Abstract
The Tec family kinase Btk plays an important role in the regulation of phospholipase Cγ2 (PLCγ2) downstream of the collagen receptor glycoprotein VI (GPVI) in human platelets. Platelets also express a second member of this family, Tec; however, its function has not been analyzed. To address the role of Tec, we analyzed Btk-/-, Tec-/-, and Btk/Tec double-deficient (Btk-/-/Tec-/-) platelets. Tec-/- platelets exhibit a minor reduction in aggregation to threshold concentrations of collagen or the GPVI-specific agonist collagen-related peptide (CRP), whereas responses to higher concentrations are normal. Tyrosine phosphorylation of PLCγ2 by collagen and CRP is not altered in Tec-/- platelets. However, Btk-/-/Tec-/- platelets exhibit a greater reduction in PLCγ2 phosphorylation than is seen in the absence of Btk, thus revealing an important role for Tec in this situation. Furthermore, Btk-/-/Tec-/- platelets fail to undergo an increase in Ca2+, aggregation, secretion, and spreading in response to collagen or CRP, whereas they aggregate normally to adenosine diphosphate (ADP) and spread on fibrinogen. A residual GPVI signal exists in the Btk-/-/Tec-/- platelets as CRP synergizes with ADP to mediate aggregation. These results demonstrate an essential requirement for Tec and Btk in platelet activation by GPVI and reveal a functional role for Tec in the regulation of PLCγ2 in the absence of Btk. (Blood. 2003;102: 3592-3599)
Introduction
Thrombus formation and cessation of bleeding after damage to the vasculature require effective platelet function. Multiple physiologic agonists initiate or modify platelet hemostatic responses through numerous and sometimes overlapping intracellular signaling pathways downstream of G-protein-coupled and tyrosine-kinase-linked receptors.1-3 The protease thrombin and the small nucleotide adenosine diphosphate (ADP) are examples of platelet G-protein-coupled receptor agonists.1 The subendothelial matrix protein collagen activates platelets through a tyrosine kinase pathway regulated by the GPVI-FcR γ-chain receptor complex.4-7 Cross-linking of GPVI induces phosphorylation of an immunoreceptor tyrosine-based activation motif (ITAM) in the FcR γ-chain by the Src kinases Lyn and Fyn.8,9 This leads to activation of the tyrosine kinase Syk and the regulation of a downstream signaling cascade that consists of adapter proteins such as LAT and SLP-76 in combination with effector enzymes including phospholipase γ2 (PLCγ2) and phosphatidylinositol 3-kinase (PI 3-kinase).4,10 This cascade shares many similarities with signaling by immune receptors such as the B-cell antigen receptor (BCR).10,11 There are also important similarities with outside-in signaling by the major platelet integrin αIIbβ3, which includes pivotal roles for Syk, SLP-76, and PLCγ2, though significantly this receptor does not use an ITAM.12-14
Tec family kinases play important roles in signaling by ITAM receptors.15 There are 5 members in the Tec family of kinases—Tec, Btk, Itk, Bmx, and Txk/Rlk—that share a homologous structure consisting of a tyrosine kinase domain, Src homology domain 2 (SH2) and SH3 domains, and (with the exception of Txk/Rlk) a PH (Pleckstrin homology) domain coupled to a distinctive TH (Tec homology) domain.16,17 Tec kinases are regulated through phosphorylation by Src kinases, autophosphorylation, and recruitment to the membrane through interaction of their PH domain with phosphatidylinositol 3,4,5-trisphosphate (PI 3,4,5P3).18-21
The importance of Tec kinases was brought to light when the human immunodeficiency X-linked agammaglobulineamia (XLA) was linked to mutations in Btk.22 Patients with XLA do not express functional Btk and as a result experience a severe loss of mature B cells caused by a block during B-cell development. Human B-cell lines lacking functional Btk have defective BCR-dependent tyrosine phosphorylation of PLCγ2 and an influx of Ca2+ that is restored by the expression of wild-type Btk.23 Xid mice, which have a mutation in the PH domain of Btk that prevents association with PI 3,4,5P3,24 and Btk-/- mice have reduced populations of B cells, although the phenotype is less severe than for humans.25,26 The difference in severity has been attributed to the presence of the related kinase Tec in murine B cells. Significantly, mice deficient in Btk and Tec have a block in B-cell development and a severe deficiency of peripheral B cells, resembling the human XLA phenotype.27
Platelets express Tec and Btk, and each has been shown to undergo tyrosine phosphorylation in response to collagen and thrombin.28 In addition, a role for Btk in signaling downstream of GPVI has been shown using platelets from patients with XLA. XLA platelets exhibit a significant reduction in aggregation and secretion induced by collagen and the GPVI-specific agonist collagen-related peptide (CRP).29 This inhibition is thought to be the result of a substantial reduction in tyrosine phosphorylation of PLCγ2 leading to inhibition in the mobilization of intracellular Ca2+ and the activation of protein kinase C. The inhibition, however, is not complete, and responses to collagen and CRP recover at higher concentrations.
In this paper we demonstrate that Tec is able to compensate for the absence of Btk in signaling downstream of GPVI in murine platelets despite playing only a minor role in the presence of Btk. In contrast, neither tyrosine kinase plays a role in platelet spreading after adhesion to fibrinogen, providing further evidence of the distinct nature of the signaling pathways used by the integrin αIIbβ3 and GPVI.
Materials and methods
Materials and reagents
Collagen (Horm) was purchased from Nycomed (Munich, Germany). CRP (GPC[GPO]12GPC) was synthesized by Tana Laboratories (Houston, TX) and cross-linked using N-succinimidyl 3-[2-pyridyldithio]propionate (SPDP) as described previously.30,31 Heparin sodium (Monoparin, 25 000 U/mL) was obtained from CP Pharmaceuticals (Wrexham, United Kingdom). Rhodamine-conjugated phalloidin was obtained from Sigma (Poole, United Kingdom). Ca2+ sensitive dye Fluo-4AM was purchased from Molecular Probes (Cambridge BioScience, Cambridge, United Kingdom). Anti-Btk and anti-Tec polyclonal antibodies (pAbs) were kind gifts from Dr M. Thomlinson (formerly of DNAX, Palo Alto, CA). Other reagents were from previously described sources.32,33
Mice
Mice used were between 8 and 12 weeks of age. Btk-/- mice25,26 were originally obtained from The Jackson Laboratory (Bar Harbor, ME). Tec-/- and Btk-/-/Tec-/- mice were generated as previously described.27 F3 generations (C57BL/6 × 129) of wild-type, Btk-/-, and Tec-/- and Btk-/-/Tec-/- mice were maintained as separate knock-out lines.
Platelet preparation
Blood samples were drawn from the hepatic portal vein of CO2 terminally anesthetized mice. Approximately 1 mL blood was collected from each mouse into either 100 μL heparin (10 U/mL) or 100 μL acid-citrate-dextrose (ACD; 2.5% sodium citrate, 2% glucose, and 1.5% citric acid, wt/vol). Subsequent to collection, heparin-anticoagulated blood was further diluted with 200 μL heparin (10 U/mL), and ACD-anticoagulated blood was further diluted with 200 μL Tyrode buffer (pH 6.6). The blood was centrifuged at 1000 rpm (60g) for 6 minutes in a microfuge, then the supernatant and the top third of the erythrocytes were removed and centrifuged at 2000 rpm (approximately 220g) for 5 minutes to yield the platelet-rich plasma (PRP). Washed platelets were prepared as follows: PRP containing 800 nM prostacyclin was centrifuged at 2800 rpm (500g) for 10 minutes. The platelet pellet was resuspended in 1.5 mL Tyrode buffer (pH 7.3) containing 25 μL ACD. After the addition of more prostacyclin (800 nM), the platelet pellet was centrifuged again at 2800 rpm (500g) for 10 minutes. Platelets were resuspended to a concentration of 2 × 108/mL in HEPES (N-2-hydroxyethylpiperazine-N'-2-ethanesulfonic acid)-buffered Tyrode (134 mM NaCl, 2.9 mM KCl, 1 mM MgCl2, 20 mM HEPES, NaHCO3 12 mM, 5 mM glucose).
All tests, unless otherwise stated, were conducted in siliconized glass vials at 37°C in a Born aggregometer (ChronoLog, Havertown, PA) with stirring at 1200 rpm. Platelets were warmed to 37°C for 5 minutes before stimulation. The following preincubation times were used for drugs or their solvent controls: indomethacin (10 μM), 5 minutes; A3P5P (1 mM), 1 minute; AR-C67085 (1 μM), 1 minute; and PP1 (20 μM), 10 minutes.
Measurement of intracellular Ca2+
Washed murine platelets (4 × 108/mL) were loaded with the Ca2+-sensitive dye Fluo-4 by incubation with the cell-permeant acetoxymethyl (am) derivative (2.5 μM Fluo-4AM for 40 minutes, maintaining the suspension temperature at 22°C). Loaded platelets were washed again to remove remaining extracellular Fluo-4 and, after resuspension to a concentration of 2 × 108/mL, left for 20 minutes before experimentation. Experiments measuring Ca2+ changes in a platelet suspension were performed using a Molecular Devices Flexstation (Wokingham, United Kingdom) with temperature maintained at 37°C. The software, SOFTmax PRO version 4.3 (Molecular Devices, Wokingham, United Kingdom), was used for data capture and analysis.
Phosphorylation studies
Stimulations with CRP and collagen were conducted in suspension, and experiments were terminated by adding an equal volume of ice-cold 2 × lysis buffer to 500 μL platelets (1.5 × 108/mL). Stimulations with fibrinogen were conducted using a fibrinogen-coated surface, as described by Wonerow et al.14 Immunoprecipitation and Western blotting were carried out as previously described.4 Densitometry was conducted by volume analysis using Quantity One software (version 4.2.1) from BioRad (Hemel Hempstead, Herts, United Kingdom).
Flow cytometry studies
Expression of P-selectin (CD-62P) and procoagulant phospholipids was measured by flow cytometry. Platelets (10 × 109/L) were stimulated for 10 minutes with the agonist or its vehicle in the presence of lotrafiban at 37°C. A sample (50 μL) was incubated with 2 μL fluorescein isothiocyanate (FITC)-anti-P-selectin antibody or FITC-annexin V for 20 minutes before flow cytometry. Data were acquired using Cell Quest-Pro, and analysis was conducted using Cell Quest version 3.1f (BD Biosciences, San Jose, CA).
Spreading studies
Platelets (5 × 107/mL) were allowed to sediment onto either fibrinogen- or collagen-coated coverslips for 30 minutes before fixing with 3.7% paraformaldehyde and permeabilization with 0.2% Triton X-100 in phosphate-buffered saline (PBS; 140 mM NaCl, 3 mM KCl, 10 mM Na2HPO4, and 2 mM KH2PO4; pH 7.4). After 2 washes with PBS, the fixed platelets were stained for F-actin with rhodamine-conjugated phalloidin for 1 hour and were washed 3 more times with PBS before analysis. Fluorescence was visualized using a Zeiss Axiovert S100 microscope (Zeiss, Oberkochen, Germany) with a monochromatic light source and a charge-coupled device (CCD) camera, as described previously by Poole and Watson.34 Openlab version 3.0 software (Improvision Ltd, Coventry, United Kingdom) was used for image capture and subsequent analysis.
Analysis of data
Results are expressed as the mean ± SEM. Experiments were performed between 3 and 10 times. Statistical indications were made using Student t test, with P values less than .05 taken as the level of significance.
Results
Tyrosine phosphorylation of Tec and Btk by GPVI
The involvement of Tec and Btk in GPVI and αIIbβ3 signaling was investigated using Western blotting Tec and Btk immunoprecipitates from wild-type murine platelet lysates and the pan phosphotyrosine mAb 4G10. Collagen and the GPVI-selective peptide CRP stimulated the robust tyrosine phosphorylation of Tec and Btk in a concentration-dependent manner (Figure 1A). Interestingly, CRP stimulated more robust tyrosine phosphorylation of Btk than did collagen, in line with previous reports that it is a more powerful platelet agonist. On the other hand, collagen stimulated a greater increase in tyrosine phosphorylation of Tec, which may reflect a role for the integrin α2β1 in mediating this response. Activation of αIIbβ3 through a fibrinogen-coated surface was also able to stimulate substantial tyrosine phosphorylation of the 2 Tec family kinases (Figure 1A).
CRP, collagen, and fibrinogen induce phosphorylation of murine Btk and Tec in wild-type platelets, the phosphorylation of Tec is unaltered in Btk-/- platelets, and that of Btk is unaltered in Tec-/- platelets in response to CRP. (A) CRP, collagen, and fibrinogen induce phosphorylation of murine Btk and Tec in wild-type platelets. (B) Phosphorylation of Tec is unaltered in Btk-/- platelets, and that of Btk is unaltered in Tec-/- platelets in response to CRP. Wild-type (A) or Tec-/- and Btk-/- (B) murine washed platelets were stimulated for 30 seconds with CRP and 90 seconds with collagen at increasing concentrations. Platelets were stimulated by adhesion to a fibrinogen-coated surface for 45 minutes. Stimulation was terminated by the addition of ice-cold lysis buffer. Subsequently, either Btk or Tec was immunoprecipitated from the sample, and the immunoprecipitate (IP) was run on an SDS-PAGE gel before Western blotting for tyrosine phosphorylation using the specific antibody 4G10. Anti-Btk or anti-Tec antibody was used to show equal protein levels. Each gel contained samples from 1 mouse but is representative of 3 identical experiments.
CRP, collagen, and fibrinogen induce phosphorylation of murine Btk and Tec in wild-type platelets, the phosphorylation of Tec is unaltered in Btk-/- platelets, and that of Btk is unaltered in Tec-/- platelets in response to CRP. (A) CRP, collagen, and fibrinogen induce phosphorylation of murine Btk and Tec in wild-type platelets. (B) Phosphorylation of Tec is unaltered in Btk-/- platelets, and that of Btk is unaltered in Tec-/- platelets in response to CRP. Wild-type (A) or Tec-/- and Btk-/- (B) murine washed platelets were stimulated for 30 seconds with CRP and 90 seconds with collagen at increasing concentrations. Platelets were stimulated by adhesion to a fibrinogen-coated surface for 45 minutes. Stimulation was terminated by the addition of ice-cold lysis buffer. Subsequently, either Btk or Tec was immunoprecipitated from the sample, and the immunoprecipitate (IP) was run on an SDS-PAGE gel before Western blotting for tyrosine phosphorylation using the specific antibody 4G10. Anti-Btk or anti-Tec antibody was used to show equal protein levels. Each gel contained samples from 1 mouse but is representative of 3 identical experiments.
As a prelude to investigating the functional role of the 2 Tec kinases, we measured their expression by Western blotting and compared the ability of CRP to stimulate their tyrosine phosphorylation in the single kinase-deficient platelets relative to wild type. The level of expression of Btk in Tec-/- platelets was similar to that in wild-type cells, with the converse also being true for the expression of Tec in Btk-/- platelets (Figure 1B). Importantly, tyrosine phosphorylation of Tec and Btk by CRP was maintained after gene ablation of the counterpart member of the Tec family kinase (Figure 1B). The small reduction in phosphorylation of Btk observed in the absence of Tec shown in Figure 1B was not seen in all experiments. Our observations provide evidence for the regulation of Tec and Btk in signaling downstream of GPVI and αIIbβ3 in murine platelets and demonstrate that they are subject to independent expression and regulation.
Tyrosine phosphorylation in Tec familykinase-deficient platelets
We compared the ability of CRP (Figure 2A) and collagen (Figure 2B) to stimulate tyrosine phosphorylation in whole-cell lysates of Tec and Btk single- and double-deficient platelets. In agreement with previous observations, CRP and collagen stimulated a significant increase in tyrosine phosphorylation of a number of proteins in the whole-cell lysate with a major band at 70 to 75 kDa, which comigrates with a number of the proteins in the GPVI signaling cascade including Syk and SLP-76 and the 2 Tec family kinases Btk and Tec. No major qualitative differences were observed in the pattern of tyrosine phosphorylation in the genetically modified platelets, and the level of tyrosine phosphorylation of most bands was unchanged in the single- and double knock-out platelets.
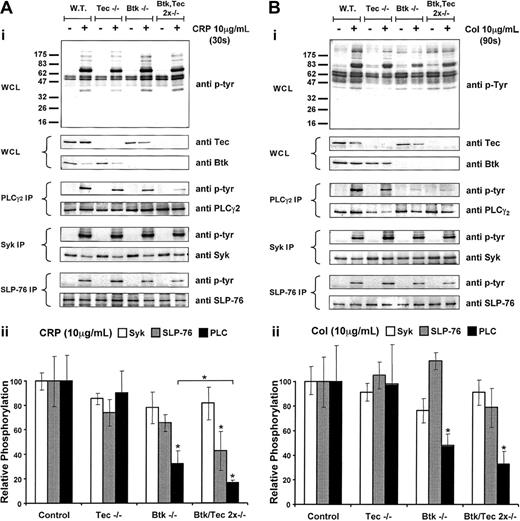
Collagen-induced whole-cell, PLCγ2, Syk, and SLP-76 tyrosine phosphorylation in BTK-/-, Tec-/-, and Btk-/-/Tec-/- platelets compared with wild type and corresponding, densitometric analysis. Murine washed platelets from the 4 genotypes—wild-type, Btk-/-, Tec-/-, and Btk-/-/Tec-/-—were stimulated with either CRP (10 μg/mL) (A) or collagen (10 μg/mL) (B) or for 30 seconds and 90 seconds, respectively. All experiments were carried out in the presence of 10 μM lotrafiban to prevent aggregation. Basal and stimulated platelet samples were lysed by the addition of ice-cold lysis buffer and then used for the whole cell lysate (WCL) or the appropriate immunoprecipitation (IP). Tyrosine phosphorylation was revealed using the pan antiphosphotyrosine antibody 4G10. The presence of Btk and Tec was shown in the WCL using antibodies against these proteins. For the IP, a corresponding reprobe of PLCγ2, Syk, or SLP-76 was used to show equal loading. Results shown are representative of at least 3 identical experiments. Densitometry by band volume analysis was conducted on the tyrosine phosphorylation (4G10)-probed IP and the reprobes from panels Ai and Bi. Graphs show the ratio of the tyrosine phosphorylation band volumes to the corresponding reprobe band volumes then normalized to the wild-type control for each blot. Panel Aii shows the variation of phosphorylation of PLCγ2 (▪), SLP-76 (▦), and Syk (□) in the various knock-out genotypes after 30-second stimulation with CRP (10 μg/mL). Panel Bii shows equivalent data after stimulation with collagen (10 μg/mL). *Significant difference (P < .05) from control or between pairs. Each part shows the average band density from at least 3 Western blots ± SEM.
Collagen-induced whole-cell, PLCγ2, Syk, and SLP-76 tyrosine phosphorylation in BTK-/-, Tec-/-, and Btk-/-/Tec-/- platelets compared with wild type and corresponding, densitometric analysis. Murine washed platelets from the 4 genotypes—wild-type, Btk-/-, Tec-/-, and Btk-/-/Tec-/-—were stimulated with either CRP (10 μg/mL) (A) or collagen (10 μg/mL) (B) or for 30 seconds and 90 seconds, respectively. All experiments were carried out in the presence of 10 μM lotrafiban to prevent aggregation. Basal and stimulated platelet samples were lysed by the addition of ice-cold lysis buffer and then used for the whole cell lysate (WCL) or the appropriate immunoprecipitation (IP). Tyrosine phosphorylation was revealed using the pan antiphosphotyrosine antibody 4G10. The presence of Btk and Tec was shown in the WCL using antibodies against these proteins. For the IP, a corresponding reprobe of PLCγ2, Syk, or SLP-76 was used to show equal loading. Results shown are representative of at least 3 identical experiments. Densitometry by band volume analysis was conducted on the tyrosine phosphorylation (4G10)-probed IP and the reprobes from panels Ai and Bi. Graphs show the ratio of the tyrosine phosphorylation band volumes to the corresponding reprobe band volumes then normalized to the wild-type control for each blot. Panel Aii shows the variation of phosphorylation of PLCγ2 (▪), SLP-76 (▦), and Syk (□) in the various knock-out genotypes after 30-second stimulation with CRP (10 μg/mL). Panel Bii shows equivalent data after stimulation with collagen (10 μg/mL). *Significant difference (P < .05) from control or between pairs. Each part shows the average band density from at least 3 Western blots ± SEM.
Btk has been shown to regulate tyrosine phosphorylation of PLCγ2 in human platelets. To investigate whether this is the case in the mouse and to compare it with the role of Tec, we immunoprecipitated PLCγ2 from Btk- and Tec-deficient platelets and measured tyrosine phosphorylation of the phospholipase using the pan antiphosphotyrosine antibody 4G10. We also performed similar studies for the 2 upstream regulators of PLCγ2, the tyrosine kinase Syk and the adapter SLP-76 (Figure 2A-B).
Tyrosine phosphorylation of PLCγ2 by CRP was reduced by more than 50% in the absence of Btk but was not significantly altered in the absence of Tec (Figures 2Aii). Further reduction (P < .05) occurred in the degree of tyrosine phosphorylation of PLCγ2 in Btk-/-/Tec-/- platelets, which lowered the level to approximately 20% of control. There was no significant reduction in tyrosine phosphorylation of Syk or SLP-76 in Tec-/- platelets and a small reduction in tyrosine phosphorylation of SLP-76 in Btk-/- cells in response to CRP (Figure 2Aii). A similar set of observations was made for collagen, though a significant decrease in phosphorylation of SLP-76 was not found (Figure 2Bii).
These results demonstrate a critical role for Btk in the regulation of tyrosine phosphorylation of PLCγ2 by collagen and CRP and are consistent with results in human XLA patients. In addition, the further reduction in tyrosine phosphorylation of PLCγ2 in the absence of Btk and Tec demonstrates a regulatory role for Tec even though this was not apparent when examining Tec-/- platelets. This suggests that Tec is able to compensate to a limited extent for the absence of Btk but that it has a minimal role in the regulation of the PLCγ2 in wild-type platelets.
Inhibition of GPVI-induced platelet aggregation in kinase-deficient platelets
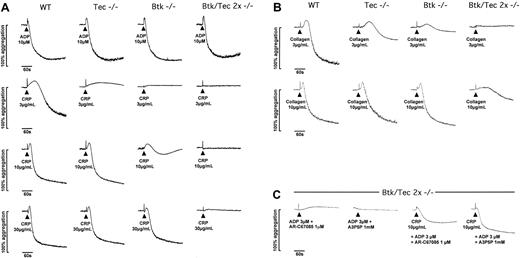
The functional consequence of the reduction in tyrosine phosphorylation of PLCγ2 was initially investigated by measuring shape change and aggregation in a Born aggregometer. This apparatus measures light transmission through a platelet suspension. An increase in optical density represents a change in platelet shape from discoid to spherical, whereas a decrease represents aggregation. Shape change precedes aggregation and is inducible by lower concentrations of stimuli. A low concentration of CRP stimulated a slow increase in optical density (shape change) followed by weak aggregation in wild-type platelets (Figure 3A). Higher concentrations of the GPVI-specific agonist stimulated rapid shape change followed by aggregation. The same profile of response was seen with a high concentration of the G-protein-coupled receptor agonist ADP (10 μM). The weak aggregation response to a low concentration of CRP (3 μg/mL) was reduced to shape change in the absence of Tec, whereas the response to higher concentrations of the peptide (10-30 μg/mL) was not altered. In comparison, the response to low (3 μg/mL) and intermediate (10 μg/mL) concentrations of CRP were abolished or severely reduced, respectively, in the absence of Btk. The response to a high concentration of CRP (30 μg/mL) was unaltered in the absence of Btk. Strikingly, the response to this high concentration of CRP (30 μg/mL) was reduced to a residual shape change response in the absence of both Tec family kinases, whereas the response to lower concentrations of the collagen peptide was completely abolished (Figure 3A). The response to a high (10 μM) and to lower concentrations of ADP (1 and 3 μM; not shown) was not altered in the single- and double-deficient platelets (Figure 3A). A differential role for Tec and Btk was also seen in the stimulation of shape change and aggregation by collagen. Aggregation to a low concentration of collagen (3 μg/mL) was more severely reduced in the absence of Btk than for Tec, but in both cases the inhibitory effect could be overcome by increasing the concentration of collagen (Figure 3B). Only a high concentration of collagen was able to stimulate a slow, partial increase in light transmission in the Btk-/-/Tec-/- platelets, possibly reflecting either a combination of α2β1-dependent adhesion and limited activation of αIIbβ3 or collagen fibril formation (Figure 3B).
Aggregation of wild-type, Tec-/-, Btk-/-, and Btk-/-/Tec-/-murine platelets in response to CRP and ADP. Traces showing the effect of loss of Tec, Btk, or both on platelet aggregation responses to (A) ADP and CRP, (B) collagen and, (C) CRP with ADP. Aggregation was conducted using platelets at a concentration of 1.5 × 108/mL in diluted heparinized platelet-rich plasma (PRP). Agonist addition was timed at 30 seconds, and subsequent monitoring using a Chronolog 490-2D aggregometer (ChronoLog) was maintained for 5 minutes. The scale indicates the percentage change in optical density where resting PRP (diluted) is set at 0% and platelet-poor plasma is equivalent to 100%. Traces are illustrative of at least 4 separate experiments.
Aggregation of wild-type, Tec-/-, Btk-/-, and Btk-/-/Tec-/-murine platelets in response to CRP and ADP. Traces showing the effect of loss of Tec, Btk, or both on platelet aggregation responses to (A) ADP and CRP, (B) collagen and, (C) CRP with ADP. Aggregation was conducted using platelets at a concentration of 1.5 × 108/mL in diluted heparinized platelet-rich plasma (PRP). Agonist addition was timed at 30 seconds, and subsequent monitoring using a Chronolog 490-2D aggregometer (ChronoLog) was maintained for 5 minutes. The scale indicates the percentage change in optical density where resting PRP (diluted) is set at 0% and platelet-poor plasma is equivalent to 100%. Traces are illustrative of at least 4 separate experiments.
Experiments were designed to investigate whether the residual response to GPVI agonists observed in the Btk-/-/Tec-/- platelets was of functional significance. This was accomplished by investigating the possible synergy of the residual response with signals from either the Gq-coupled P2Y1 or the Gi-coupled P2Y12 ADP receptors, which, on their own, cannot induce platelet aggregation (Figure 3C). An intermediate concentration of CRP (10 μg/mL), which alone was unable to induce shape change or aggregation of the Btk-/-/Tec-/- platelets, could induce substantial aggregation when used in combination with a threshold concentration of ADP (not shown). Significantly, this potentiation was seen in the presence of either a P2Y1 or a P2Y12 receptor antagonist, demonstrating that it is mediated through synergy with both Gi- and Gq/G12/13-based signaling pathways (Figure 3C).
These results show that Btk plays a greater role than Tec in platelet activation by GPVI. Significantly, however, a role for Tec was observed in response to very low concentrations of CRP and collagen despite the apparent absence of an effect in supporting tyrosine phosphorylation of PLCγ2, which possibly fell below the noise of the assay. Moreover, the additional loss of response in the double-deficient platelets over that seen in Btk-deficient platelets provides evidence for a compensatory role of Tec in supporting signals downstream of GPVI. Evidence of a functionally relevant (albeit weak) Btk/Tec-independent pathway of platelet activation downstream of GPVI was demonstrated by the ability of CRP to induce aggregation on costimulation with either P2Y1 or P2Y12 ADP receptors.
Critical role for Tec and Btk in Ca2+ mobilization and α-granule secretion downstream of GPVI
We have investigated whether the reduction in tyrosine phosphorylation of PLCγ2 and the inhibition of aggregation was associated with an inhibition of Ca2+ mobilization and a more general loss of platelet activation by monitoring α-granule secretion and expression of procoagulant activity. CRP stimulated a concentration-dependent (3-30 μg/mL) increase in Ca2+ elevation above basal in wild-type platelets but not in Btk-/-/Tec-/- cells (Figure 4). In contrast, the elevation of Ca2+ by thrombin was not altered in the double-deficient platelets (Figure 4).
Inhibition of Ca2+ elevation in Btk-/-/Tec-/-in response to CRP. Traces showing Ca2+ elevation induced by thrombin (3 U/mL) and CRP (3, 10, and 30 μg/mL) in wild-type and Btk-/-/Tec-/- platelets. The Ca2+ elevation traces are all from the same experiment and were performed using a Fluo-4-loaded, washed platelet suspension in a Molecular Devices Flexstation with temperature maintained at 37°C. Agonist addition was automated and conducted at 20 seconds after the initiation of monitoring. Platelets were in the presence of lotrafiban (10 μM) to prevent aggregation. The scale is in arbitrary units derived from the intensity of fluorescence emission from the Fluo-4. Traces shown are representative of 3 parallel experiments.
Inhibition of Ca2+ elevation in Btk-/-/Tec-/-in response to CRP. Traces showing Ca2+ elevation induced by thrombin (3 U/mL) and CRP (3, 10, and 30 μg/mL) in wild-type and Btk-/-/Tec-/- platelets. The Ca2+ elevation traces are all from the same experiment and were performed using a Fluo-4-loaded, washed platelet suspension in a Molecular Devices Flexstation with temperature maintained at 37°C. Agonist addition was automated and conducted at 20 seconds after the initiation of monitoring. Platelets were in the presence of lotrafiban (10 μM) to prevent aggregation. The scale is in arbitrary units derived from the intensity of fluorescence emission from the Fluo-4. Traces shown are representative of 3 parallel experiments.
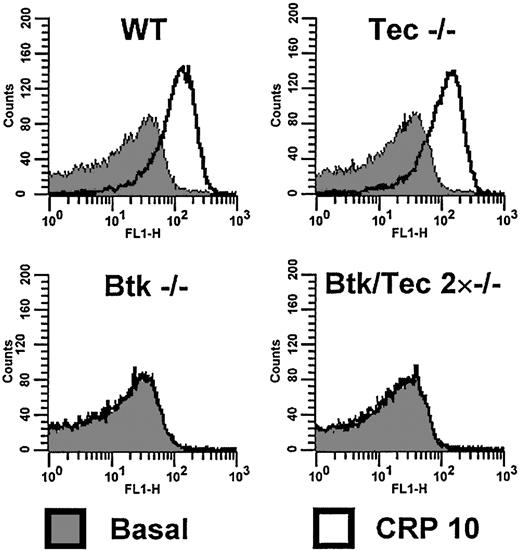
An FITC-conjugated antibody, P-selectin, was used to measure the marker for α-granule secretion. P-selectin exposure induced by CRP (10 μg/mL) was not altered in the absence of Tec but was abolished in Btk-/- and Btk-/-/Tec-/- platelets (Figure 5). In contrast, the response to thrombin was not altered in either the single- or the double-deficient platelets (not shown). CRP (10 μg/mL) stimulated a marked increase in annexin V binding, a marker of procoagulant activity. The response to CRP was not significantly altered in the absence of Tec but was abolished in the absence of Btk and in the double-deficient platelets (not shown), suggesting that Tec is unable to substitute for the absence of Btk in the regulation of α-granule secretion. This could be because of the requirement for a greater degree of activation of PLC by GPVI for α-granule secretion than, for example, aggregation,35 although we cannot rule out a specific role for Btk in regulating this response.
Btk-/-/Tec-/- platelets do not show surface expression of P-selectin after stimulation by CRP, in contrast to wild type. Washed platelets in the presence of lotrafiban (10 μM) and at a concentration of 1 × 107/mL were incubated with vehicle or CRP (10 μg/mL) for 10 minutes before dilution to 1 × 106/mL. Basal or stimulated platelets were stained for 30 minutes with an FITC-conjugated anti-P-selectin antibody. The relative fluorescence of the stained platelets was investigated by flow cytometry and is displayed as a histogram of counts versus fluorescence intensity. Graphs show that in wild-type and Tec-/- platelets there is a significant shift of the population to higher intensities of fluorescence, indicating P-selectin expression on the platelet surface. This shift is not seen with Btk-/- and Btk-/-/Tec-/- platelets, which show no change in distribution on stimulation. This experiment was conducted 5 times, and the histograms shown are representative of these.
Btk-/-/Tec-/- platelets do not show surface expression of P-selectin after stimulation by CRP, in contrast to wild type. Washed platelets in the presence of lotrafiban (10 μM) and at a concentration of 1 × 107/mL were incubated with vehicle or CRP (10 μg/mL) for 10 minutes before dilution to 1 × 106/mL. Basal or stimulated platelets were stained for 30 minutes with an FITC-conjugated anti-P-selectin antibody. The relative fluorescence of the stained platelets was investigated by flow cytometry and is displayed as a histogram of counts versus fluorescence intensity. Graphs show that in wild-type and Tec-/- platelets there is a significant shift of the population to higher intensities of fluorescence, indicating P-selectin expression on the platelet surface. This shift is not seen with Btk-/- and Btk-/-/Tec-/- platelets, which show no change in distribution on stimulation. This experiment was conducted 5 times, and the histograms shown are representative of these.
Impaired spreading of platelets deficient in Tec and Btk on collagen but not on fibrinogen
There are many similarities between the signaling cascades used by GPVI and the major platelet integrin αIIbβ3. This includes critical roles for Src and Syk family kinases, the adapter SLP-76, and PLCγ2. We have demonstrated tyrosine phosphorylation of Btk and Tec in platelets that had undergone adhesion to fibrinogen (Figure 1A), suggesting that it may also play a significant role in the integrin-based signaling pathway. To investigate this further, we compared spreading of Btk-/-, Tec-/-, and Btk-/-/Tec-/- platelets on fibrinogen and collagen. These studies were also performed in the presence of the Src family kinase inhibitor PP1 (20 μM) or the G-protein-coupled receptor agonist ADP. The αIIbβ3 antagonist lotrafiban was used in the collagen experiments to prevent additional activation through the integrin.
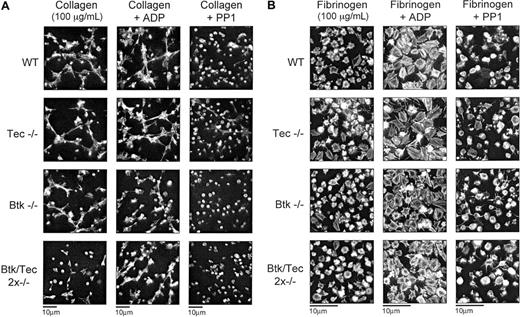
The spreading of wild-type platelets along the length of collagen fibers is not altered in the presence of ADP but is substantially inhibited in the presence of PP1 (Figure 6A). In the latter case, most platelets remain round, although a few filopodial extensions can be seen. A similar set of results is seen for the Btk-/- and the Tec-/- platelets. For both sets of mutant platelets, spreading on collagen is not significantly different than it is for wild-type platelets, and it is substantially inhibited by PP1 but unaffected by ADP (Figure 6A). Strikingly, a very different picture is seen with the double-deficient platelets. Spreading on collagen is dramatically inhibited but is restored to the same level as controls by addition of ADP (Figure 6A). A residual degree of spreading is seen in the double-deficient platelets on collagen compared with the effect of PP1, indicating that a limited degree of signaling has occurred.
Btk-/-/Tec-/- platelets display reduced spreading responses on a collagen monolayer but exhibit normal responses on fibrinogen. Murine platelets from the 4 genotypes in PRP were allowed to sediment for 30 minutes onto either (A) collagen or (B) fibrinogen-coated coverslips. The sedimentation was allowed to occur as control, in the presence of ADP (3 μM) or with inhibition of Src kinases by PP1 (20 μM). After 30 minutes the sedimented samples were fixed using 3.2% formaldehyde, subsequently washed with PBS, and stained for F-actin using rhodamine-phalloidin. (A) Wild-type (WT), Btk-/-, and Tec-/- but not Btk-/-/Tec-/- platelets spread along collagen fibers. With the addition of ADP all genotypes showed spreading, and in the presence of PP1 all genotypes displayed inhibition. Platelets sedimenting on collagen were in the presence of 10 μM lotrafiban to remove the effects of GPIIbIIIa. (B) All genotypes show spreading on fibrinogen with or without ADP. Pictures displayed are small fields of view from separate experiments obtained using a × 100 objective and are representative of 3 experiments.
Btk-/-/Tec-/- platelets display reduced spreading responses on a collagen monolayer but exhibit normal responses on fibrinogen. Murine platelets from the 4 genotypes in PRP were allowed to sediment for 30 minutes onto either (A) collagen or (B) fibrinogen-coated coverslips. The sedimentation was allowed to occur as control, in the presence of ADP (3 μM) or with inhibition of Src kinases by PP1 (20 μM). After 30 minutes the sedimented samples were fixed using 3.2% formaldehyde, subsequently washed with PBS, and stained for F-actin using rhodamine-phalloidin. (A) Wild-type (WT), Btk-/-, and Tec-/- but not Btk-/-/Tec-/- platelets spread along collagen fibers. With the addition of ADP all genotypes showed spreading, and in the presence of PP1 all genotypes displayed inhibition. Platelets sedimenting on collagen were in the presence of 10 μM lotrafiban to remove the effects of GPIIbIIIa. (B) All genotypes show spreading on fibrinogen with or without ADP. Pictures displayed are small fields of view from separate experiments obtained using a × 100 objective and are representative of 3 experiments.
Platelets that have undergone spreading on fibrinogen have a morphology distinct from that seen on fibrous collagen. Although platelets spread along the length of a collagen fiber, they show only a limited formation of lamellipodia and filopodia on fibrinogen (Figure 6B), in agreement with previous reports on murine platelets.12 This is in contrast to results for human platelets that show more extensive spreading on fibrinogen. In the presence of ADP, however, the formation of lamellipodia is more extensive, and stress fibers can be readily seen (Figure 6B). Despite the use of a relatively high density of platelets, there was no evidence of platelet clumping or aggregate formation. PP1 substantially inhibited spreading on fibrinogen, with the platelets remaining round as seen on collagen in the presence of the Src family kinase inhibitor. Significantly, the absence of Btk, Tec, or both kinases had no effect on spreading in the absence or presence of ADP or PP1 (Figure 6B).
These results provide evidence for a combined role of Btk and Tec in mediating spreading on fibrillar collagen, but they demonstrate that the presence of one or the other is sufficient to support this response. In contrast, neither Tec family kinase appears to play a role in actin-fiber-dependent spreading on fibrinogen despite reports of the tyrosine phosphorylation of Btk.
Discussion
This study has investigated the role of Tec and Btk in the activation of platelets by GPVI and the integrin αIIbβ3. The work on GPVI signaling extends previous studies in XLA patients that demonstrated an important role for Btk in the regulation of PLCγ2 downstream of the immunoglobulin receptor. The present results confirm this observation in murine platelets and show that the remaining functional activity in platelets in the absence of Btk can be explained by the presence of the related tyrosine kinase Tec. Interestingly, although Tec appears to play, at best, only a minor role in GPVI signaling in the presence of Btk, it is able to play a substantial role in the absence of Btk, but it is unable to fully compensate for the absence of this kinase. Strikingly, and in contrast to the roles of Btk and Tec in GPVI signaling, we found no evidence to suggest roles for the 2 kinases in outside-in signaling by the integrin αIIbβ3 despite induction of their phosphorylation by this pathway. This adds to the growing list of differences in the signaling cascades used by GPVI and αIIbβ3 in spite of a significant number of similarities.
Tec and Btk are tyrosine phosphorylated in wild-type murine platelets after stimulation with collagen and CRP, thereby demonstrating that they are regulated downstream of the GPVI-FcR γ-chain complex. Murine platelets deficient in either Tec or Btk do not show a significant change in the degree of tyrosine phosphorylation of the remaining kinase. Similarly, tyrosine phosphorylation of Syk is not altered in platelets deficient in Btk, Tec, or both Tec family kinases, consistent with a role for Syk upstream of their regulation. There is, however, a small reduction in tyrosine phosphorylation of the adapter SLP-76 in the absence of Btk and Tec in CRP-stimulated platelets, suggesting that the adapter is partially regulated downstream of the 2 Tec kinases. This is in agreement with a previous observation in SH2 domain containing inositol phosphatase (SHIP)-deficient platelets demonstrating that an increase in tyrosine phosphorylation of Btk is accompanied by a small increase in tyrosine phosphorylation of SLP-76.33 The most dramatic effect of the absence of Tec and Btk is seen on the tyrosine phosphorylation of PLCγ2. There is a dramatic but incomplete abolition of tyrosine phosphorylation of the phospholipase in Btk-/- platelets, which is further reduced in Btk-/-/Tec-/- platelets. This reveals an important role for Tec in mediating tyrosine phosphorylation of PLCγ2. Interestingly, tyrosine phosphorylation of PLCγ2 is not significantly altered in the absence of Tec alone, suggesting that its ability to phosphorylate the phospholipase is masked by the presence of Btk. A minor role for Tec in mediating PLCγ2 tyrosine phosphorylation in wild-type platelets, however, cannot be ruled out because of the relative insensitivity of the methodology used to assess phosphorylation. Indeed, evidence that Tec may play a minor role in mediating tyrosine phosphorylation of PLCγ2 in wild-type platelets is illustrated by the partial loss of aggregation to a low, submaximal concentration of CRP, as shown in Figure 3A. Alternatively, the partial loss of response may be attributed to a second, uncharacterized action of Tec.
The observation that Btk plays a more significant role than Tec in platelets is similar to the role in B cells, in which a reduction in pre-B-cell and peripheral B-cell numbers is seen in the absence of Btk but not in the absence of Tec.27 However, a dramatic reduction in pre-B-cell numbers and additional defects in peripheral B cells are seen in Btk/Tec double-deficient animals,27 indicating that Tec functions in the absence of Btk downstream of the B-cell receptor complex. Thus, our results in platelets strongly parallel the observations made in the B-cell lineage and again indicate that the absence of Btk unmasks roles for Tec kinase downstream of an ITAM-containing receptor complex (this time the GPVI receptor complex).
The observation of a dramatic reduction in tyrosine phosphorylation of PLCγ2 phosphorylation adds to a growing body of evidence that Tec family kinases directly phosphorylate and regulate the phospholipase.23,36 The question that emerges is why tyrosine phosphorylation of PLCγ2 is not abrogated in the absence of both Tec kinases. There are 2 likely explanations. The first is that platelets may express another member of the Tec family of kinases, such as Itk, Bmx, or Txk. Although we have been unable to detect the presence of any of these kinases in platelets using specific antibodies, this approach is ultimately dependent on the sensitivity of the antibodies and does not provide definitive evidence against a minor role for any of the kinases in mediating phosphorylation. This would require experimentation on the relevant knock-out mouse. The second explanation for the residual tyrosine phosphorylation is that it is mediated by a separate family of tyrosine kinase, such as one or more members of the Src or Syk family of kinases.23 However, it should be borne in mind that this residual phosphorylation may not give rise to activation.
It is well established that tyrosine phosphorylation of PLCγ2 is required for activation of the phospholipase. Additionally, studies in PLCγ2-/- platelets have shown a critical role for the phospholipase in mediating responses to collagen. The observation that a dramatic reduction in tyrosine phosphorylation of PLCγ2 leads to impairment of functional responses, including loss of Ca2+ elevation, α-granule secretion, exposure of procoagulant lipids, shape change, and aggregation is consistent with this role. Significantly, however, there is a residual shape change response in Btk and Tec double-deficient platelets to CRP that is able to synergize with ADP acting through the Gq-coupled P2Y1 receptor or the Gi-coupled P2Y12 receptor to convert threshold responses to substantial aggregation. The mechanism of the synergy is unclear. It could be mediated through a residual activation of PLCγ2 or by the activation of other effector pathways, such as small G proteins or PI 3-kinase. We speculate that this synergy may explain the absence of a major bleeding phenotype in the Btk/Tec double-deficient mice. In comparison patients who lack all GPVI-induced signaling because of a deficiency in the collagen receptor exhibit an intermediate increase in bleeding times, and their platelets are refractory to activation by collagen, though the nature of their clinical symptoms is mild. Therefore, the ability of Tec to compensate for the absence of Btk and the ability of GPVI-induced, Btk/Tec-independent signals to synergize with other pathways are both likely to contribute to the absence of reports of increases in bleeding times in XLA patients.
An increasing body of evidence supports a significant role for many of the proteins in the GPVI signaling cascade in outside-in signaling by the integrin αIIbβ3. This includes Src and Syk family tyrosine kinases, the adapter SLP-76, and PLCγ2.12,13 On the other hand, there are a significant number of differences in the 2 signaling cascades, notably with respect to the role of the FcR γ-chain and the membrane adapter LAT, neither of which plays a role downstream of the integrin.14,37 We have demonstrated for the first time (Figure 1A) that Btk and Tec are tyrosine phosphorylated downstream of αIIbβ3 in murine platelets that have undergone spreading on fibrinogen, thereby adding to the growing list of proteins that are tyrosine phosphorylated downstream of the 2 glycoprotein receptors. Despite this increase in tyrosine phosphorylation, however, we were unable to observe a defect in spreading of Btk/Tec double-deficient platelets on fibrinogen in contrast to the marked inhibition, but not complete abolition, of spreading on collagen. This may reflect the fact that tyrosine phosphorylation of Btk and Tec lies downstream of the spreading response or that other pathways are able to mask their roles in spreading. Interestingly, the spreading defect on collagen could be rescued by stimulation with ADP in a way similar to that seen with aggregation. This rescue is also likely to contribute to the apparent absence of a bleeding problem in the Btk/Tec double-deficient platelets.
In conclusion, we have confirmed the role of Btk in the regulation of PLCγ2 downstream of GPVI and have identified for the first time a role of Tec in this signaling pathway, which is unmasked in the absence of Btk. Significantly, the ablation of Btk and Tec dramatically inhibits platelet activation through GPVI, although a minor response remains that is able to synergize with G-protein-coupled receptor stimuli to mediate aggregation and spreading. We speculate that this synergy may limit the deleterious effect of the double knock-out on bleeding.
Prepublished online as Blood First Edition Paper, July 3, 2003; DOI 10.1182/blood-2003-04-1142.
Supported by the British Heart Foundation and Wellcome Trust (B.T.A., S.P.W.) and by the START Program of the Austrian Research Fund (W.E.). B.T.A. was a British Heart Foundation research student. S.P.W. holds a British Heart Foundation Chair.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Gudrun Werner (Novartis Research Institute, Vienna, Austria) for the use of the Flexstation for Ca2+ measurements and Dr Elisabeth Koller (Institute of Physiology, University of Vienna, Austria) for the use of the Born aggregometer. Furthermore, we thank Romana Maschek for help in the initial genotyping of the mice and Dr Yotis Senis for help with the phosphorylation studies.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal