells did not support transendothelial migration (TEM) of T cells in vitro. Re-expression of different ICAM-1 mutants in the ICAM-1-/-ICAM-2-/- endothelioma line bEndI1/2.1 or in the ICAM-1-/- endothelioma line bEndI1.1 demonstrated that the extracellular domain of ICAM-1 suffices to support T-cell adhesion while the presence of the cytoplasmic tail was strictly required for TEM. Surprisingly, tyrosine phosphorylation of endothelial ICAM-1 was not necessary for TEM of T cells or for Rho guanosine triphosphatase (RhoGTPase) activation. Furthermore, cytoplasmic deletion mutants of ICAM-1 were unable to mediate RhoGTPase activation. Thus, our data demonstrate that the cytoplasmic tail of endothelial ICAM-1—independently from tyrosine phosphorylation—is essential for supporting TEM of T lymphocytes, while Rho signaling is involved in endothelial cells. (Blood. 2003;102:3675-3683)
Introduction
Interaction of T cells with the vascular endothelium is a critical step during T-cell extravasation from blood into tissue in immunosurveillance and inflammation. Cell adhesion molecules (CAMs) expressed on the T-cell and endothelial cell surfaces play essential roles in this intercellular interaction. The intercellular adhesion molecule 1 (ICAM-1) and ICAM-2, members of the immunoglobulin superfamily, and their β2-integrin ligand lymphocyte function-associated antigen 1 (LFA-1), expressed on all leukocytes,1,2 are important for firm attachment of leukocytes to, and their subsequent migration across, the endothelium.3 A role of endothelial ICAM-1 and ICAM-2 in LFA-1-mediated T-cell adhesion to endothelial cells as well as their transendothelial migration (TEM) has been firmly established by a number of in vitro studies.4-8
In vivo expression patterns of ICAM-1 and ICAM-2 are distinct but overlapping. Overall tissue distribution of ICAM-2 is more restricted than that of ICAM-1. Both ICAMs are expressed at low levels on most leukocytes. ICAM-2 is constitutively expressed on all vascular endothelium, including high endothelial venules at much higher levels than ICAM-1.9 ICAM-1 expression is strongly inducible by inflammatory cytokines, whereas ICAM-2 was reported to be down-regulated by inflammatory cytokines.10 On the basis of the endothelial expression patterns of ICAM-1 and ICAM-2, it has been hypothesized that ICAM-2 mediates leukocyte traffic into noninflamed tissue, that is, lymphocyte recirculation during immunosurveillance, whereas up-regulated levels of ICAM-1 may increase leukocyte extravasation at sites of inflammation.11
Strikingly, ICAM-1- or ICAM-2-deficient mice are both viable and show relatively mild defects in their immune responses. ICAM-1-deficient mice suffer from a moderate leukocytosis while activation and migration of leukocytes to places of inflammation are reduced, resulting in impaired immune and inflammatory responses.12 On the other hand, they are resistant to septic shock13 and show attenuated ischemia reperfusion injury14 owing to reduced leukocyte-endothelial interaction. Interestingly, ICAM-2-deficient mice do not show any impairment in lymphocyte homing or development of leukocytes, but rather demonstrated a critical role for ICAM-2 in eosinophil trafficking.15 Recently, an additional function for human endothelial ICAM-2 in mediating the TEM of dendritic cells (DCs) via interaction with a novel DC-specific ligand DC-specific ICAM-grabbing nonintegrin (DC-SIGN) has been demonstrated.16
Analysis of the involvement of endothelial ICAM-1 and ICAM-2 in T-cell extravasation in vivo has been complicated even in ICAM-1-/- or ICAM-2-/- mice by the fact that both molecules are also involved in immune cell activation. To delineate the functional involvement of endothelial ICAM-1 versus ICAM-2 in TEM of T lymphocytes in vitro, we have established endothelial cell lines deficient for both ICAM-1 and ICAM-2 from ICAM-1-/-ICAM-2-/- mice. Here we show that the extracellular domain of ICAM-1 is sufficient to support T-cell adhesion, while TEM was strictly dependent on the presence of the cytoplasmic tail of ICAM-1, probably by delivering Rho-dependent signals into endothelial cells.
Materials and methods
Antibodies and reagents
All antibodies used in this study have been previously described in detail and were either purified from hybridoma supernatants as described7,8,17 or purchased. The hybridoma 29G1 (antimouse ICAM-1) was purchased from American Type Culture Collection (Rockville, MD). The hybridomas 25ZC7 (antimouse ICAM-1) and 9DB3 (antimouse vascular cell adhesion molecule 1 [VCAM-1]) were from D. Vestweber (Münster, Germany); YNI/1.7 (antimouse ICAM-1), MECA-367 (antimouse mucosal addressin CAM 1 [MAdCAM-1]), MECA-79 (antimouse peripheral lymph node addressin [PNAd]), MJ7/18 (antimouse endoglin), MECA-32 (antimouse endothelium), and 9B5/Hermes-1 (antihuman CD44) were from E. C. Butcher (Stanford, CA). Mec13.3 (antimouse platelet endothelial CAM 1 [PECAM-1]) was provided by E. Dejana (Milan, Italy), and GC51 (antimouse PECAM-1) by B. Imhof (Geneva, Switzerland). The 3C4 (MIC2/4; antimouse ICAM-2) was purchased from BD Biosciences (Heidelberg, Germany), mouse antiphosphotyrosine monoclonal antibody (mAb) clone 4G10 from Upstate Biotechnology (Lake Placid, NY), rabbit antiphosphotyrosine stress-activated protein kinase/c-Jun N-terminal kinase (anti-P-SAPK/JNK) from New England Biolabs (Schwalbach, Germany), and horseradish peroxidase (HRP)-conjugated antimouse, antirat, or antirabbit immunoglobulin G (IgG) from Pierce (Chester, United Kingdom). Electrochemiluminescence (ECL) reagents were obtained from Amersham International (Amersham, Buckinghamshire, United Kingdom).
T cells
The proteolipid protein (PLP)-specific T-helper 1 (TH1) memory/effector T-cell lines SJL.PLP2 to SJL.PLP9 have been described in detail earlier.18
Endothelioma lines
The ICAM-1-/-ICAM-2-/- brain endothelioma cell line bEndI1/2.1 was established by infection of primary brain endothelial cells derived form ICAM-1-/-ICAM-2-/- mice with a recombinant retrovirus coding for the Polyomavirus middle-T oncogene19 as described before.7,8,20 ICAM-1-/-ICAM-2-/- mice were obtained by cross-breeding the ICAM-1- and the ICAM-2-deficient mice.13,15 The bEnd.5, serving as a wild-type control, bEndI1.1, and bEnd I1.1-ICAM-1 were described before.7 Although these endotheliomas are derived from primary brain endothelium, they do not maintain specific blood-brain barrier characteristics such as a permeability barrier or complex tight junctions and can therefore be considered a model for microvascular endothelium in general.
Generation of ICAM-1 mutants and an ICAM-1/ICAM-2 chimera
A pBluescript plasmid clone coding for the full-length open reading frame of murine ICAM-1 was used as a template to generate the ICAM-1 mutants by polymerase chain reaction (PCR) mutagenesis and PCR fragment amplification, which were subcloned into the retroviral vector pBABEpuro. Oligonucleotides used were as follows: RL01, cct gat gtc gac tca gcg gtt ata aac ata aga ggc; RL02, cgt gat ggc agc tag ctt tgt ttt t; RL03, gcc aga gaa aga tac gta tat tca agc tgc aga agg ctc agg; RL04, cgc aat taa ccc tca cta aag g; RL06, ggc agg agt cga ctc cag cag gct cag gg; RL11, ggt aca tac gtg tgc cat gc; RL13, ccg tat gtc gac tca gcg gtt aaa aac aaa gct agc. PCR mutagenesis of internal DNA sequences was based on a 2-step PCR procedure using the proofreading VENT DNA-polymerase (New England Biolabs) and combinations of mutagenesis primers with 2 peripheral primers. In the first round, the mutagenesis primer and the opposite peripheral primer were used. After purification, the generated PCR fragment was used as a megaprimer together with both peripheral primers for the second round of PCR. A detailed description of the generation of the individual mutants is available on the Blood website; see the Supplemental Document link at the top of the online article. All fragments generated by PCR were sequenced to verify the DNA sequence.
The ICAM-1/ICAM-2 chimeric construct I1EI2C (ICAM-1 extracellular, ICAM-2 cytoplasmic) was created as follows: The cytoplasmic domain of ICAM-2 (amino acids [aa] 199-277) was obtained from an expressed sequence tag (EST) clone (Genbank accession no. AA028405) in pT7T3D-Pac as an NcoI/NotI fragment and ligated in frame with NcoI/NotI-digested pBluescript ICAM-1 (to remove the transmembrane and cytoplasmic domains of ICAM-1 domain starting from aa 478). The pBluescript I1EI2C was ligated as an EcoR/NotI-blunt fragment into EcoRI/SalI-blunt cut pBABEpuro to produce the final I1EI2C expression construct.
Retroviral transduction of endothelioma lines
The resulting plasmids were transfected into the retrovirus packaging cell line GP+E86,21 and stable clones were selected with puromycin (2 μg/mL) or, in the case of the ICAM-2 construct, with hygromycin (50 μg/mL) as described.7 The resulting GP+E86 supernatants were used to infect bEndI1/2.1 or bEndI1.1 twice beginning at two-thirds confluency following standard procedures. Clones were grown to passage 5, and expression of ICAM-1, ICAM-1 mutants, or ICAM-2 in retransfected endotheliomas was analyzed by fluorescence-activated cell sorter (FACS) analysis and immunofluorescence.
Flow cytometry
FACS analysis was performed exactly as described before.17 Flow cytometric analysis was performed on a FACScan with the use of CellQuest software (Becton Dickinson, Heidelberg, Germany).
Adhesion and transmigration assays
ICAM-1 cross-linking
Cross-linking of endothelial ICAM-1 was performed exactly as described previously.22-24 The external domain of ICAM-1 was ligated by rat antimouse ICAM-1 (5 μg/mL 25ZC7 plus 5 μg/mL YNI) followed by cross-linking with goat-antirat IgG (Jackson Immunochemicals, Raritan, NJ) for 15 minutes.
Western blotting
Western blotting of immunoprecipitated samples or cell lysates was performed exactly as described.22-24 Immunoprecipitates or cell lysates were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) before transfer to nitrocellulose membranes. After blocking (5% fat-free milk in phosphate-buffered saline [PBS] for 2 hours), membranes were incubated overnight at 4°C with primary antibody in PBS/5% bovine serum albumin (BSA) (4G10 at 1:5000; 25ZC7 at 10 μg/mL; anti-P-SAPK/JNK at 1:1000); washed with PBS/0.1% Tween 20; and incubated 1 hour with an HRP-conjugated goat antimouse, antirat, or antirabbit IgG (1:5000; Pierce). Blots were developed by means of the ECL system (Amersham International) according to the manufacturer's instructions and exposed to x-ray film.
Rho activation
Glutatione S-transferase (GST)-rhotekin was expressed from pGEX-2T-rhotekin in Escherichia coli for 5 hours with the use of 1 mM isopropyl-beta-D-thiogalactopyranoside (IPTG) (Gibco/BRL, Paisley, United Kingdom) and used to precipitate activated rho from the endothelioma lysates as previously described.25,26 Rho proteins were labeled by adenosine diphosphate (ADP) ribosylation as previously described.23
Statistical analysis
Within each assay, parameters were tested in at least triplicate. The number of repeats of the individual assays is indicated in “Results.” Quantitative data are given as mean values ± standard deviation (SD). For analysis of differences between the assays, one-way analysis of variance (ANOVA) followed by unpaired Student t test and Tukey-Kramer correction for repeated measurements was performed with the use of the software InStat (Cancom, Jettingen-Scheppach, Germany), with results considered significant as follows: P value less than .05, significant; P value less than .01, very significant, and P value less than .001, extremely significant.
Results
Establishment and phenotype of ICAM-1-/-ICAM-2-/- endothelioma lines
Brain endothelioma lines from ICAM-1-/-ICAM-2-/- mice were established by retroviral transduction of primary microvascular brain endothelial cell cultures with the Polyomavirus middle-T oncogene. Infected cells were selected in the presence of neomycin until pure endothelioma cultures free of contaminating cells such as pericytes, astrocytes, or fibroblasts were obtained. Endothelioma cells were passaged several times and used for assays from passages 10 to 20. The established endothelioma lines retained their endothelial morphology and showed contact inhibition upon confluency (data not shown). ICAM-1-/-ICAM-2-/- endotheliomas did not reveal any differences in morphology or in growth rates when compared with either wild-type (bEnd5) or ICAM-1-deficient (bEndI1.1) endotheliomas derived from brain (data not shown and Reiss et al7 ). Thus, the newly established ICAM-1-/-ICAM-2-/- endothelioma lines can be considered representative for ICAM-1/ICAM-2 double-deficient microvascular endothelium in vitro.
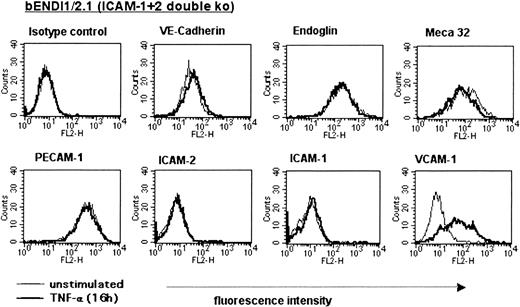
To verify their endothelial nature, the surface phenotype of the newly established ICAM-1-/-ICAM-2-/- brain endothelioma lines was investigated by FACS analysis (Figure 1). ICAM-1-/-ICAM-2-/- endotheliomas showed similar cell surface expression levels of endoglin, VE-cadherin, PECAM-1, and the endothelial-specific MECA-32 antigen as the wild-type brain endothelioma lines.7 One representative cell line, bEndI1/2.1 (brainEndothelioma-ICAM-1-/-ICAM-2-/-.1st line), is shown in Figure 1. Thus, the lack of ICAM-1 and ICAM-2 expression did not cause changes in the expression of other endothelial CAMs These defined phenotypes unequivocally characterized these cell lines as pure endothelial cells. Upon stimulation with TNF-α, bEndI1/2.1 showed up-regulation of E- and P-selectin after 4 hours (data not shown) and up-regulation of VCAM-1 after 16 hours on their surface, whereas the expression of VE-cadherin and PECAM-1 did not change (Figure 1). Expression of MAdCAM-1 or expression of the peripheral addressin, as defined by positive immunostaining for the MECA-79 epitope, was not observed (data not shown).
Phenotype of the ICAM-1/ICAM-2 double-deficient bEndI1/2.1. The phenotype of unstimulated versus TNF-α-stimulated (16 hours) bEndI1/2.1 as determined by FACS analysis is shown. Endothelial cells are scatter gated on live cells; overlays compare surface expression of unstimulated (thin lines) versus stimulated (thick lines) bEndI1/2.1.
Phenotype of the ICAM-1/ICAM-2 double-deficient bEndI1/2.1. The phenotype of unstimulated versus TNF-α-stimulated (16 hours) bEndI1/2.1 as determined by FACS analysis is shown. Endothelial cells are scatter gated on live cells; overlays compare surface expression of unstimulated (thin lines) versus stimulated (thick lines) bEndI1/2.1.
Deficiency of ICAM-1 and ICAM-2 protein expression in bEndI1/2.1 was additionally confirmed by immunofluorescence staining for intracellular expression of ICAM-1 and ICAM-2, respectively (data not shown). bEndI1/2.1 does not support transendothelial migration (TEM) of PLP-specific T cells
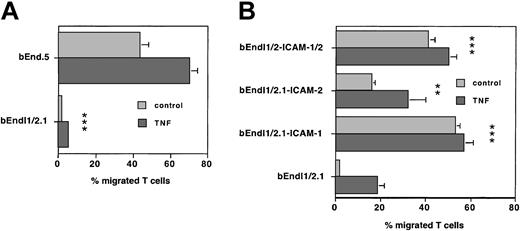
TEM of PLP-specific T cells across unstimulated ICAM-1-/-ICAM-2-/- bEndI1/2.1 and wild-type bEnd5 was always investigated simultaneously within the same experiments. Figure 2A shows one representative experiment that was repeated 6 times with qualitatively identical results. During a 4-hour time period, 36.3% ± 6.3% of T cells spontaneously migrated across a monolayer of bEnd5, whereas migration of T cells across bEndI1/2.1 over the same time period was negligible (1.7% ± 0.6%, n = 7, P < .01). Migration of SJL.PLP3 across TNF-α-stimulated bEnd5 increased to 59.4% ± 9.6%, whereas migration across TNF-α-stimulated bEnd I1/2.1 only increased to 10.8% ± 7.8% (n = 7, P < .01), respectively. Thus, migration of T cells across bEndI1/2.1 when compared with unstimulated or TNF-α-stimulated wild-type endothelium, was dramatically reduced by 97.3% ± 1.8% and 81.7% ± 1.3%, respectively. Taken together, in the absence of endothelial ICAM-1 and ICAM-2, TEM of PLP-specific T lymphocytes in vitro was almost completely abolished.
ICAM and T-cell TEM. Endothelial ICAM-1 and ICAM-2 are required for TEM of T cells. (A) One representative experiment comparing TEM of the T-cell line SJLB.PLP3 across unstimulated and stimulated bEnd5 and ICAM-1-/-ICAM-2-/- bEndI1/2.1 is shown. Transmigration assays were performed with the use of 6.5-mm Costar transwells (Corning Costar, Bodenheim, Germany) with 5-μm pore size. T lymphocytes were collected for cell counting from the lower chamber and the bottom of the filter. Bars represent mean ± SD (n = 3). TEM of T cells across bEndI1/2.1 is reduced to an extremely low level (***P < .001) when compared with bEnd5. This assay was reproduced 6 times. (B) One representative experiment is shown comparing transmigration of SJLB.PLP3 across bEndI1/2.1 deficient for ICAM-1 and ICAM-2; bEndI1/2.1-ICAM-1 (bEndI1/2.1 transduced with ICAM-1); bEndI1/2.1-ICAM-2 (bEndI1/2.1 transduced with ICAM-2); and bEndI1/2.1-ICAM-1/ICAM-2 (bEndI1/2.1 transduced with ICAM-1 plus ICAM-2). Re-expression of ICAM-1 or both ICAM-1 and ICAM-2 in bEndI1/2.1 reconstitutes TEM of PLP-specific T cells to extremely significant levels (***P < .001), and re-expression of ICAM-2 reconstitutes TEM to very significant levels (**P < .01) when compared with migration across bEndI1/2.1. This assay was reproduced 4 times. Bars represent mean ± SD (n = 3).
ICAM and T-cell TEM. Endothelial ICAM-1 and ICAM-2 are required for TEM of T cells. (A) One representative experiment comparing TEM of the T-cell line SJLB.PLP3 across unstimulated and stimulated bEnd5 and ICAM-1-/-ICAM-2-/- bEndI1/2.1 is shown. Transmigration assays were performed with the use of 6.5-mm Costar transwells (Corning Costar, Bodenheim, Germany) with 5-μm pore size. T lymphocytes were collected for cell counting from the lower chamber and the bottom of the filter. Bars represent mean ± SD (n = 3). TEM of T cells across bEndI1/2.1 is reduced to an extremely low level (***P < .001) when compared with bEnd5. This assay was reproduced 6 times. (B) One representative experiment is shown comparing transmigration of SJLB.PLP3 across bEndI1/2.1 deficient for ICAM-1 and ICAM-2; bEndI1/2.1-ICAM-1 (bEndI1/2.1 transduced with ICAM-1); bEndI1/2.1-ICAM-2 (bEndI1/2.1 transduced with ICAM-2); and bEndI1/2.1-ICAM-1/ICAM-2 (bEndI1/2.1 transduced with ICAM-1 plus ICAM-2). Re-expression of ICAM-1 or both ICAM-1 and ICAM-2 in bEndI1/2.1 reconstitutes TEM of PLP-specific T cells to extremely significant levels (***P < .001), and re-expression of ICAM-2 reconstitutes TEM to very significant levels (**P < .01) when compared with migration across bEndI1/2.1. This assay was reproduced 4 times. Bars represent mean ± SD (n = 3).
ICAM-1 and ICAM-2 reconstitute TEM of PLP-specific T cells across bEndI1/2.1
We next investigated whether re-expression of ICAM-1 and/or ICAM-2 on the cell surface of bEndI1/2.1 would re-establish TEM of T cells. For this purpose, ICAM-1, ICAM-2, or both were retrovirally transduced into bEndI1/2.1 and derivative cell lines of bEndI1/2.1 expressing ICAM-1 (bEndI1/2.1-ICAM-1), ICAM-2 (bEndI1/2.1-ICAM-2), or both ICAM-1 plus ICAM-2 (bEndI1/2.1-ICAM-1/2) were established. Surface expression of both, ICAM-1 and ICAM-2, was verified by FACS analysis and immunofluorescence staining with the use of 3 different anti-ICAM-1 monoclonal antibodies and 1 anti-ICAM-2 monoclonal antibody. Additionally, the presence of ICAM-1 and ICAM-2 protein was confirmed by Western blot analysis (data not shown). Ectopic expression of ICAM-1 and/or ICAM-2 affected neither the expression of other endothelial cell surface molecules, such as endoglin, the MECA-32 antigen, VE-cadherin, nor the inducibility of E- and P-selectin or VCAM-1 as investigated by FACS analysis (data not shown).
In direct comparisons of the TEM of PLP-specific T cells across unstimulated or TNF-α-stimulated bEndI1/2.1 with their migration across unstimulated or TNF-α-stimulated bEndI1/2.1-ICAM-1, surface expression of ICAM-1 itself was found to be sufficient to re-establish TEM of T cells from 1.7% ± 0.6% to 43.3% ± 15.6% across unstimulated bEndI1/2.1 and from 10.8% ± 7.8% to 45.7% ± 12.8% across stimulated bEndI1/2.1 (n = 5, P < .001; also Figure 2B). In contrast, cell surface expression of ICAM-2 in bEndI1/2.1-ICAM-2 restored spontaneous migration of PLP-specific T cells only to 9.2% ± 4.6% across unstimulated and to 30.3% ± 5.2% across stimulated bEndI1/2-ICAM-2 (n = 5, P < .01). TEM of SJL.PLP3 across bEndI1/2.1-ICAM-1/2 expressing both ICAM-1 and ICAM-2 established TEM rates of SJL.PLP3 to 26.1% ± 12.0% and 38.4% ± 11.0% across unstimulated and stimulated bEndI1/2.1, respectively (n = 4, P < .01), levels that are similar to those observed across bEndI1/2.1-ICAM-1. Thus, surface expression of ICAM-1, but not of ICAM-2, was sufficient to achieve migration rates of PLP-specific T cells comparable to those obtained across wild-type endothelium. Furthermore, ICAM-1, and to a lower degree ICAM-2, can reconstitute TEM of T cells in the absence of the other ICAM molecule whereby ICAM-1 in the absence of ICAM-2, but not ICAM-2 in the absence of ICAM-1, reconstituted TEM to wild-type migration levels.
After TNF-α stimulation of bEndI1/2.1 or bEndI1/2-ICAM-2, that is, in the absence of ICAM-1, an increased transendothelial migration of PLP-specific T cells was occasionally observed in comparison with their migration across unstimulated endothelium and was found to be mediated via high-affinity α4-integrin on the PLP-specific T cells and endothelial VCAM-1 (data not shown).
Constitutive expression of ICAM-1 mutants in brain endothelioma lines bEndI1/2.1 and bEndI1.1
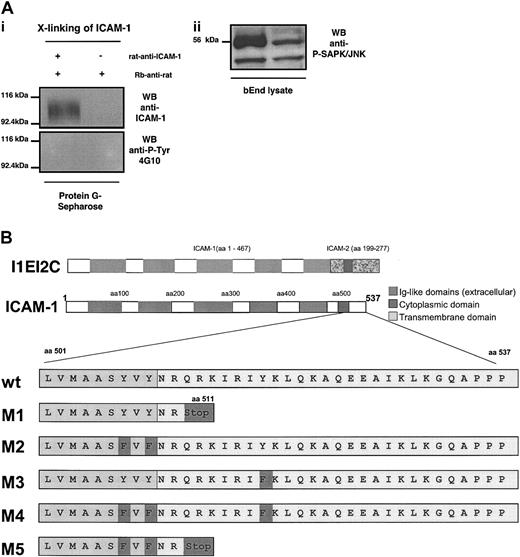
Because our data pointed to a dominant role of endothelial ICAM-1 in mediating the TEM of PLP-specific T cells, we next asked which part of endothelial ICAM-1 was relevant for this process. To address a potential contribution of tyrosine phosphorylation of the cytoplasmic or putative transmembrane parts of endothelial ICAM-1 in TEM of T cells, bEnd5 cells were cross-linked with antimouse ICAM-1 mAbs and rabbit antirat mAb. ICAM-1 present in the immunocomplexes precipitated from these preparations was not tyrosine phosphorylated (Figure 3A). However, ICAM-1 cross-linking did lead to SAPK/JNK activation, which has previously been shown to be activated in response to ICAM-1 cross-linking on rat brain endothelial cells.27
Lack of tyrosine phosphorylation of endothelial ICAM-1 and mutants of murine ICAM-1. (A) The bEnd5 cells were cross-linked with rat antimouse ICAM-1 mAb followed by rabbit-antirat IgG. Immunocomplexes were precipitated by protein G sepharose. Whereas ICAM-1 cross-linking did not lead to tyrosine phosphorylation of endothelial ICAM-1 (i) it did induce activation of SAPK/JNK within the endothelioma cells (ii). (B) Mutants of murine ICAM-1 (M1 to M5) were created by PCR mutagenesis as described in “Materials and methods.” M1 and M5 lack the cytoplasmic tail. M2, M3, and M4 harbor point mutations, changing tyrosines to phenylalanines at the cytoplasmic tail (M3 and M4) or at the border of the transmembrane domain as indicated. The amino acid sequences of the ICAM-1 mutants are shown in comparison with the wild-type sequence. Mutated amino acids are highlighted in dark gray. The ICAM-1/ICAM-2 chimeric construct was obtained by conventional cloning via restricion enzyme digestions. Correct sequences and base exchanges were verified by DNA sequencing.
Lack of tyrosine phosphorylation of endothelial ICAM-1 and mutants of murine ICAM-1. (A) The bEnd5 cells were cross-linked with rat antimouse ICAM-1 mAb followed by rabbit-antirat IgG. Immunocomplexes were precipitated by protein G sepharose. Whereas ICAM-1 cross-linking did not lead to tyrosine phosphorylation of endothelial ICAM-1 (i) it did induce activation of SAPK/JNK within the endothelioma cells (ii). (B) Mutants of murine ICAM-1 (M1 to M5) were created by PCR mutagenesis as described in “Materials and methods.” M1 and M5 lack the cytoplasmic tail. M2, M3, and M4 harbor point mutations, changing tyrosines to phenylalanines at the cytoplasmic tail (M3 and M4) or at the border of the transmembrane domain as indicated. The amino acid sequences of the ICAM-1 mutants are shown in comparison with the wild-type sequence. Mutated amino acids are highlighted in dark gray. The ICAM-1/ICAM-2 chimeric construct was obtained by conventional cloning via restricion enzyme digestions. Correct sequences and base exchanges were verified by DNA sequencing.
To investigate the involvement of the extracellular versus the cytoplasmic part and the potential contribution of tyrosine phosphorylation of the cytoplasmic or putative transmembrane parts of endothelial ICAM-1 in TEM of PLP-specific T cells, 6 different mutants of murine ICAM-1 named M1, M2, M3, M4, M5, and I1EI2C were created (Figure 3B). M1 is characterized by a deletion of the cytoplasmic tail of murine ICAM-1. The mutants M2, M3, and M4 harbor point mutations changing tyrosines to phenylalanines at the border of the putative transmembrane domain (M2, M4) or within the cytoplasmic tail of ICAM-1 (M3, M4). M5 combines the point mutations of M2 with the cytoplasmic deletion of M1 (Figure 3B). I1EI2C consists of the extracellular part of ICAM-1 (aa 1-476) and the transmembrane and cytoplasmic part of ICAM-2 (aa 199-277; Figure 3B).
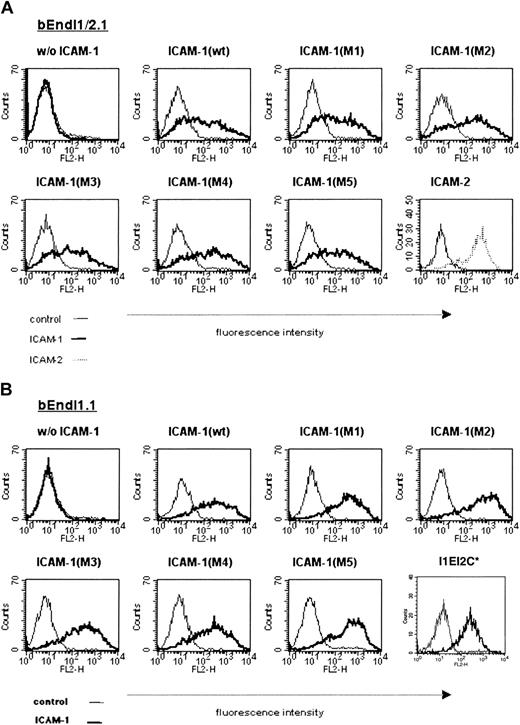
These murine ICAM-1 mutants were introduced into bEndI1/2.1 by retroviral transduction, and derivative cell lines expressing the mutant M1 (bEndI1/2.1-ICAM-1-M1), M2 (bEndI1/2.1-ICAM-1-M2), M3 (bEndI1/2.1-ICAM-1-M2), M4 (bEndI1/2.1-ICAM-1-M4), M5 (bEndI1/2.1-ICAM-1-M5), and bEndI1/2.1-I1EI2C were established (Figure 4A) (also in data not shown). To investigate a possible contribution of endogenously expressed ICAM-2, all ICAM-1 mutants were additionally transduced into the ICAM-1-deficient brain endothelioma cell line bEndI1.1, giving rise to the derivative cell lines bEndI1-ICAM-1-M1, bEndI1-ICAM-1-M2, bEndI1-ICAM-1-M3, bEndI1-ICAM-1-M4, bEndI1-ICAM-1-M5, and bEndI1-I1EI2C, respectively (Figure 4B).
Comparable surface expression levels of ICAM-1 wild type and ICAM-1 mutants on bEndI1/2.1 and bEndI1.1. The surface expression levels of ICAM-1 wild type and ICAM-1 mutants transduced into bEndI1/2.1 (A) and bEndI1.1 (B) is shown as determined by FACS analysis. Endothelial cells are scatter gated on live cells; overlays show specific staining for ICAM-1 (thick lines) compared with control antibody staining (thin lines). In panel A, the surface expression level for ICAM-2 transduced into bEndI1/2.1 is included. In panel B, the surface expression level for the ICAM-1/ICAM-2 chimera I1EI2C is included. *This staining was performed separately.
Comparable surface expression levels of ICAM-1 wild type and ICAM-1 mutants on bEndI1/2.1 and bEndI1.1. The surface expression levels of ICAM-1 wild type and ICAM-1 mutants transduced into bEndI1/2.1 (A) and bEndI1.1 (B) is shown as determined by FACS analysis. Endothelial cells are scatter gated on live cells; overlays show specific staining for ICAM-1 (thick lines) compared with control antibody staining (thin lines). In panel A, the surface expression level for ICAM-2 transduced into bEndI1/2.1 is included. In panel B, the surface expression level for the ICAM-1/ICAM-2 chimera I1EI2C is included. *This staining was performed separately.
Comparable surface expression levels of the ICAM-1 mutants on bEndI1/2.1- and bEndI1.1-derived cell lines were investigated by FACS analysis with the use of the anti-ICAM-1 monoclonal antibodies 29G.1, 25ZC7, and YN1/1.7 (Figure 4). Expression of VE-cadherin, PECAM-1, endoglin, and the MECA-32 antigen and inducibility of VCAM-1 upon stimulation with TNF-α was not affected when compared with the respective parental cell line (data not shown).
The cytoplasmic tail of endothelial ICAM-1 is important for the TEM of PLP-specific T cells
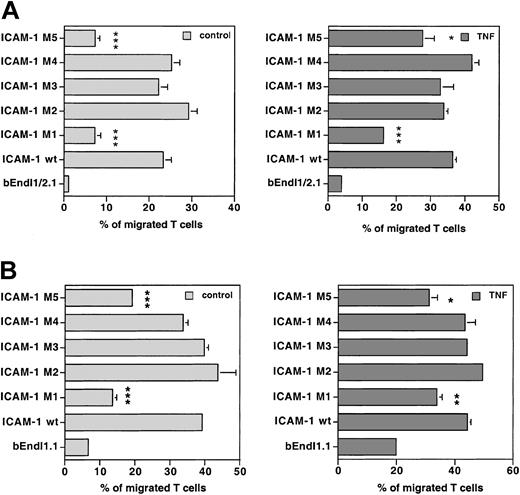
The brain endothelioma lines bEndI1/2.1 and bEndI1.1 re-expressing wild-type ICAM-1 or different ICAM-1 mutants were compared for their capacity to support TEM of PLP-specific T cells. To qualitatively and quantitatively compare T-cell migration across endothelial monolayers expressing different ICAM-1 mutants, TEM of T cells across the parental cell line and the daughter cell lines expressing the individual mutants were always investigated within the same experiment. To ascertain constant and comparable cell surface expression levels for the different ICAM-1 mutants on all daughter cell lines within each assay, FACS analysis for ICAM-1 was always performed in parallel with individual TEM assays. The ICAM-1 mutants M2, M3, and M4 harboring point mutations of tyrosines to phenylalanines (Figure 3) fully re-established TEM of PLP-specific T cells across unstimulated and TNF-α-stimulated bEndI1/2.1 to wild-type levels (Figure 5A) (wt, 42.3% ± 15.6% and 45.7% ± 12.8%, respectively; M2, 41.5% ± 13.5% and 45.1% ± 13.8%; M3, 26.1% ± 7.4% and 37.3% ± 9.2%; M4, 39.8% ± 11.8% and 47.6% ± 2.2%; n = 5; P > .05, wt versus individual mutants). A similar result was obtained, when investigating the migration of PLP-specific T cells across unstimulated and TNF-α-stimulated bEndI1.1, still expressing endogenous ICAM-2, retransduced with wt ICAM-1 or ICAM-1 mutants (Figure 5B) (wt, 42.9% ± 7.9% and 47.6% ± 13.6%; M2, 48.4% ± 13.6% and 55.1% ± 19.3%; M3, 37.9% ± 5.1% and 49.1% ± 15.9%; M4, 37.8% ± 8.6 and 55.3% ± 14.0; n = 4; P > .05, wt versus the mutants). In contrast, the ICAM-1 mutants M1 and M5 (Figure 3B) only partially restored TEM of PLP-specific T cells across bEndI1/2.1 (Figure 5A) and bEndI1.1 (Figure 5B). TEM of PLP-specific T cells across unstimulated and TNF-α-stimulated bEndI1/2.1-ICAM-1-M1 was 18.0% ± 8.9% and 26.6% ± 7.3% (n = 5; P < .01 versus wt), and across unstimulated and TNF-α-stimulated bEndI1/2.1-ICAM-1-M5, it was 8.1% ± 1.2% and 3.1% ± 0.3% (n = 4, P < .05 versus wt), respectively. Concommitantly, the TEM of T cells in the presence of endogenous ICAM-2 across unstimulated and TNF-α-stimulated bEndI.1-ICAM-1-M1 was found to be 31.2% ± 9.0% and 28.6% ± 9.2% (n = 4, P < .01 and P > .05 versus wt), and across unstimulated and TNF-α-stimulated bEndI1.1-ICAM-1-M5, it was 33.7% ± 3.2% and 32.5% ± 2.6% (n = 4, P < .05 and P > .05 versus wt), respectively. Thus, despite the fact that within each individual assay TEM across TNF-α-stimulated bEndI1.1 retransduced with either M1 or M5 versus wt ICAM-1 was significantly different, statistical analysis over the sum of all assays calculated this difference in contrast to all other cases as not quite significant. We consider this to be due to the inherent variabilities in endothelial cell activation states resulting in differences in absolute numbers of migrating T cells in between the assays and thus an apparent loss of significance in the migration rates across stimulated bEndI1.1 retransduced with M1 or M5.
Comparison of TEM of PLP-specific T cells in the 2 bEnd cell lines. TEM of PLP-specific T cells was compared across bEndI1/2.1 (A) or bEndI1.1 (B) re-expressing wild-type ICAM-1 or ICAM-1 mutants. One representative experiment, performed as described in Figure 2A, directly compared TEM of SJLB.PLP3 across unstimulated and TNF-α-stimulated bEndI1/2.1 (A) or bEndI1.1 (B) with their TEM across the respective daughter endothelioma lines transduced with wild-type (wt) ICAM-1 or the ICAM-1 mutants M1, M2, M3, M4, and M5. The ICAM-1 mutants M2, M3, and M4 harboring mutations of tyrosines to phenylalanines re-established TEM of PLP-specific T cells across unstimulated and TNF-α-stimulated bEnd I1/2.1 and bEndI1.1 comparable to wild-type ICAM-1 (panels A, B). In contrast, the cytoplasmic deletion mutants M1 and M5, although restoring some TEM of PLP-specific T cells across bEnd I1/2.1 and bEndI1.1, were unable to reconstitute TEM to wild-type levels. Bars represent mean ± SD (n = 3); significant differences of wt versus mutant are marked as follows: *P < .05, significant; **P < .01, very significant; ***P < .001, extremely significant.
Comparison of TEM of PLP-specific T cells in the 2 bEnd cell lines. TEM of PLP-specific T cells was compared across bEndI1/2.1 (A) or bEndI1.1 (B) re-expressing wild-type ICAM-1 or ICAM-1 mutants. One representative experiment, performed as described in Figure 2A, directly compared TEM of SJLB.PLP3 across unstimulated and TNF-α-stimulated bEndI1/2.1 (A) or bEndI1.1 (B) with their TEM across the respective daughter endothelioma lines transduced with wild-type (wt) ICAM-1 or the ICAM-1 mutants M1, M2, M3, M4, and M5. The ICAM-1 mutants M2, M3, and M4 harboring mutations of tyrosines to phenylalanines re-established TEM of PLP-specific T cells across unstimulated and TNF-α-stimulated bEnd I1/2.1 and bEndI1.1 comparable to wild-type ICAM-1 (panels A, B). In contrast, the cytoplasmic deletion mutants M1 and M5, although restoring some TEM of PLP-specific T cells across bEnd I1/2.1 and bEndI1.1, were unable to reconstitute TEM to wild-type levels. Bars represent mean ± SD (n = 3); significant differences of wt versus mutant are marked as follows: *P < .05, significant; **P < .01, very significant; ***P < .001, extremely significant.
After TNF-α stimulation of bEndI1/2.1 or bEndI1.1 retransduced with M1 or M5, here in the absence of the cytoplasmic tail of ICAM-1, increased transendothelial migration of PLP-specific T cells was found to be mediated via high affinity α4-integrin on the PLP-specific T cells and endothelial VCAM-1 (data not shown).
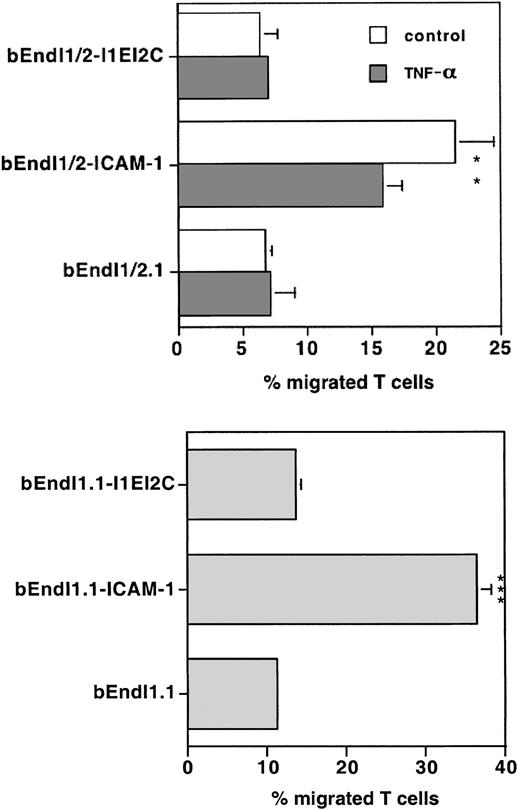
The importance of the cytoplasmic tail of endothelial ICAM-1 in mediating TEM of T cells was, however, further underlined in a separate set of experiments where we could demonstrate that a chimeric molecule carrying the extracellular domain of ICAM-1 and the transmembrane and cytoplasmic domains of ICAM-2 (I1EI2C) does not reconstitute TEM of T cells across bEndI1/2.1 (Figure 6). Surface expression levels of ICAM-1 could be achieved only on 35% of bEndI1/2.1 transduced with I1EI2C (data not shown). To rule out that low surface expression might lead to low TEM rates across bEndI1/2.1-I1EI2C, we also transduced I1EI2C into bEndI1.1, and surface expression levels were found to be comparable to those of the other ICAM-1 mutants (Figure 4B). In both bEndI1/2.1 and bEndI1.1, the chimeric molecule I1EI2C was not able to restore TEM of T cells, although it was found to re-establish adhesion of T cells to the endothelium almost to wild-type levels (data not shown).
Comparison of TEM of PLP-specific T cells across bEndI1/2.1 or bEndI1.1 expressing the ICAM-1/ICAM-2 chimera I1EI2C. TEM of SJL.PLP2 across TNF-α-stimulated and unstimulated bEndI1/2.1 or across bEndI1.1 with their TEM across the respective daughter endothelioma lines transduced with wild-type (wt) ICAM-1 or the ICAM-1/ICAM-2 chimera I1EI2C is shown. The chimeric I1EI2C was found to be unable to reconstitute TEM to wild-type levels. Bars represent mean ± SD (n = 3). Significant differences in I1EI2C versus wt are marked as follows: **P < .01, very significant; ***P < .001, extremely significant.
Comparison of TEM of PLP-specific T cells across bEndI1/2.1 or bEndI1.1 expressing the ICAM-1/ICAM-2 chimera I1EI2C. TEM of SJL.PLP2 across TNF-α-stimulated and unstimulated bEndI1/2.1 or across bEndI1.1 with their TEM across the respective daughter endothelioma lines transduced with wild-type (wt) ICAM-1 or the ICAM-1/ICAM-2 chimera I1EI2C is shown. The chimeric I1EI2C was found to be unable to reconstitute TEM to wild-type levels. Bars represent mean ± SD (n = 3). Significant differences in I1EI2C versus wt are marked as follows: **P < .01, very significant; ***P < .001, extremely significant.
Taken together, these results indicate that the cytoplasmic tail of endothelial ICAM-1 facilitates TEM of T cells across unstimulated and TNF-α-stimulated endothelium independent of tyrosine phosphorylation and cannot be replaced by the cytoplasmic tail of ICAM-2.
The cytoplasmic tail of endothelial ICAM-1 is not necessary for the adhesion of PLP-specific T cells to brain endothelium in vitro
To define whether attenuated TEM of T cells across bEndI1/2.1 and bEndI1.1 expressing the ICAM-1 mutants M1 and M5 might be a consequence of reduced T-cell adhesion, we assessed the adhesion of PLP-specific T cells to unstimulated and TNF-α-stimulated bEndI1/2.1 and bEndI1.1 relative to derivative cell lines expressing wild-type or mutant ICAM-1 proteins.
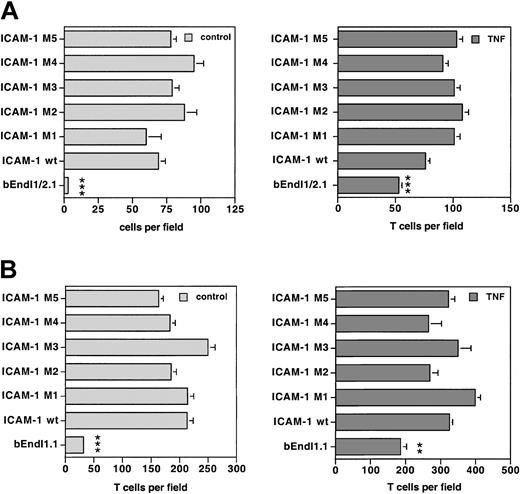
To quantitatively compare T-cell adhesion to the different ICAM-1 mutants, adhesion of T cells to the parental cell line and the derivative cell lines expressing the individual mutants was investigated within the very same experiments. Additionally, to ascertain constant and comparable cell surface expression for the different ICAM-1 variants, FACS analysis for ICAM-1 was always performed in parallel. Although, within each assay slight variations in the adhesion of PLP-specific T cells to the different ICAM-1 variants on either bEndI1/2.1 and bEndI1.1 were found, taken together all ICAM-1 mutants re-established T-cell adhesion on either bEndI1/2.1 (Figure 7A) or bEndI1.1 (Figure 7B) to levels indistinguishable from wild-type ICAM-1. Adhesion of T cells to TNF-α-stimulated bEnds was generally found to be increased and was mediated mainly via VCAM-1 and E-selectin as shown before.7 Thus, whereas the extracellular domain of endothelial ICAM-1 is sufficient to mediate T-cell adhesion to brain endothelium in vitro, the cytoplasmic tail of ICAM-1 is essential for the process of TEM.
Comparison of the adhesion of T cells to bEndI1/2.1 or bEndI1.1 re-expressing ICAM-1 or its mutants. A representative experiment directly comparing the adhesion of SJLB.PLP3 to unstimulated and TNF-α-stimulated bEndI1/2.1 (A) or bEndI1.1 (B) with their adhesion to the respective daughter cell lines retransduced with wild-type (wt) ICAM-1 or the ICAM-1 mutants M1, M2, M3, M4, or M5 is shown. Adhesion assays were performed with the use of 16-well Nunc glass chamber slides and analyzed by video-associated light microscopy (NIH Image software; National Institutes of Health, Bethesda, MD) counting for 5 predefined fields per well bound cells on the endothelial surface. Neither deletion of the cytoplasmic tail (M1 and M5) of ICAM-1 nor mutations of tyrosines to phenylalanines (M2, M3, M4) lead to a statistically significantly different adhesion of T cells when compared with adhesion to wild-type ICAM-1. Bars represent mean ± SD (n = 5). Significant differences were only observed for the parental cells versus wt ICAM-1 and each mutant and are marked as follows: **P < .01, very significant; ***P < .001, extremely significant. These results were reproduced twice.
Comparison of the adhesion of T cells to bEndI1/2.1 or bEndI1.1 re-expressing ICAM-1 or its mutants. A representative experiment directly comparing the adhesion of SJLB.PLP3 to unstimulated and TNF-α-stimulated bEndI1/2.1 (A) or bEndI1.1 (B) with their adhesion to the respective daughter cell lines retransduced with wild-type (wt) ICAM-1 or the ICAM-1 mutants M1, M2, M3, M4, or M5 is shown. Adhesion assays were performed with the use of 16-well Nunc glass chamber slides and analyzed by video-associated light microscopy (NIH Image software; National Institutes of Health, Bethesda, MD) counting for 5 predefined fields per well bound cells on the endothelial surface. Neither deletion of the cytoplasmic tail (M1 and M5) of ICAM-1 nor mutations of tyrosines to phenylalanines (M2, M3, M4) lead to a statistically significantly different adhesion of T cells when compared with adhesion to wild-type ICAM-1. Bars represent mean ± SD (n = 5). Significant differences were only observed for the parental cells versus wt ICAM-1 and each mutant and are marked as follows: **P < .01, very significant; ***P < .001, extremely significant. These results were reproduced twice.
The intracellular domain of ICAM-1 is essential for ICAM-1-mediated Rho activation
Activation of Rho guanosine triphosphatase (RhoGTPase) was found to be important for ICAM-1-mediated TEM of T cells.23 Therefore, we investigated whether cross-linking of ICAM-1 and its mutants on bEndI1/2.1 would result in activation of endothelial Rho proteins as previously described for rat brain endothelial cells.23 The bEnd I1.2 cells show some basal activiy of Rho that could be bound and therefore precipitated by rhotekin (Figure 8). Cross-linking of wt ICAM-1 with a specific antimouse ICAM-1 mAb resulted in the activation of endothelial Rho proteins above this basal activity (Figure 8). The mutants M2, M3, and M4 harboring the tyrosine-to-phenylalanine substitutions were also capable of inducing Rho activation to levels indistinguishable from wt ICAM-1. However, cross-linking of either M1, M5, or I1EI2C, each lacking the cytoplasmic tail of ICAM-1, were incapable of supporting increased activation of Rho following cross-linking of ICAM-1 (Figure 8).
Activation of RhoGTPases following ICAM-1 cross-linking on bEndI1/2.1 re-expressing ICAM-1 wild type or its mutants. Endothelial cells were cross-linked with antimouse ICAM-1 followed by goat antirat IgG (RAM) for 15 minutes. Cross-linked cells were lysed and activated Rho proteins (guanosine triphosphate [GTP]-bound form) precipitated with GST-rhotekin. Total Rho proteins and GST-rhotekin-precipitated Rho proteins were subsequently [32P]ADP-ribosylated, resolved on 15% SDS-PAGE, and exposed to autoradiographic film. L indicates lysate; rh, precipitated GST-rhotekin.
Activation of RhoGTPases following ICAM-1 cross-linking on bEndI1/2.1 re-expressing ICAM-1 wild type or its mutants. Endothelial cells were cross-linked with antimouse ICAM-1 followed by goat antirat IgG (RAM) for 15 minutes. Cross-linked cells were lysed and activated Rho proteins (guanosine triphosphate [GTP]-bound form) precipitated with GST-rhotekin. Total Rho proteins and GST-rhotekin-precipitated Rho proteins were subsequently [32P]ADP-ribosylated, resolved on 15% SDS-PAGE, and exposed to autoradiographic film. L indicates lysate; rh, precipitated GST-rhotekin.
Discussion
A critical role for endothelial ICAM-1 and ICAM-2 in T-cell adhesion and transendothelial migration (TEM) has previously been demonstrated by several in vitro studies using monoclonal antibodies against ICAM-1 and ICAM-2.4,5 Using ICAM-1-deficient endothelioma lines, we previously demonstrated that the important role of ICAM-1 in TEM could be dissociated from its role in T-cell adhesion to endothelium.7,8 Furthermore, in the absence of ICAM-1, endothelial ICAM-2 maintained residual TEM of T cells.7,8 Supporting our previous observations that both endothelial ICAM-1 and ICAM-2 are necessary for TEM of T cells, we now demonstrate that brain endothelioma lines lacking both ICAM-1 and ICAM-2 no longer support TEM of antigen-specific T cells, which we have previously shown to migrate in an LFA-1 dependent manner across central nervous system (CNS) microvessels in vivo.28
Retroviral transduction of ICAM-1-/-ICAM-2-/- bEndI1/2.1 with wild-type ICAM-1 reconstituted TEM of T cells to levels entirely comparable to those across wild-type endothelium, whereas retroviral transduction of ICAM-2 only partially reconstituted TEM of T cells across the endothelial monolayer, regardless of its stimulation stage. Additionally, endothelial ICAM-1 and ICAM-2 were not additive in their ability to re-establish TEM of T cells across bEnd I1/2.1 as retroviral transduction of both ICAM-1 and ICAM-2 into bEnd I1/2.1 did not further increase TEM of T cells beyond that across bEndI1/2.1 retransduced with ICAM-1 alone. The difference in migration rates mediated via ICAM-1 or ICAM-2 and their lack in additivity was not attributable to surface expression levels of both molecules, which quite resembled the in vivo situation, where ICAM-2 levels on endothelial cells are characteristically much higher as compared with ICAM-1 even on inflamed endothelium.2 Rather, our data suggest differences in the ability of endothelial ICAM-1 and ICAM-2 to deliver signals into the endothelial cells required for TEM of T cells, as the chimeric molecule I1EI2C, where the transmembrane and cytoplasmic part of ICAM-1 was replaced by the respective part of ICAM-2, failed to reconstitute TEM of T cells across bEndI1/2.1 and bEndI1.1.
The role of endothelial ICAM-1 has generally been envisaged as an extracellular docking molecule for lymphocyte attachment to the endothelium. To investigate whether this suffices for its role in mediating TEM of T cells, we set out to dissect the relative contribution of the extracellular versus the intracellular part of endothelial ICAM-1 in TEM of T cells. To this end, ICAM-1 mutants lacking the cytoplasmic tail or harboring point mutations of 3 tyrosines that abrogate potential phophorylation sites were created and expressed in both the ICAM-1-/-ICAM-2-/- brain endothelioma line bEndI1/2.1 and the ICAM-1-deficient brain endothelioma line bEndI1.1. All mutants reconstituted adhesion of T cells equally well in both cell lines back to wild-type levels, demonstrating that the extracellular part of ICAM-1 is sufficient to support adhesion of T cells to endothelium. In contrast, TEM of T lymphocytes was supported only by wild-type ICAM-1 and mutants containing the entire cytoplasmic tail of ICAM-1, regardless of possible tyrosine phosphorylation sites, and not by mutants lacking the cytoplasmic tail of ICAM-1. These findings suggest that efficient TEM requires intracellular events within endothelial cells, which are coordinated by the cytoplasmic tail of endothelial ICAM-1 and are independent of its tyrosine phosphorylation status or the presence of endogenous ICAM-2.
The cytoplasmic tail of ICAM-1 was previously demonstrated to be required for the fibrinogen-dependent migration of neutrophils across ICAM-1-transfected Chinese hamster ovary (CHO) cells.29 Interestingly, although the cytoplasmic tail of ICAM-1 lacks intrinsic kinase activity or known protein-protein interaction domains that could recruit known downstream signaling components, ligation of endothelial ICAM-1 with fibrinogen was found to lead to the binding of the Src homology domain 2-containing tyrosine phosphatase 2 (SHP-2) to the cytoplasmic tail of ICAM-1 in a phosphotyrosine-dependent manner.30 These observations are in apparent contrast to our findings demonstrating that tyrosine phosphorylation of the transmembrane or the cytoplasmic tyrosines of endothelial ICAM-1 is not involved in delivering the signals required for TEM of T cells across unstimulated or stimulated brain endothelium. The evidence is 2-fold: ICAM-1 mutants lacking all potential juxtamembrane and intracellular tyrosine phosphorylation sites fully reconstituted TEM of T cells to wild-type levels. Additionally, engagement of endothelial ICAM-1 by antibody cross-linking on brain endothelioma lines, although delivering signals into the endothelial cells as demonstrated by the activation of SAPK/JNK, did not lead to tyrosine phosphorylation of ICAM-1.
In rat brain endothelial cell lines, ICAM-1 was shown to activate specific signaling pathways, which can be distinguished from those elicited by ICAM-1 in astrocytes.31 Engagement of ICAM-1 on rat brain endothelium by either antibody cross-linking or T-cell adhesion was shown to result in the activation of p60src and the phosphorylation of cytoskeletal-associated proteins such as the p60src substrate cortactin,32 but also others such as paxillin, p130Cas, focal adhesion kinase (FAK), and SAPK/JNK. In a similar approach, engagement of endothelial ICAM-1 has been demonstrated to induce a reorganization of the cytoskeleton to form stress fibers and ICAM-1 association with the Triton X-100 insoluble fraction, suggesting a link between ICAM-1 and the cytoskeleton in endothelial cells.23,24 Formation of stress fibers and phosphorylation of paxillin, p130Cas, and FAK was demonstrated to depend on Ca2+-mediated signals and on p38 mitogen-activated protein kinase (p38 MAPK) activity as well as on the activation of the small guanosine triphosphate (GTP)-binding protein Rho.23,27,33 In our present study, we could demonstrate that the intracellular part of ICAM-1 is the essential upstream component of the signaling cascade initiated through LFA-1-mediated adhesion of T lymphocytes to endothelial ICAM-1 subsequently controlling TEM. Whereas wt ICAM-1 and ICAM-1 mutants lacking the potential juxtamembrane and intracellular tyrosine phosphorylation sites could initiate activation of endothelial Rho proteins upon antibody-mediated ICAM-1 cross-linking, ICAM-1 mutants lacking the cytoplasmic part of ICAM-1 were incapable of activating endothelial Rho. This observation is consistent with previous results demonstrating that inhibition of Rho proteins by pretreatment of endothelial cells with C3-transferase resulted in a substantial inhibition of TEM of T cells without affecting T-cell adhesion to the endothelium.23 Additionally, the chimeric molecule I1EI2C was incapable of activating Rho proteins, indicating that in endothelial cells the cytoplasmic part of ICAM-1 but not of ICAM-2 can stimulate Rho activation. Our observations support previous findings in which cross-linking of endothelial ICAM-1 but not of endothelial ICAM-2 was demonstrated to lead to RhoA activation and stress-fiber formation.34 Thus, the cytoplasmic tail of endothelial ICAM-1—independently of tyrosine phosphorylation—seems to trigger rearrangement of the endothelial actin cytoskeleton, probably via a Rho-dependent pathway that is necessary for TEM of T cells.23,29 ICAM-1 has been reported to bind a number of intracellular proteins such as α-actinin,35 glyceraldehyde-3-phosphate dehydrogenase,36 and ezrin.37 However, it is not clear if any of these molecules can transduce signals from the intracellular domain of endothelial ICAM-1. As members of the ezrin/radixin/moesin (ERM) family can initiate activation of Rho through interaction with Rho GDP-dissociation inhibitor (Rho-GDI),38 it is tempting to speculate that upon T-cell adhesion to endothelium the cytoplasmic tail of endothelial ICAM-1 connects to the cytoskeleton via proteins of the ERM family, which initiate cytoskeletal rearrangements in a Rho-dependent fashion enabling TEM of T cells. So far, evidence for a direct association of ezrin and moesin with endothelial ICAM-1 upon antibody-mediated cross-linking is lacking.39
Taken together, our data clearly demonstrate that the cytoplasmic tail of endothelial ICAM-1 is necessary for TEM of T cells. The molecular events involved in T-cell adhesion can be distinguished from those involved in TEM. The extracellular part of endothelial ICAM-1 is clearly sufficient for mediating adhesion of T cells to the endothelium, and the cytoplasmic tail of ICAM-1 is required for TEM of T cells without any necessity for phosphorylation of juxtamembrane or cytoplasmic tyrosines. To understand how the cytoplasmic tail of endothelial ICAM-1 delivers the specific signals necessary for TEM into endothelial cells, an in-depth characterization of the intracellular binding partners for ICAM-1 in endothelial cells will be necessary.
Prepublished online as Blood First Edition Paper, July 31, 2003; DOI 10.1182/blood-2003-02-0358.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The outstanding technical assistance of Gabi Hoch is gratefully acknowledged. We owe great thanks to Urban Deutsch and Friedeman Kiefer for critically discussing the manuscript and to Dietmar Vestweber for continuous support.

![Figure 8. Activation of RhoGTPases following ICAM-1 cross-linking on bEndI1/2.1 re-expressing ICAM-1 wild type or its mutants. Endothelial cells were cross-linked with antimouse ICAM-1 followed by goat antirat IgG (RAM) for 15 minutes. Cross-linked cells were lysed and activated Rho proteins (guanosine triphosphate [GTP]-bound form) precipitated with GST-rhotekin. Total Rho proteins and GST-rhotekin-precipitated Rho proteins were subsequently [32P]ADP-ribosylated, resolved on 15% SDS-PAGE, and exposed to autoradiographic film. L indicates lysate; rh, precipitated GST-rhotekin.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/10/10.1182_blood-2003-02-0358/6/m_h82235214008.jpeg?Expires=1763459941&Signature=WYRWXragymtc6p90jWZZYz123UNv8GC6UiqLDV7aLnUw7DNlu3NFmbUtTtGhQA6LmcXciJLtpPpxIA1aGs1waFa4VK2hF2s6vAwhvV~7TEjva5XRsLNAjn-B2G-0ZcEdTMr-3dyceigshQwwOcmZfTunx6IBmvglzi~dEeAIlZsQzt2VWZpPVisRHWCCl4YlhwBQ~nvopjDUad0KM8uqzBqUnbXgu-j8d-C0rWagUD0If2eCg-tAAX0q~9JSWeKsJafkFO5nFozzd6hy82b~XpOsIEQ1zHVxZyUeUxVCYWL4VPG6QEdt~3akvQKr3fJNv9JKFTxKdTkVXnVXyeGGbA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal